Hyperspectral Core-Logging for Past Primary Productivity Assessment
Abstract
1. Introduction
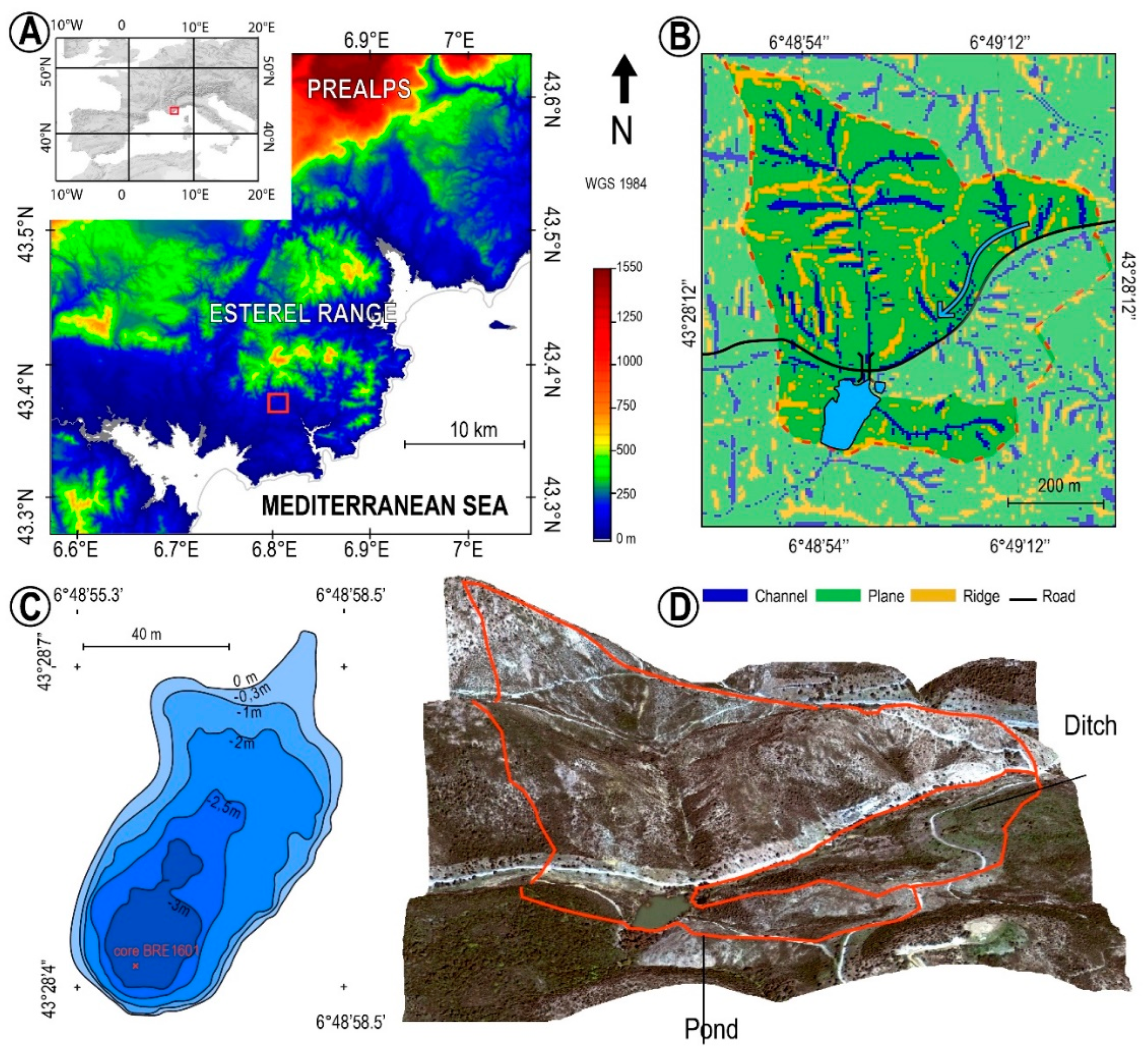
2. Study Site
3. Materials and Methods
3.1. Core Sampling
3.2. High Pressure Liquid Chromatography for Pigment Analysis
3.3. Hyperspectral Image Analysis
3.3.1. Analysis Protocols: Simulation of a Matrix Effect
3.3.2. Acquisition
3.4. Index Categories
4. Results and Interpretation
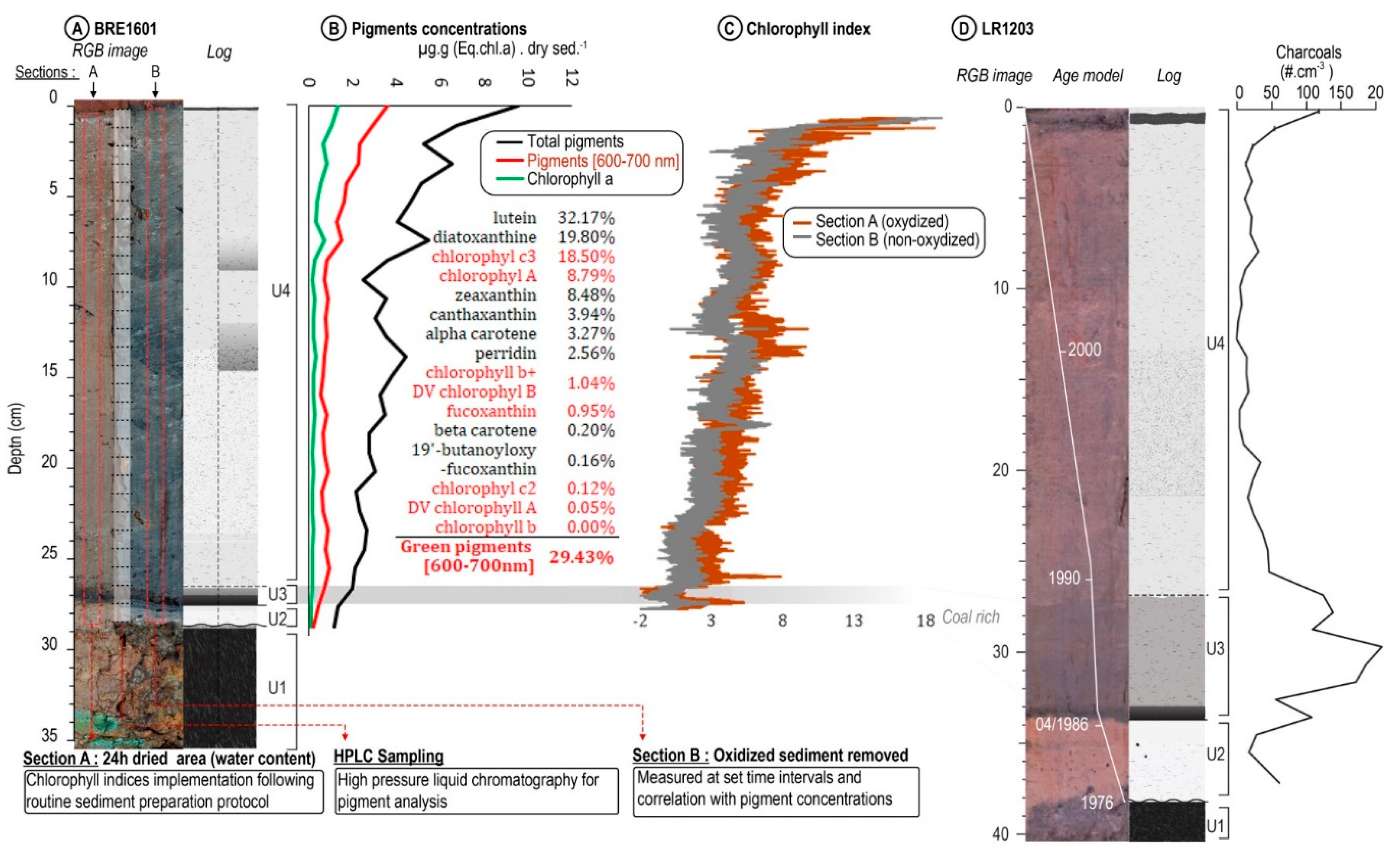
4.1. Sedimentology of BRE1601 and LR1203 Cores
4.2. Pigment Concentrations by HPLC
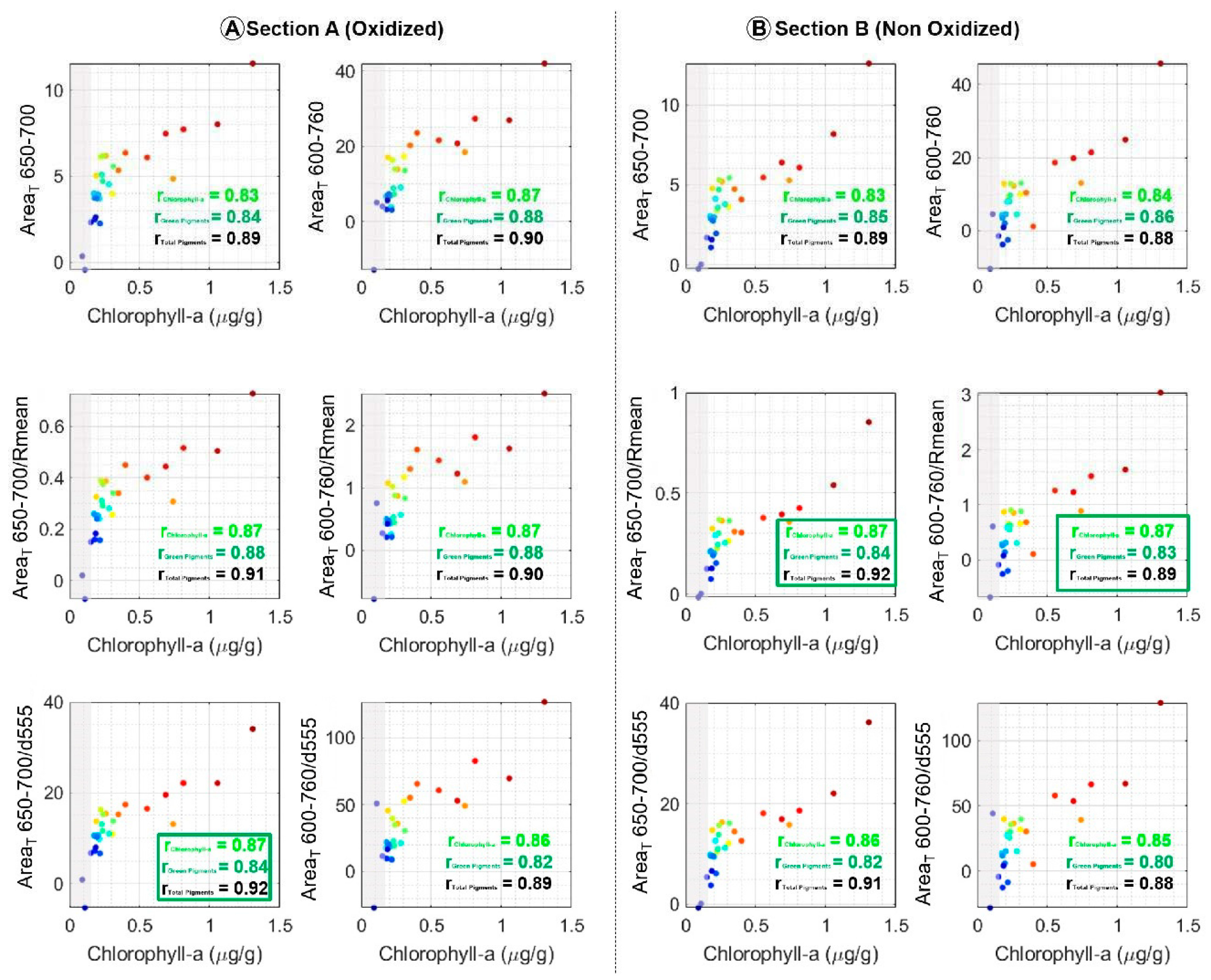
4.3. Comparison of Indices with Chlorophyll-a Concentration
5. Discussion
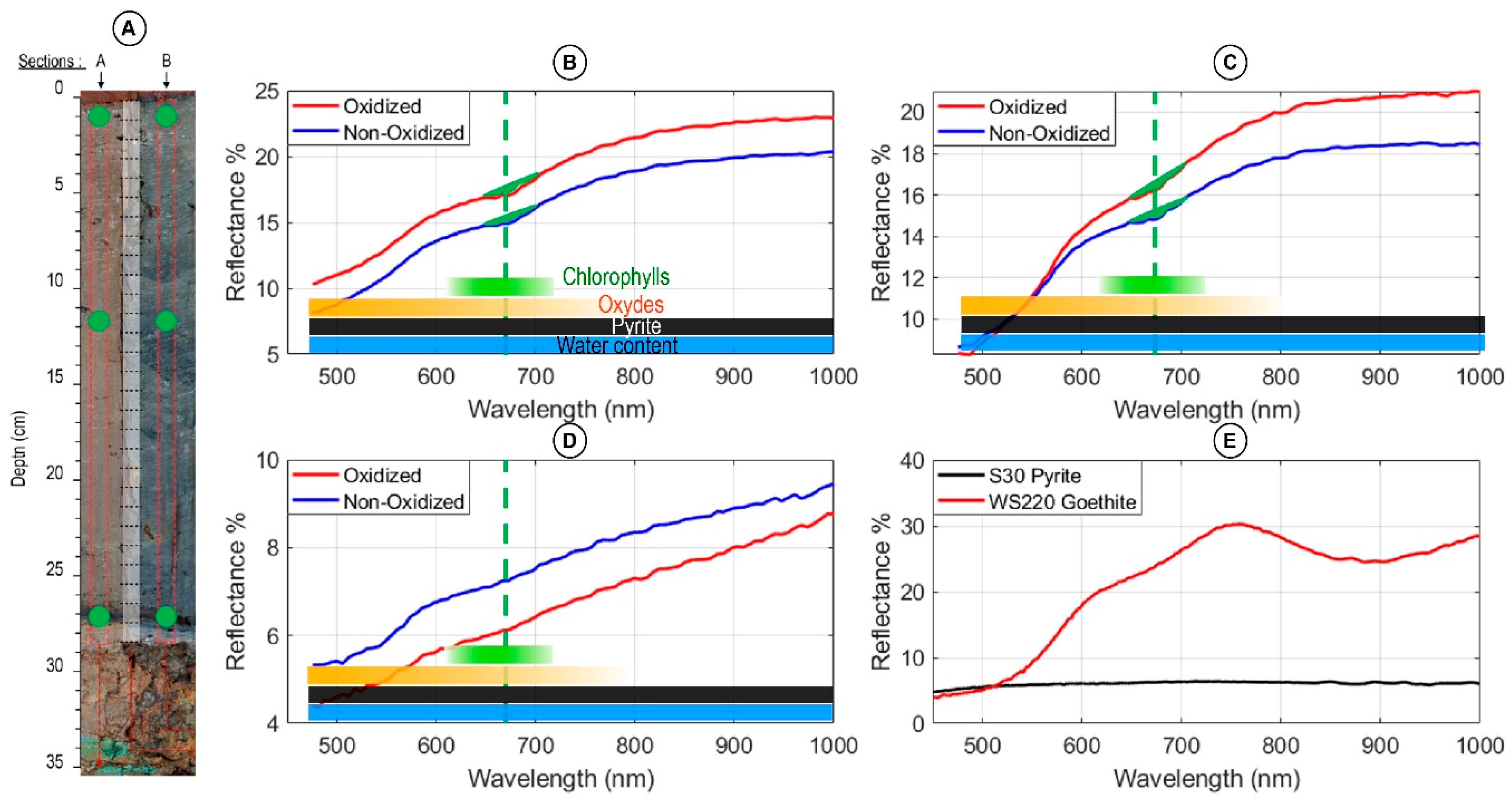
5.1. Impact of Sediment Oxidation
5.2. Matrix Effect Correction: Normalization of Indices
5.2.1. Normalization by the Average Spectrum: Rmean
5.2.2. Normalization by the Signature in Oxides: d555
5.3. The Problem of Quantification
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brantley, S.L.; Goldhaber, M.B.; Vala Ragnarsdottir, K. Crossing disciplines and scales to understand the critical zone. Elements 2007, 3, 307–314. [Google Scholar] [CrossRef]
