Did Human Dispersal into Europe Cause the Continent-Wide Extinction of the Pig Sus strozzii at 1.8 Ma?—Review of a Debate
Abstract
1. Introduction
2. Materials and Methods
3. The History of the “Suid Gap”: Relevant Literature That Has Not Been Cited
4. Life History Traits in the Suoidea
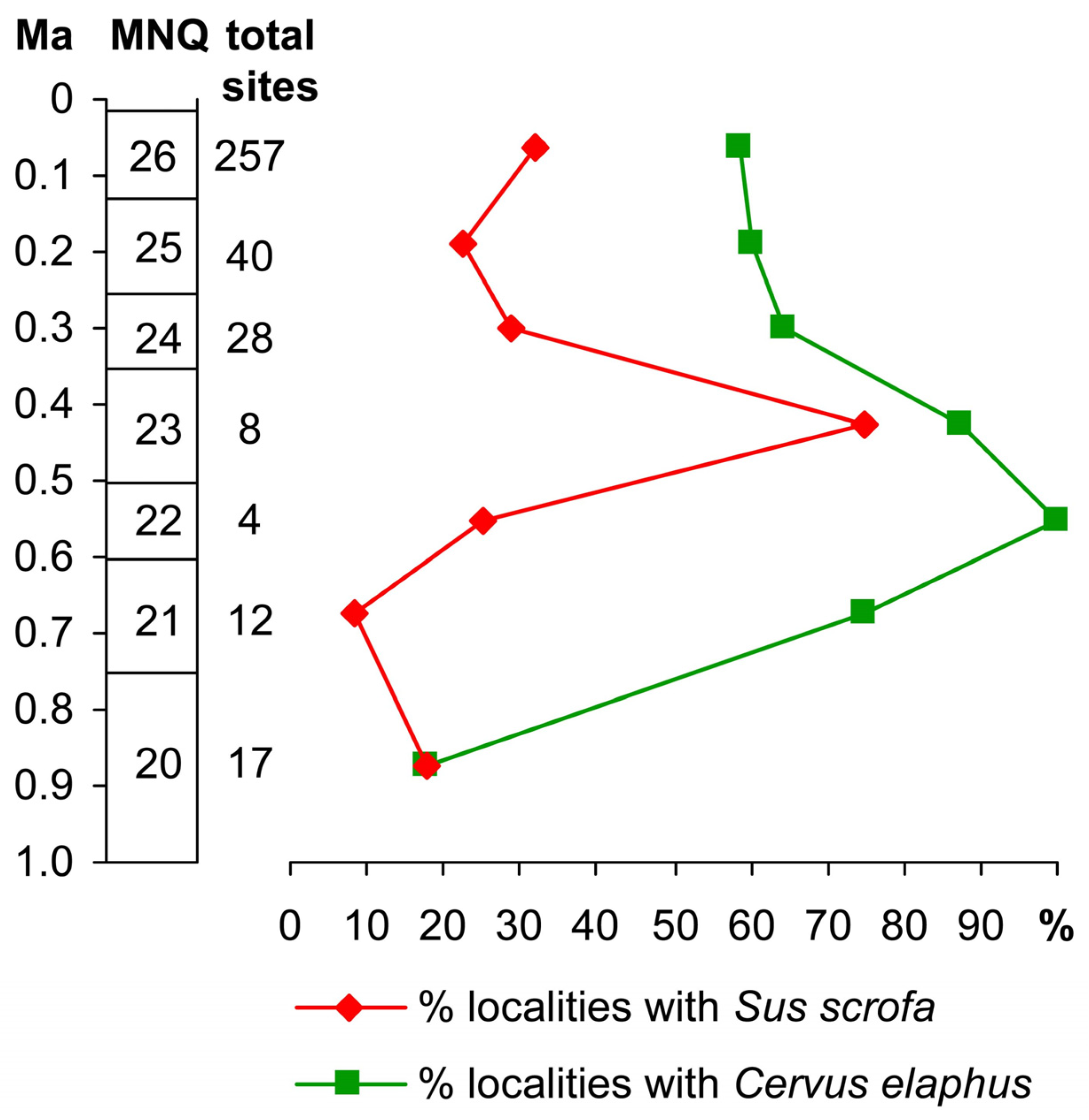
5. r-Selection and Pig Abundance in the Fossil Record
6. Are Pigs Abundant in the Pleistocene European Fossil Record?
7. Pigs Became Rare in Europe Long Before the Pleistocene
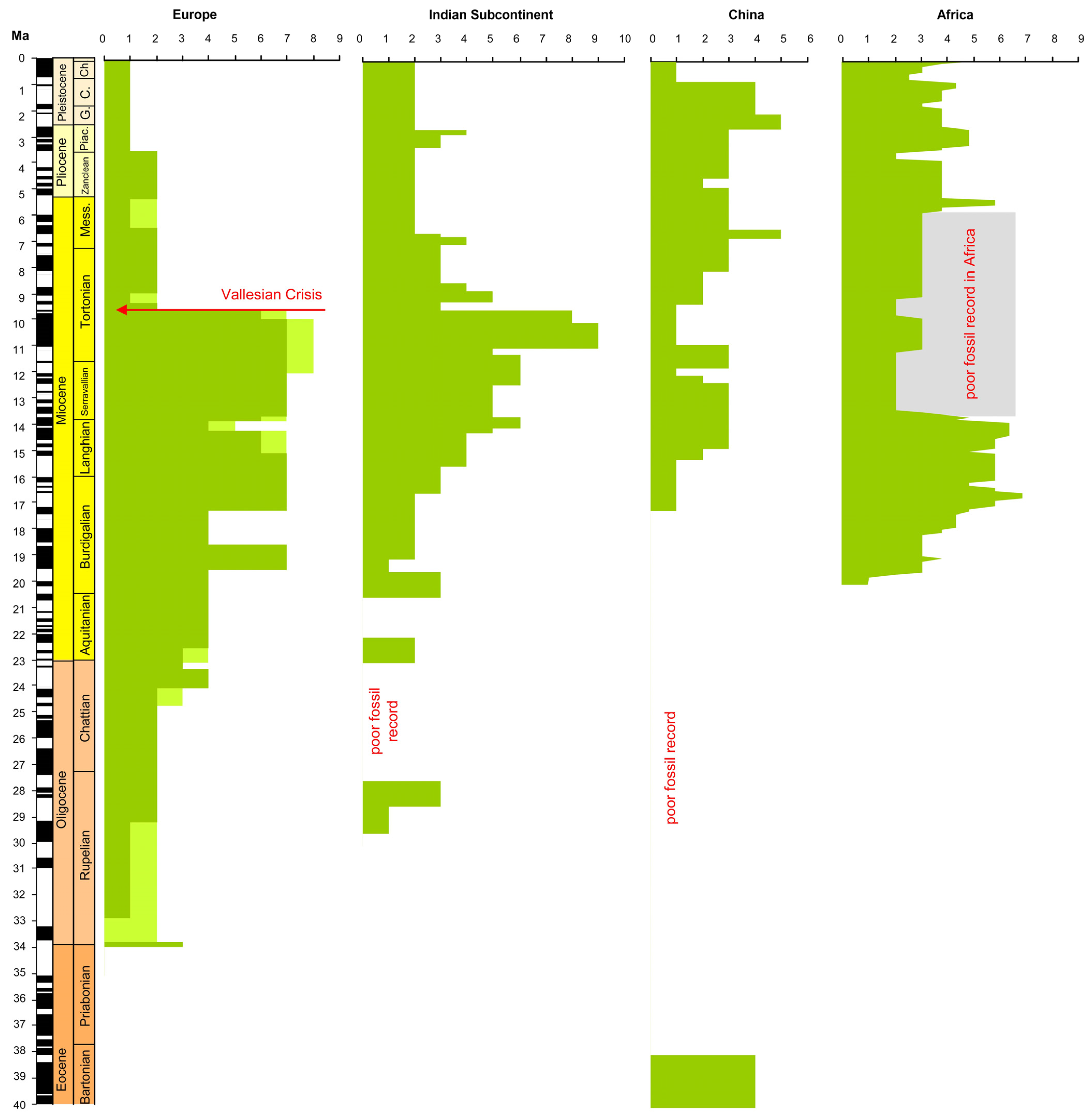
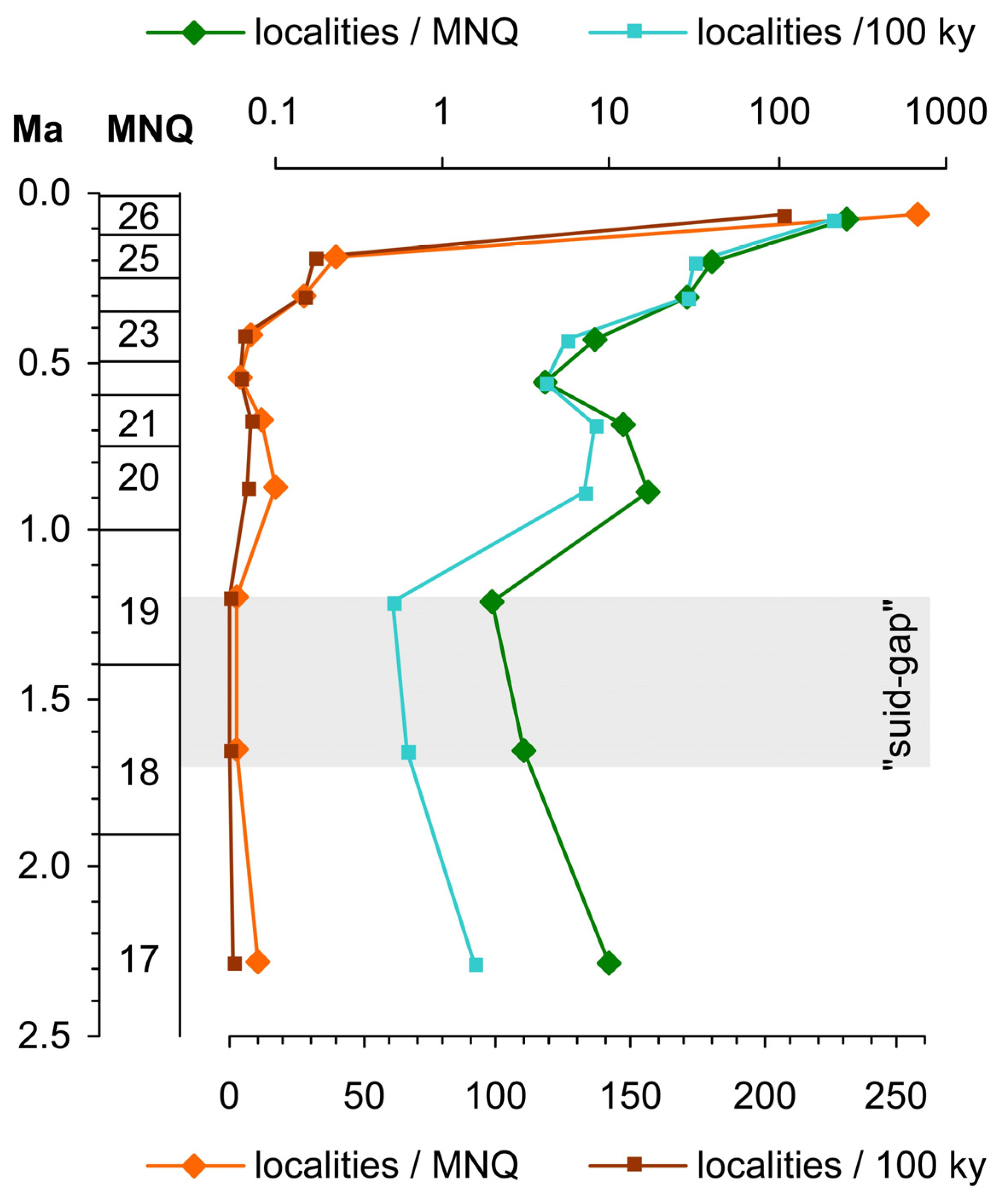
8. The Time of the “Suid Gap” Is Under-Represented in the Fossil Record
9. Is the Link with Human Ecology Real?
10. If the “Suid Gap” Existed, When Would It Have Started?
11. What Is the Importance of the “Suid Gap”?
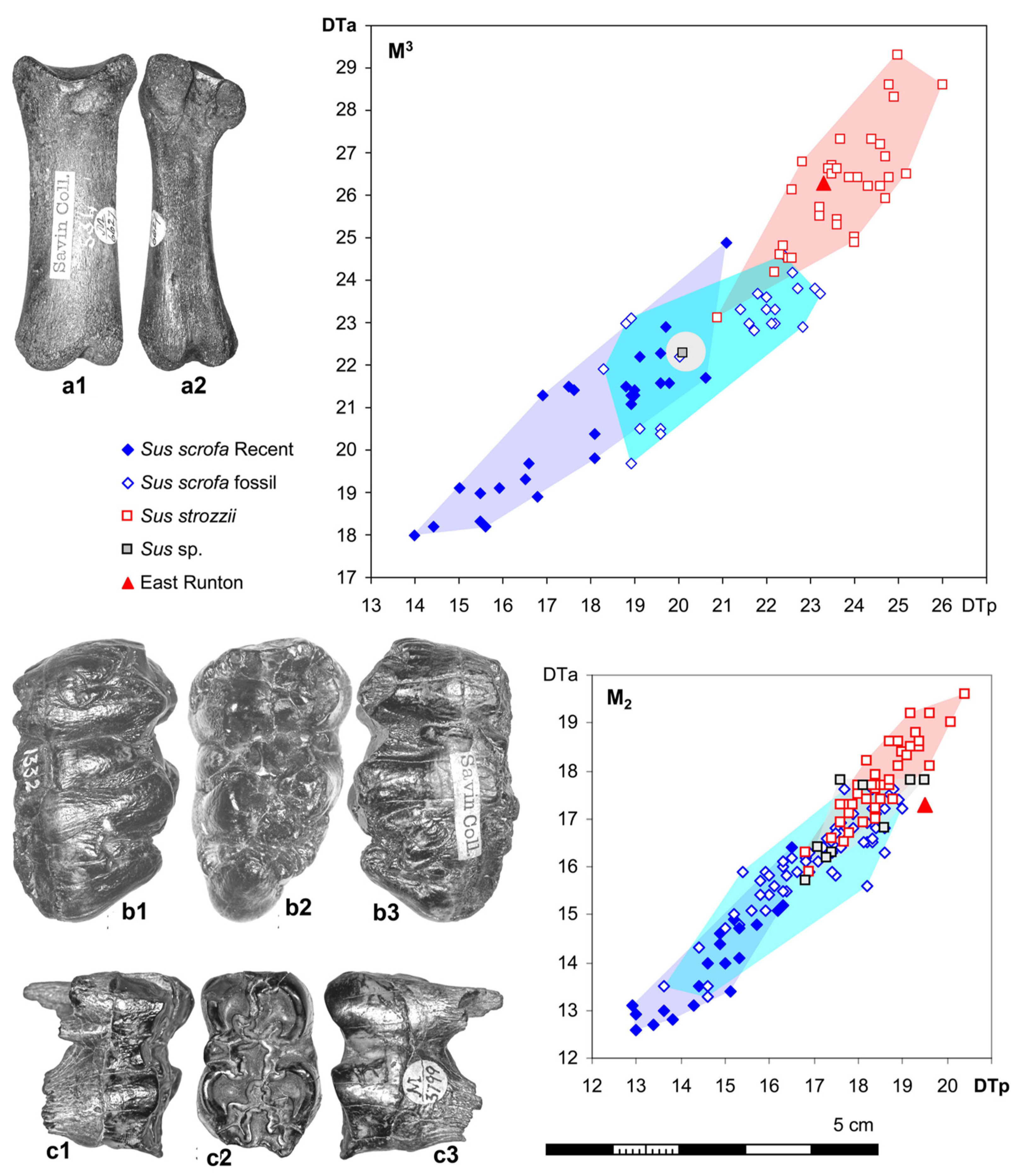
12. Are There Pigs from the “Suid Gap”?—Whom to Believe?
13. Sus strozzii from East Runton
14. The Sites That Should Prove the Absence of Pigs
15. Biostratigraphic Marker for the Epi-Villafranchian?
16. Discussion
- (1)
- pigs are r-selected and therefore
- (2a)
- they are best represented by fossils of deciduous dentition,
- (2b)
- their fossils are abundant,
- (3)
- therefore, if we do not find their fossils, they were absent,
- (4)
- therefore, they were absent between 1.8 and 1.2 Ma,
- (5)
- this was because they were displaced by humans,
- (6)
- the absence of pigs dates the period between 1.8 and 1.2 Ma,
- (7)
- since pigs were absent from 1.8 Ma onward, humans were present.
17. Future Directions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUT | Aristotle University of Thessaloniki |
| AVPM | Accademia Valdarnese del Poggio, Montevarchi |
| CENIEH | Centro Nacional de Investigación sobre la Evolución Humana, Burgos |
| CK | Coll. Kerkhoff |
| CMH | Coll. Mikko Haaramo, Helsinki |
| FSB | Forschungstelle Bilzingsleben, Friedrich Schiller-Universität Jena, Bilzingsleben |
| HUJ | Hebrew University, Jerusalem |
| IGF | Istituto di Geologia, now Museo di Storia Naturale, Firenze |
| IPHES | Institut Català de Paleoecologia Humana I Evolució Social, Tarragona |
| IQW | Institut für Quartärpaläontologie, now Forschungsstation für Quartärpaläontologie of the Senckenberg Forschungsinstitut und Naturmuseum Frankfurt, Weimar |
| MGL | Museum Guimet, Lyon |
| MNB | Museum für Naturkunde, Berlin |
| MNCN | Museo Nacional de Ciencias Naturales, Madrid |
| MNI | minimum number of individuals |
| MNPE | Musée national de Préhistoire, les Eyzies |
| NBC | Naturalis Biodiversity Center, Leiden |
| NHM | Natural History Museum, London |
| NMB | Naturhistorisches Museum, Basel |
| NMMa | Natuurhistorisch Museum, Maastricht |
| NMMi | Naturhistorisches Museum, Mainz |
| PIMUZ | Paläontologisches Institut und Museum der Universität, Zürich |
| SMNK | Staatliches Museum für Naturkunde, Karlsruhe |
| UCBL | Université Claude Bernard, Lyon |
| UCM | Universidad Complutense, Madrid |
References
- Stehlin, H.G. Ueber die Geschichte des Suiden-Gebisses. Abh. Schweiz. Paläontol. Ges. 1899–1990, 26, 1–527. [Google Scholar]
- Martínez-Navarro, B.; Madurell-Malapeira, J.; Ros-Montoya, S.; Espigares, M.-P.; Medin, T.; Hortolà, P.; Palmqvist, P. The Epivillafranchian and the arrival of pigs into Europe. Quat. Int. 2015, 389, 131–138. [Google Scholar] [CrossRef]
- Medin, T.; Martínez-Navarro, B.; Madurell-Malapeira, J.; Figueirido, B.; Kopaliani, G.; Rivals, F.; Kiladze, G.; Palmqvist, P.; Lordkipanidze, D. The bears from Dmanisi and the first dispersal of early Homo out of Africa. Sci. Rep. 2019, 9, 17752. [Google Scholar] [CrossRef]