- Friedrich, J.; Janssen, F.; Aleynik, D.; Bange, H.W.; Boltacheva, N.; Çagatay, M.N.; Dale, A.W.; Etiope, G.; Erdem, Z.; Geraga, M.; et al. Investigating hypoxia in aquatic environments: Diverse approaches to addressing a complex phenomenon. Biogeosciences 2014, 11, 1215–1259. [Google Scholar] [CrossRef]
- Jenny, J.P.; Francus, P.; Normandeau, A.; Lapointe, F.; Perga, M.E.; Ojala, A.; Schimmelmann, A.; Zolitschka, B. Global spread of hypoxia in freshwater ecosystems during the last three centuries is caused by rising local human pressure. Glob. Chang. Biol. 2016, 22, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Battarbee, R.W.; Anderson, N.J.; Bennion, H.; Simpson, G.L. Combining limnological and palaeolimnological data to disentangle the effects of nutrient pollution and climate change on lake ecosystems: Problems and potential. Freshw. Biol. 2012, 57, 2091–2106. [Google Scholar] [CrossRef]
- Mills, K.; Schillereff, D.; Saulnier-Talbot, É.; Gell, P.; Anderson, N.J.; Arnaud, F.; Dong, X.; Jones, M.; McGowan, S.; Massaferro, J.; et al. Deciphering long-term records of natural variability and human impact as recorded in lake sediments: A palaeolimnological puzzle. Wiley Interdiscip. Rev. Water 2017, 4, e1195. [Google Scholar] [CrossRef]
- Schneider, T.; Rimer, D.; Butz, C.; Grosjean, M. A high-resolution pigment and productivity record from the varved Ponte Tresa basin (Lake Lugano, Switzerland) since 1919: Insight from an approach that combines hyperspectral imaging and high-performance liquid chromatography. J. Paleolimnol. 2018, 60, 381–398. [Google Scholar] [CrossRef]
- Lee, M.; Shevliakova, E.; Malyshev, S.; Milly, P.C.D.; Jaffé, P.R. Climate variability and extremes, interacting with nitrogen storage, amplify eutrophication risk. Geophys. Res. Lett. 2016, 43, 7520–7528. [Google Scholar] [CrossRef]
- Wolfe, A.P.; Hobbs, W.O.; Birks, H.H.; Briner, J.P.; Holmgren, S.U.; Ingólfsson, Ó.; Kaushal, S.S.; Miller, G.H.; Pagani, M.; Saros, J.E.; et al. Stratigraphic expressions of the Holocene–Anthropocene transition revealed in sediments from remote lakes. Earth Sci. Rev. 2013, 116, 17–34. [Google Scholar] [CrossRef]
- Holmgren, S.U.; Bigler, C.; Ingólfsson, Ó.; Wolfe, A.P. The Holocene-Anthropocene transition in lakes of western spitsbergen, Svalbard (Norwegian high arctic): Climate change and nitrogen deposition. J. Paleolimnol. 2010, 43, 393–412. [Google Scholar] [CrossRef]