- Cherin, M.; Sorbelli, L.; Crotti, M.; Iurino, D.A.; Sardella, R.; Souron, A. New material of Sus strozzii (Suidae, Mammalia) from the Early Pleistocene of Italy and a phylogenetic analysis of suines. Quat. Sci. Rev. 2018, 194, 94–115. [Google Scholar] [CrossRef]
- Cherin, M.; Alba, D.M.; Crotti, M.; Menconero, S.; Moullé, P.-É.; Sorbelli, L.; Madurell-Malapeira, J. The post-Jaramillo persistence of Sus strozzii (Suidae, Mammalia) in Europe: New evidence from the Vallparadís Section (NE Iberian Peninsula) and other coeval sites. Quat. Sci. Rev. 2020, 233, 106234. [Google Scholar] [CrossRef]
- Iannucci, A. The Occurrence of Suids in the Post-Olduvai to Pre-Jaramillo Pleistocene of Europe and Implications for Late Villafranchian Biochronology and Faunal Dynamics. Quaternary 2024, 7, 11. [Google Scholar] [CrossRef]
- Martínez-Navarro, B.; Madurell-Malapeira, J.; Ros-Montoya, S.; Espigares, M.P.; Rodríguez-Gómez, G.; Rook, L.; Palmqvist, P. The Late Villafranchian Absence of Pigs in Europe. Comment on Iannucci, A. The Occurrence of Suids in the Post-Olduvai to Pre-Jaramillo Pleistocene of Europe and Implications for Late Villafranchian Biochronology and Faunal Dynamics. Quaternary 2024, 7, 51. [Google Scholar] [CrossRef]
- Iannucci, A. On the Inconsistency of the “Suid Gap” Hypothesis and Its Inappropriate Biochronological Use in Dating the Localities of Orce (Venta Micena, Barranco León D, and Fuente Nueva 3). Reply to Martínez-Navarro et al. Comment on “Iannucci, A. The Occurrence of Suids in the Post-Olduvai to Pre-Jaramillo Pleistocene of Europe and Implications for Late Villafranchian Biochronology and Faunal Dynamics. Quaternary 2025, 8, 8. [Google Scholar] [CrossRef]
- Van der Made, J. Listriodontinae (Suidae, Mammalia), their evolution, systematics and distribution in time and space. Meded. Werkgr. Tert. Kwartaire Geol. 1996, 33, 253–254. [Google Scholar]
- Pilgrim, G.E. The Fossil Suidae of India. In Memoirs of the Geological Survey of India; Government of India, Central Publication Branch: Calcutta, India, 1926; Volume 8, pp. 1–65. [Google Scholar]
- Azzaroli, A. Filogenesi e Biologia di Sus Strozzii e di Sus Minor; Tipografia Moderna: La Spezia, Italy, 1954; Volume 48, pp. 41–71. [Google Scholar]
- Azzaroli, A. Villafranchian correlations based on large mammals. G. Geol. 1967, 35, 111–131. [Google Scholar]
- Azzaroli, A. The Villafranchian stage in Italy and the Plio-Pleistocene boundary. G. Geol. 1977, 41, 61–79. [Google Scholar]
- Azzaroli, A.; De Giuli, C.; Ficcarelli, G.; Torre, D. Late Pliocene to early Mid-Pleistocene mammals in Eurasia: Faunal succession and dispersal events. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1988, 66, 77–100. [Google Scholar] [CrossRef]
- Kurtén, B. Pleistocene Mammals of Europe; Weidenfeld & Nicolson: London, UK, 1968; pp. 1–317. [Google Scholar]
- Guérin, C. Première biozonation du Pléistocène européen, principal résultat biostratigraphique de l‘étude des Rhinocerotidae (Mammalia, Perissodactyla) du Miocène terminal au Pléistocène Supérieur d’Europe occidentale. Geobios 1982, 15, 593–598. [Google Scholar] [CrossRef]
- Faure, M.; Guérin, C. Sus strozzii et Sus scrofa, deux mammifères artiodactyles, marquers des paleoenvironnements. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1984, 48, 215–228. [Google Scholar] [CrossRef]
- Van der Made, J.; Moyá-Solá, S. European Suinae (Artiodactyla) from the Late Miocene onwards. Bolletino Soc. Paleontol. Ital. 1989, 28, 329–339. [Google Scholar]
- Faure, M.; Guérin, C. La grande faune d’Europe occidental au Pléistcène moyen et supérieur et ses potentialités d’informationen préhistoire. Mém. Société Géologique Fr. 1992, 160, 77–84. [Google Scholar]
- Guérin, C.; Patou-Mathis, M. Introduction. Limites et problèmes de chronologie. In Les Grands Mammifères Plio-Pléistocènes d’Europe; Guérin, C., Patou-Mathis, M., Eds.; Masson: Paris, France; Milan, Italy; Barcelona, Spain, 1996; pp. 1–11. [Google Scholar]