- Florian, C.R.; Miller, G.H.; Fogel, M.L.; Wolfe, A.P.; Vinebrooke, R.D.; Geirsdóttir, Á. Algal pigments in Arctic lake sediments record biogeochemical changes due to Holocene climate variability and anthropogenic global change. J. Paleolimnol. 2015, 54, 53–69. [Google Scholar] [CrossRef]
- Waters, C.N.; Steffen, W.; Waters, C.N.; Zalasiewicz, J.; Zalasiewicz, J.; Summerhayes, C.; Summerhayes, C.; Barnosky, A.D.; Barnosky, A.D.; Poirier, C.; et al. The Anthropocene is functionally and stratigraphically distinct from the Holocene. Science 2016, 351, aad2622. [Google Scholar] [CrossRef] [PubMed]
- Giguet-Covex, C.; Arnaud, F.; Poulenard, J.; Enters, D.; Reyss, J.-L.; Millet, L.; Lazzaroto, J.; Vidal, O. Sedimentological and geochemical records of past trophic state and hypolimnetic anoxia in large, hard-water Lake Bourget, French Alps. J. Paleolimnol. 2010, 43, 171–190. [Google Scholar] [CrossRef]
- Winegardner, A.K.; Legendre, P.; Beisner, B.E.; Gregory-Eaves, I. Diatom diversity patterns over the past c. 150 years across the conterminous United States of America: Identifying mechanisms behind beta diversity. Glob. Ecol. Biogeogr. 2017, 26, 1303–1315. [Google Scholar] [CrossRef]