- Guérin, C. Famille des Suidae. In Les Grands Mammifères Plio-Pléistocènes d’Europe; Guérin, C., Patou-Mathis, M., Eds.; Masson: Paris, France; Milan, Italy; Barcelona, Spain, 1996; pp. 40–43. [Google Scholar]
- Guérin, C.; Faure, M. The wild boar (Sus scrofa priscus) from the post-Villafranchian lower Pleistocene of Untermassfeld. In Das Pleistozän von Untermassfeld bei Meiningen (Thüringen) Teil 1; Kahlke, R.D., Ed.; Dr. Rudolf Habelt GMBH: Bonn, Germany, 1997; pp. 375–383, pls. 63–67. [Google Scholar]
- Van der Made, J.; Rosell, J.; Blasco, R. Faunas from Atapuerca at the Early–Middle Pleistocene limit: The ungulates from level TD8 in the context of climatic change. Quat. Int. 2017, 433, 296–346. [Google Scholar] [CrossRef]
- Iannucci, A. New results on suids from the Early Pleistocense site of Untermassfeld. In The Pleistocene of Untermassfeld Near Meiningen (Thüringen, Germany) Part 5; Kahlke, R.D., Ed.; Dr. Rudolf Habelt GMBH: Bonn, Germany, 2022; pp. 1339–1354. [Google Scholar]
- von Koenigswald, W.; Tobien, H. Bemerkungen zur Alterstellung der pleistozänen Mosbach-Sande bei Wiesbaden. Geol. Jahrb. Hesse 1987, 115, 227–237. [Google Scholar]
- Daams, R.; Freudenthal, M. The Ramblian and the Aragonian: Limits, subdivision, geographical and temporal extension. In European Neogene Mammal Chronology; Lindsay, E.H., Fahlbusch, V., Mein, P., Eds.; Plenum Press: New York, NY, USA; London, UK, 1990; pp. 51–59. [Google Scholar]
- Agustí, J.; Moyá-Solá, S.; Pons Moyà, J. La sucesión de mamíferos en el Pleistoceno inferior de Europa: Proposición de una nueva escala bioestratigráfica. Paleontol. Evol. 1987, 1, 287–295. [Google Scholar]
- Van der Made, J. Sus nanus nov. sp. a Pliocene dwarf pig from Capo Figari (Sardinia). Bolletino Soc. Paleontol. Ital. 1988, 27, 367–378. [Google Scholar]
- Van der Made, J. A range chart for European Suidae and Tayassuidae. Paleontol. Evol. 1990, 23, 99–104. [Google Scholar]
- Van der Made, J. Iberian Suoidea. Paleontol. Evol. 1990, 23, 83–97. [Google Scholar]
- Van der Made, J. Los Suoidea de la Península Ibérica. In Avances en el Conocimiento del Terciario Ibérico; Calvo, J.P., Morale, J., Eds.; Universidad Complutense de Madrid, Museo Nacional de Ciencias Naturales, Museo de Cuenca: Madrid, Spain; Cuenca, Spain, 1997; pp. 109–112. [Google Scholar]
- Kostopoulos, D.S.; Konidaris, G.E.; Amanatidou, M.; Chitoglou, K.; Fragkioudakis, E.; Gerakakis, N.; Giannakou, V.; Gkeme, A.; Kalaitzi, C.; Tsakalidis, C.; et al. The new fossil site Krimni-3 in Mygdonia Basin and the first evidence of a giant ostrich in the Early Pleistocene of Greece. PalZ 2023, 97, 147–161. [Google Scholar] [CrossRef]
- Meijaard, E.; d’Huart, J.P.; Oliver, W.L.R. Family Suidae (pigs). In Handbook of the Mammals of the World. II Hoofed Mammals; Wilson, D.E., Mittemeier, R.A., Eds.; Lynx: Barcelona, Spain, 2011; pp. 248–291. [Google Scholar]
- Bogin, B. Evolutionary Hypotheses for Human Childhood. Yearb. Phys. Anthropol. 1997, 40, 63–89. [Google Scholar] [CrossRef]
- Smith, B.H. Dental Development and the Evolution of Life History in Hominidae. Am. J. Phys. Anthropol. 1991, 86, 157–174. [Google Scholar] [CrossRef]
- Bermúdez de Castro, J.M. El Chico de la Gran Dolina: En los Origenes de lo Humano; Editorial Critica: Barcelona, Spain, 2009; pp. 1–295. [Google Scholar]
- Mason, D.R. Dentition and age determination of the Warthog Phacochoerus aethiopicus in Zululand, South Africa. Koedoe 1984, 27, 79–119. [Google Scholar] [CrossRef]
- Van der Made, J.; Choudhary, D.; Singh, N.P.; Sharma, K.M.; Singh, N.A.; Patnaik, R. Listriodon dukkar sp. nov. (Suidae, Artiodactyla, Mammalia) from the late Miocene of Pasuda (Gujarat, India): The decline and extinction of the Listriodontinae. PalZ 2022, 96, 355–383. [Google Scholar] [CrossRef]
- Krushka, D. Über die Evolution des Gehirns in der Ordnung Artiodactyla Owen, 1848, insbesondere der Teilordnumg Suina Gray, 1868. Z. Für Säugetierkd 1970, 35, 214–238. [Google Scholar]
- Kruska, D. Über das Gehirn des Zwergwildschweins, Sus (Porcula) salvanius Hodgson, 1847 Ein Beitrag zur Problematik vergleichender Hirnuntersuchungen bei Säugetieren unterschiedlicher Körpergröße1. J. Zool. Syst. Evol. Res. 1983, 20, 1–12. [Google Scholar] [CrossRef]
- Iannucci, A.; Gasparik, M.; Sardella, R. First report of Sus strozzii (Suidae, Mammalia) from the Early Pleistocene of Hungary (Dunaalmás) and species distinction based on deciduous teeth. Sci. Nat. 2020, 107, 5. [Google Scholar] [CrossRef]
- Harris, J.M.; White, T.D. Evolution of the Plio-Pleistocene African Suidae. Trans. Am. Philos. Soc. 1979, 69, 1–128. [Google Scholar] [CrossRef]
- Pickford, M. Les Suoidea (Artiodactyla) de Sansan: Systématique, paléoécologie, biogéographie et biochronologie. Mém. Muséum. Natl. Hist. Nat. 2012, 203, 249–277. [Google Scholar]
- Hellmund, M. Schweineartige (Suina, Artiodactyla, Mammalia) aus oligo-miozänen Fundstellen Deutschlands, der Schweiz und Frankreichs II. Revision von Palaeochoerus POMEL 1847 und Propaleochoerus STEHLIN 1899 (Tayassuidae). Stuttg. Beitr. Naturkunde Ser. B Geol. Paläontol 1992, 189, 1–75. [Google Scholar]
- Van der Made, J. Suoidea from the Lower Miocene of Cetina de Aragón, Spain. Rev. Esp. Paleontol. 1994, 9, 1–23. [Google Scholar]
- Hellmund, M. Schweineartige (Suina, Artiodactyla, Mammalia) aus oligo-miozänen Fundstellen Deutschlands, der Schweiz und Frankreichs, I. Hyotherium meissneri (Suidae) aus dem Untermiozän von Ulm-Westtangente (Baden-Würtenberg). Stuttg. Beitr. Naturkunde Ser. B Geol. Paläontol 1991, 176, 1–69. [Google Scholar]
- Van der Made, J. Aureliachoerus from Oberdorf and other Aragonian pigs from Styria. Ann. Naturhistorisches Mus. Wien. 1998, 99A, 225–277. [Google Scholar]
- Van der Made, J. The pigs and ‘Old World peccaries’ (Suidae and Palaeochoeridae, Suoidea, Artiodactyla) from the Miocene of Sandelzhausen (southern Germany): Phylogeny and an updated classification of the Hyotheriinae and Palaeochoeridae. Paläontol. Z. 2010, 84, 43–121. [Google Scholar] [CrossRef]
- Hünermann, K.A. Die Suidae (Mammalia, Artiodactyla) aus den Dinotheriensanden (Unterpliozän + Pont) Rheinhessens (Südwestdeutschland). Schweiz. Paläontol. Abh. 1968, 86, 1–96, plate 1. [Google Scholar]
- Fortelius, M.; Van der Made, J.; Bernor, R.L. Middle and Late Miocene Suoidea of Central Europe and the Eastern Mediterranean: Evolution, Biogeography and Paleoecology. In The Evolution of Western Eurasian Neogene Mammal Faunas; Bernor, R.L., Fahlbusch, V., Mittmann, H.W., Eds.; Columbia University Press: New York, NY, USA; Chichester, UK, 1996; pp. 344–377. [Google Scholar]
- Van der Made, J.; Han, D.f. Suoidea from the hominoid locality Lufeng (Yunnan, China). Proc. K. Ned. Akad. Wet. 1994, 97, 27–82. [Google Scholar]
- Van der Made, J. The fossil pig from the Upper Miocene of Dorn Dürkheim in Germany. Cour. Forschungsinsitut Senckenberg. 1997, 197, 205–230. [Google Scholar]
- Van der Made, J.; Montoya, P.; Alcalá, L. Microstonyx (Suidae, Mammalia) from the Upper Miocene of Spain. Geobios 1992, 25, 395–413. [Google Scholar] [CrossRef]
- Guérin, C. Les rhinocéros (Mammalia, Perissodactyla) du Miocène terminal au Pléistocène supérieur en Europe occidentale. Comparaisons avec les espèces actuelles. Doc. Lab. Géologie Lyon. 1980, 79, 1181–1185. [Google Scholar]