- Zalasiewicz, J.A.N.; Williams, M.; Steffen, W.; Crutzen, P. The new world of the anthropocene. Environ. Sci. Technol. 2010, 44, 2228–2231. [Google Scholar] [CrossRef] [PubMed]
- Van Exem, A.; Debret, M.; Copard, Y.; Vannière, B.; Sabatier, P.; Marcotte, S.; Laignel, B.; Reyss, J.-L.; Desmet, M. Hyperspectral core logging for fire reconstruction studies. J. Paleolimnol. 2018, 59, 297–308. [Google Scholar] [CrossRef]
- Van Exem, A.; Debret, M.; Copard, Y.; Verpoorter, C.; De Wet, G.; Lecoq, N.; Sorrel, P.; Werner, A.; Roof, S.; Laignel, B.; et al. New source-to-sink approach in an arctic catchment based on hyperspectral core-logging (Lake Linné, Svalbard). Quat. Sci. Rev. 2019, 203, 128–140. [Google Scholar] [CrossRef]
- Jacq, K.; Perrette, Y.; Fanget, B.; Sabatier, P.; Coquin, D.; Martinez-Lamas, R.; Debret, M.; Arnaud, F. High-resolution prediction of organic matter concentration with hyperspectral imaging on a sediment core. Sci. Total Environ. 2019, 663, 236–244. [Google Scholar] [CrossRef]