- van de Weerd, A.; Daams, R. Quantititative composition of rodent faunas in the Spanish Neogene and paleoecological implications. Proc. K. Ned. Akad. Wet. Ser. B 1978, 81, 448–473. [Google Scholar]
- Van der Made, J. Ungulates from Atapuerca-TD6. J. Hum. Evol. 1999, 37, 389–413. [Google Scholar] [CrossRef] [PubMed]
- Sesé, C.; Soto, E. Catálogo de los yacimientos de vertebrados del Pleistoceno en las terrazas se los ríos Jarama y Manzanares. In Bifaces y Elefantes; Panera Gallego, J., Rubio Jara, S., Eds.; Museo Arqueológico Regional: Alcalá de Henares, Spain, 2002; pp. 430–457. [Google Scholar]
- Van der Made, J. An updated range chart for the Oligocene to recent west Eurasian Suoidea. Variation in species richness. Paleontol. Evol. 2022, 9, 34–36. [Google Scholar]
- Agustí, J.; Moyá-Solá, S. Mammal extinctions in the Vallesian (Upper Miocene). In Extinction Events in Earth History; Kauffman, E.G., Walliser, O.H., Eds.; Springer: Berlin/Heidelberg, Germany, 1990; pp. 425–432. [Google Scholar]
- Suc, J.P.; Fauquette, S.; Bessedik, M.; Bertini, A.; Zheng, Z.; Clauzon, G.; Suballyova, D.; Diniz, F.; Quézel, P.; Feddi, N.; et al. Neogene vegetation changes in West European and West circum-Mediterranean areas. In The Evolution of Neogene Terrestrial Ecostystems in Europe; Agustí, J., Rook, L., Andrews, P., Eds.; Cambridge University Press: Cambridge, UK, 1999; pp. 378–388. [Google Scholar]
- Agustí, J.; Sanz de Siria, A.; Garcés, M. Explaining the end of the hominoid experiment in Europe. J. Hum. Evol. 2003, 45, 145–153. [Google Scholar] [CrossRef]
- Van der Made, J. Climatical changes and species diversity in Suoidea. In Proceedings of the Abstracts, International Union for Quaternary Research, XIII International Congress, Beijing, China, 2–9 August 1991; p. 365. [Google Scholar]
- Van der Made, J. Migrations and climate. Cour. Forschungsinstitut Senckenberg. 1992, 153, 27–39. [Google Scholar]
- Van der Made, J. Late Pleistocene European and Late Miocene African accelerations of faunal change in relation to the climate and as a background to human evolution. Quat. Int. 2014, 326–327, 431–447. [Google Scholar] [CrossRef]
- Van der Made, J. Biometric trends in the Tetraconodontinae, a subfamily of pigs. Trans. R. Soc. Edinb. Earth Sci. 1999, 89, 199–225. [Google Scholar] [CrossRef]
- Buffon, G.L. Leclerc. In Las Épocas de la Naturaleza; Alianza Editorial: Madrid, Spain, 1997; pp. 1–429. [Google Scholar]
- Matthew, W.D. Climate and evolution. Second edition, revised and enlarged with critical additions by the author and others and a bibliography of his scientific works. Spec. Publ. N. Y. Acad. Sci. 1939, 1, 221–223. [Google Scholar]
- Van der Made, J.; Boulaghraief, K.; Chelli-Cheheb, R.; Cáceres, I.; Harichane, Z.; Sahnouni, M. The last North African hipparions—Hipparion decline and extinction follows a common pattern. Neues Jahrb. Geol. Paläontol. Abh. 2022, 303, 39–87. [Google Scholar] [CrossRef] [PubMed]
- Han, D.F. Artiodactyla fossils from Liucheng Gigantopithecus Cave in Guangxi. Mem. Inst. Vertebr. Palaeontol. Palaeoanthropology Acad. Sin. 1987, 18, 135–208, pls. 1–12. [Google Scholar]
- Huang, W.; Fang, Q. Wushan Hominid Site; Ocean Press: Beijing, China, 1991; pp. 1–230. [Google Scholar]
- Echassoux, A.; Moigne, A.M.; Moullé, P.M.; Li, T.Y.; Tang, X.B.; Li, W.S. Les faunes de grands mammifères du site de l’homme Yunxian. In Le Site de L’Homme de Yunxian; de Lumley, H., Li, T.Y., Eds.; CNRS Éditions: Paris, France, 2008; pp. 253–364. [Google Scholar]
- Liu, L. Chinese fossil Suoidea Systematics, Evolution, and Paleoecology. Ph.D. Thesis, University of Helsinki, Helsinki, Finland, 2003. Available online: https://helda.helsinki.fi/server/api/core/bitstreams/d923a2ad-491a-4f4e-aba5-4257148a6bf5/content (accessed on 18 March 2025).
- Andrews, P. Evolution and environment in the Hominoidea. Nature 1992, 360, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Begun, D.R. The Real Planet of the Apes: A New Story of Human Origins [Internet]; Princeton University Press: Princeton, NJ, USA, 2016; pp. 1–246. Available online: https://www.jstor.org/stable/j.ctt21c4v71 (accessed on 15 April 2025).
- Stuart, A.J. Pleistocene Vertebrates in the British Isles; Longman: London, UK; New York, NY, USA, 1982; pp. 1–212. [Google Scholar]
- Von Koenigswald, W.; Heinrich, W.D. Mittelpleistozäne Säugetierfaunen aus Mitteleuropa—Der Versuch einer biostratigraphischen Zuordnung. Kaupia 1999, 9, 53–112. [Google Scholar]
- Kowalski, K. Katalog Ssaków Pleijstocenu Polski; Polska Adkademia Nauk: Warshaw, Poland; Breslavia, Poland, 1959; pp. 1–267. [Google Scholar]
- Gliozzi, E.; Abbazzi, L.; Argenti, P.; Azzaroli, A.; Caloi, L.; Di Stefano, G.; Capasso Barbato, L.; Di Stefano, G.; Ficarelli, G.; Kotsakis, T.; et al. Biochronology of selected mammals, molluscs and ostracods from the Middle Pliocene to the Late Pleistocene in Italy: The state of the art. Riv. Ital. Paleontol. E Stratigr. 1997, 103, 369–388. [Google Scholar]
- Masini, F. I Bovini Villafranchiani Dell’ Italia. Ph.D. Thesis, Universtità Consorziate, Modena, Italy, Bologna, Italy, Rome, Italy, Florence, Italy, 1989. [Google Scholar]
- Abbazzi, L.; Benvenuti, M.; Rook, L.; Masini, F. Biochronology of the Mugello intermontane basin (Northern Apennines, Italy). Il Quat. 1995, 8, 5–10. [Google Scholar]
- De Giuli, C. Late Villafranchian faunas of Italy: The Selvella local fauna in the southern Chiana Valley—Umbria. Palaeontogr. Ital. 1986, 74, 11–50. [Google Scholar]
- Duval, M.; Arnold, L.J.; Bahain, J.-J.; Parés, J.M.; Demuro, M.; Falguères, C.; Shao, Q.; Voinchet, P.; Arnaud, J.; Berto, C.; et al. Re-examining the earliest evidence of human presence in western Europe: New dating results from Pirro Nord (Italy). Quat. Geochronol. 2024, 82, 101519. [Google Scholar] [CrossRef]
- Van der Made, J. Biogeography and climatic change as a context to human dispersal out of Africa and within Eurasia. Quat. Sci. Rev. 2011, 30, 1353–1367. [Google Scholar] [CrossRef]
- Genov, P. Food Composition of Wild Boar in North-eastern and Western Poland. Acta Theriol 1981, 26, 185–205. [Google Scholar] [CrossRef]
- Ditchkoff, S.S.; Mayer, J.J. Wild Pig Food Habits. In Wild Pigs: Biology; Damage, Control Techniques, and Management; Mayer, J.J., Brisbin, I.L., Eds.; Savanna River National Laboratory: Aitken, SC, USA, 2009; pp. 105–143. [Google Scholar]
- Cao, H.; Yang, X.; Peng, C.; Wang, Y.; Guo, Q.; Su, H. Winter Dietary Analysis Reveals the Foraging Differences of Wild Boar (Sus scrofa) in Different Regions of a Karst Mountainous Area. Animals 2023, 13, 727. [Google Scholar] [CrossRef] [PubMed]
- Zorn, M.I.; Van Gestel, C.A.M.; Eijsackers, H. Species-specific earthworm population responses in relation to flooding dynamics in a Dutch floodplain soil. Pedobiologia 2005, 49, 189–198. [Google Scholar] [CrossRef]
- Melis, C.; Szafrańska, P.A.; Jędrzejewska, B.; Bartoń, K. Biogeographical variation in the population density of wild boar (Sus scrofa ) in western Eurasia. J. Biogeogr. 2006, 33, 803–811. [Google Scholar] [CrossRef]
- Mellars, P.; French, J.C. Tenfold Population Increase in Western Europe at the Neandertal–to–Modern Human Transition. Science 2011, 333, 623–627. [Google Scholar] [CrossRef]
- Rodríguez, J.; Willmes, C.; Sommer, C.; Mateos, A. Sustainable human population density in Western Europe between 560.000 and 360.000 years ago. Sci. Rep. 2022, 12, 6907. [Google Scholar] [CrossRef]
- Rodríguez, J.; Mateos, A.; Martín-González, J.A.; Rodríguez-Gómez, G. How rare was human presence in Europe during the Early Pleistocene? Quat. Int. 2015, 389, 119–130. [Google Scholar] [CrossRef]
- Huguet, R.; Rodríguez-Álvarez, X.P.; Martinón-Torres, M.; Vallverdú, J.; López-García, J.M.; Lozano, M.; Terradillos-Bernal, M.; Expósito, I.; Ollé, A.; Santos, E.; et al. The earliest human face of Western Europe. Nature 2025, 640, 707–713. [Google Scholar] [CrossRef]
- Carbonell, E.; Bermúdez De Castro, J.M.; Parés, J.M.; Pérez-González, A.; Cuenca-Bescós, G.; Ollé, A.; Mosquera, M.; Huguet, R.; Van Der Made, J.; Rosas, A.; et al. The first hominin of Europe. Nature 2008, 452, 465–469. [Google Scholar] [CrossRef]
- Martin, P.S.; Klein, R.G. (Eds.) Quaternary Extinctions: A Prehistoric Revolution; University of Arizona Press: Tucson, AZ, USA, 1989; pp. 1–892. [Google Scholar]
- Napoleone, G.; Albianelli, A.; Azzaroli, A.; Mazzini, M. Dating the late Villafranchian (Pliocene) vertebrate collections of the Upper Valdarno (Tuscany, Italy) by the magnetochronologic ramowrk of the basin fill. Bolletino Della Soc. Paleontol. Ital. 2003, 42, 301–313. [Google Scholar]
- Toro-Moyano, I.; Martínez-Navarro, B.; Agustí, J.; Souday, C.; Bermúdez De Castro, J.M.; Martinón-Torres, M.; Fajardo, B.; Duval, M.; Falguères, C.; Oms, O.; et al. The oldest human fossil in Europe, from Orce (Spain). J. Hum. Evol. 2013, 65, 1–9. [Google Scholar] [CrossRef]
- Duval, M.; Falguères, C.; Bahain, J.; Grün, R.; Shao, Q.; Aubert, M.; Hellstrom, J.; Dolo, J.; Agusti, J.; Martínez-Navarro, B.; et al. The challenge of dating early pleistocene fossil teeth by the combined uranium series–electron spin resonance method: The Venta Micena palaeontological site (Orce, Spain). J. Quat. Sci. 2011, 26, 603–615. [Google Scholar] [CrossRef]
- Muttoni, G.; Scardia, G.; Kent, D.V. A critique of evidence for human occupation of Europe older than the Jaramillo subchron (∼1 Ma): Comment on ‘The oldest human fossil in Europe from Orce (Spain)’ by. J. Hum. Evol. 2013, 65, 746–749. [Google Scholar] [CrossRef] [PubMed]
- Muttoni, G.; Kent, D.V.; Scardia, G.; Martin, R.A. Bottleneck at Jaramillo for human migration to Iberia and the rest of Europe? J. Hum. Evol. 2015, 80, 187–190. [Google Scholar] [CrossRef][Green Version]
- Torres, T.; Llamas, J.F.; Canoira, L.; García-Alonso, P.; García-Cortés, A.; Mansilla, H. Amino acid chronology of the Lower Pleistocene deposits of Venta Micena (Orce; Granada, Andalusia, Spain). Org. Geochem. 1997, 86, 85–97. [Google Scholar] [CrossRef]
- Ortíz, J.E.; Torres, T.; Llamas, J.F.; Canoira, L.; García-Alonso, P.; García de la Morena, M.A.; Lucini, M. Datación de yacimientos paleontológicos de la cuenca de Guadix-Baza (sector de Cúllar-Baza, Granada, España) y primera estimación de la edad dela apertura de la cuenca mediante el método de racimzación de aminoácidos. Geogaceta 2000, 28, 109–112. [Google Scholar]
- Poidevin, J.L.; Cantagrel, J.M.; G.U.E.R.P.P.A. Un site unique du Plio-pléistocène en Europe: Le plateau de Perrier (Puy-de-Dôme). Rev. Sci. Nat. Auvergne 1984, 50, 87–95. [Google Scholar] [CrossRef]
- Oms, O.; Parés, J.M.; Martínez-Navarro, B.; Agustí, J.; Toro, I.; Martínez-Fernández, G.; Turq, A. Early human occupation of Western Europe: Paleomagnetic dates for two paleolithic sites in Spain. Proc. Natl. Acad. Sci. USA 2000, 97, 10666–10670. [Google Scholar] [CrossRef]
- Martínez Navaro, B. Revisión Sistemática y Estudio Cuantitativo de la Fauna de Macromamíferos del Yacimiento de Venta Micena (Orce, Granada). Ph.D. Thesis, Universitat Autònoma de Barcelona, Barcelona, Spain, 1991. [Google Scholar]
- Palmqvist, P.; Martínez-Navarro, B.; Arribas, A. Prey selection by terrestrial carnivores in a lower Pleistocene paleocommunity. Paleobiology 1996, 22, 514–534. [Google Scholar] [CrossRef]
- Vekua, A. Die Wirbeltierfauna des Villafranchiums von Dmanisi und ihre biostratigraphische Bedeutung. Jahrb. Röm. Ger. Zentralmuseums 1995, 42, 77–180. [Google Scholar]
- Freudenthal, M. Neogene vertebrates from the Gargano Peninsula, Italy. Scr. Geol. 1971, 3, 1–10. [Google Scholar]
- De Giuli, C.; Masini, F.; Torre, D. The latest Villafranchan faunas in Italy: The Pirro Nord fauna (Apricena, Gargano). Palaeontogr. Ital. 1987, 74, 51–62. [Google Scholar]
- De Giuli, C.; Torre, D. A microfauna with Allphaiomys pliocaenicus from Gargao, southern Italy. Palaeontogr. Ital. 1984, 73, 116–128. [Google Scholar]
- Arzarello, M.; Peretto, C.; Moncel, M.H. The Pirro Nord site (Apricena, Fg, Southern Italy) in the context of the first European peopling: Convergences and divergences. Quat. Int. 2015, 389, 255–263. [Google Scholar] [CrossRef]
- Lacombat, F.; Abbazzi, L.; Ferretti, M.P.; Martínez-Navarro, B.; Moullé, P.-E.; Palombo, M.-R.; Rook, L.; Turner, A.; Valli, A.M.-F. New data on the Early Villafranchian fauna from Vialette (Haute-Loire, France) based on the collection of the Crozatier Museum (Le Puy-en-Velay, Haute-Loire, France). Quat. Int. 2008, 179, 64–71. [Google Scholar] [CrossRef]
- Lacombat, F. Les grands Mammifères fossiles du Velay Les collections paléontologiques du Plio-Pleistocene du musée Crozatier, le Puy-en-Velay. Ann. Amis Mus. Crozatier 2005, 13–14, 201–208. [Google Scholar]
- Guérin, C. Préface. le Puy-en-Velay. Ann. Amis Mus. Crozatier 2005, 13–14, 11–13. [Google Scholar]
- Van der Made, J.; Moullé, P.E. Listriodon splendens . Ann. Amis Mus. Crozatier 2005, 13–14, 56–57. [Google Scholar]
- Van der Made, J.c.f. “Microstonyx”. Ann. Amis Mus. Crozatier 2005, 13–14, 58–59. [Google Scholar]
- Heintz, E. Les Cervidés villafranchiens de France et d’Espagne. Memoires Mus. Natl. Hist. Nat. Sér. 5 Sci. Terre. 1970, 32. vol. 1, 1–303, pls 1–40, vol. 2, 1–206. [Google Scholar]
- Couthures, J.; Pastre, J.F. Chronostratigraphie du Plio-PIéistocène d’Auvergne et du Velay: Nouveaux apports des datations radiométriques et du paléomagnétisme. Bull. Assoc. Fr. Pour L’étude Quat. 1983, 20, 9–18. [Google Scholar] [CrossRef]
- Lydekker, R. Catalogue of the Fossil Mammalia in the British Museum (Natural History). In Part II Containing the Order Ungulata, Suborder Artiodactyla; British Museum (Natural History): London, UK, 1885; pp. 1–324. [Google Scholar]
- Lister, A.M. The stratigraphical significance of deer species in the cromer forest-bed formation. J. Quat. Sci. 1993, 8, 95–108. [Google Scholar] [CrossRef]
- Fidalgo, D.; Rosas, A.; Madurell-Malapeira, J.; Pineda, A.; Huguet, R.; García-Tabernero, A.; Cáceres, I.; Ollé, A.; Vallverdú, J.; Saladie, P. A review on the Pleistocene occurrences and palaeobiology of Hippopotamus antiquus based on the record from the Barranc de la Boella Section (Francolí Basin, NE Iberia). Quat. Sci. Rev. 2023, 307, 108034. [Google Scholar] [CrossRef]
- Van der Made, J.; Dimitrijević, V. Eucladoceros montenegrensis n. sp. and other Cervidae from the Lower Pleistocene of Trlica (Montenegro). Quat. Int. 2015, 389, 90–118. [Google Scholar] [CrossRef]
- Koufos, G.; Kostopulos, D. Biochronology and succession of the Plio-Pleistocene macromammalian localities of Greece. Mém. Trav. Inst. Montp. 1997, 21, 619–634. [Google Scholar]
| Number Specimens | % of Expected | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Species | Locality | Source | D2-4 | P2-4 | M1-2 | M3 | D2-4 | P2-4 | M1-2 | M3 |
| Choermorus | Sansan | [43] | 6 | 56 | 65 | 31 | 6 | 57 | 100 | 95 |
| Propalaeochoerus sp. | Tomerdingen | [44] | 36 | 75 | 130 | 40 | 18 | 38 | 100 | 62 |
| Listriodontinae | >350 occurrences | [9] | 258 | 1192 | 1033 | 733 | 12 | 54 | 70 | 100 |
| Bunolistriodon meidamon | Pasalar | [9] | 30 | 82 | 71 | 44 | 23 | 62 | 81 | 100 |
| Listriodon splendens | Pasalar | [9] | 72 | 186 | 126 | 87 | 28 | 71 | 72 | 100 |
| Hyotherium meisneri | Cetina de Aragón | [45] | 5 | 30 | 32 | 18 | 9 | 56 | 89 | 100 |
| Hyotherium major | Ulm-Westtangente | [46] | 21 | 57 | 72 | 30 | 19 | 53 | 100 | 100 |
| Hyotherium soemmeringi | Styria | [47] | 46 | 164 | 149 | 69 | 21 | 73 | 100 | 93 |
| Conohyus simorrensis | Styria | [47] | 5 | 73 | 55 | 33 | 5 | 74 | 83 | 100 |
| All Suidae | Styria | [47] | 15 | 128 | 134 | 66 | 4 | 32 | 100 | 99 |
| Hyotherium soemmeringi | Sandelzhausen | [48] | 46 | 164 | 149 | 69 | 21 | 73 | 100 | 93 |
| Hyotherium soemmeringi | South German Molasse | [49] | 1 | 16 | 31 | 15 | 2 | 34 | 100 | 97 |
| Parachleuastochoerus steinheimensis | Deinotheriensande | [49] | 12 | 45 | 38 | 41 | 10 | 37 | 46 | 100 |
| Propotamochoerus palaeochoerus | Deinotheriensande | [49] | 5 | 65 | 132 | 156 | 1 | 14 | 42 | 100 |
| All Suidae | Deinotheriensande | [49] | 17 | 110 | 170 | 197 | 3 | 19 | 43 | 100 |
| Parachleuastochoerus | Rudabánya | [50] | 1 | 16 | 17 | 9 | 4 | 59 | 94 | 100 |
| Propotamochoerus palaeochoerus | Rudabánya | [50] | 43 | 55 | 72 | 28 | 40 | 51 | 100 | 78 |
| Propotamochoerus wui | Lufeng | [51] | 14 | 69 | 87 | 43 | 11 | 53 | 100 | 99 |
| Hippopotamodon | Dorn Dürkheim | [52] | 29 | 121 | 70 | 88 | 11 | 46 | 40 | 100 |
| Hippopotamodon | Spain | [53] | 15 | 57 | 54 | 40 | 13 | 48 | 68 | 100 |
| Sus strozzii | many | * | 33 | 160 | 140 | 81 | 14 | 66 | 86 | 100 |
| Sus sp. | Vallonnet | * | 2 | 50 | 38 | 9 | 4 | 88 | 100 | 47 |
| Sus sp. | Vallparadís | [5] | 2 | 15 | 12 | 8 | 8 | 63 | 75 | 100 |
| Sus scrofa | Mosbach | * | 6 | 23 | 38 | 23 | 9 | 33 | 83 | 100 |
| Sus scrofa | Taubach | * | 3 | 23 | 52 | 35 | 3 | 22 | 74 | 100 |
| Sus scrofa | Pinilla del Valle—Camino | * | 3 | 32 | 23 | 15 | 7 | 71 | 77 | 100 |
| mean (no doubles) | 11 | 52 | 85 | 93 | ||||||
| M3 Sus | M3 Cervidae | Cervidae/Sus | Species | Collection | |
|---|---|---|---|---|---|
| Pinilla del Valle | 8 | 15 | 1.88 | Dama dama | UCM |
| Taubach | 22 | 5 | 0.36 | Capreolus | IQW |
| Ehringsdorf | 2 | 34 | 17.00 | Cervus elaphus | IQW |
| Bilzingsleben | 3 | 126 | 42.00 | Cervus elaphus | FBFSUJ |
| Petralona | 4 | 56 | 14.00 | Dama | AUT |
| Mosbach | 18 | >>31 * | >>1.722 | Cervus elaphus | NMMi |
| Mauer | 4 | 55 | 13.75 | Cervus elaphus | SMNK |
| Voigtstedt | 1 | 12 | 12.00 | Cervus elaphus | IQW |
| Süssenborn | 2 | 16 | 8.00 | Cervus elaphus | IQW |
| Untermassfeld | 1 | 48 | 48.00 | Eucladoceros giulii | IQW |
| Vallonnet | 9 | 69 | 7.67 | Dama vallonnetensis | MPRM |
| Ubeidiya | 2 | 33 | 16.50 | Dama | HUJ |
| Upper Valdarno | 25 | 46 | 1.84 | Dama nestii | IGF |
| Olivola | 4 | 17 | 4.25 | Dama nestii | IGF |
| Tegelen | 2 | 16 | 8.00 | Dama rhenana | NBC, MNB, NMMa |
| mean | 13.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van der Made, J. Did Human Dispersal into Europe Cause the Continent-Wide Extinction of the Pig Sus strozzii at 1.8 Ma?—Review of a Debate. Quaternary 2025, 8, 26. https://doi.org/10.3390/quat8020026
van der Made J. Did Human Dispersal into Europe Cause the Continent-Wide Extinction of the Pig Sus strozzii at 1.8 Ma?—Review of a Debate. Quaternary. 2025; 8(2):26. https://doi.org/10.3390/quat8020026
Chicago/Turabian Stylevan der Made, Jan. 2025. "Did Human Dispersal into Europe Cause the Continent-Wide Extinction of the Pig Sus strozzii at 1.8 Ma?—Review of a Debate" Quaternary 8, no. 2: 26. https://doi.org/10.3390/quat8020026
APA Stylevan der Made, J. (2025). Did Human Dispersal into Europe Cause the Continent-Wide Extinction of the Pig Sus strozzii at 1.8 Ma?—Review of a Debate. Quaternary, 8(2), 26. https://doi.org/10.3390/quat8020026