- Jacq, K.; Giguet-Covex, C.; Sabatier, P.; Perrette, Y.; Fanget, B.; Coquin, D.; Debret, M.; Arnaud, F. High-resolution grain size distribution of sediment core with hyperspectral imaging. Sediment. Geol. 2019, 393–394, 105536. [Google Scholar] [CrossRef]
- Jacq, K.; Rapuc, W.; Benoit, A.; Coquin, D.; Fanget, B.; Perrette, Y.; Sabatier, P.; Wilhelm, B.; Debret, M.; Arnaud, F. Sedimentary structure discrimination with hyperspectral imaging in sediment cores. Sci. Total Environ. 2022, 817, 152018. [Google Scholar] [CrossRef]
- Butz, C.; Grosjean, M.; Fischer, D.; Wunderle, S.; Tylmann, W.; Rein, B. Hyperspectral imaging spectroscopy: A promising method for the biogeochemical analysis of lake sediments. J. Appl. Remote Sens. 2015, 9, 096031. [Google Scholar] [CrossRef]
- Butz, C.; Grosjean, M.; Poraj-Górska, A.; Enters, D.; Tylmann, W. Sedimentary Bacteriopheophytin a as an indicator of meromixis in varved lake sediments of Lake Jaczno, north-east Poland, CE 1891–2010. Glob. Planet. Chang. 2016, 144, 109–118. [Google Scholar] [CrossRef]
- Butz, C.; Grosjean, M.; Goslar, T.; Tylmann, W. Hyperspectral imaging of sedimentary bacterial pigments: A 1700-year history of meromixis from varved Lake Jaczno, northeast Poland. J. Paleolimnol. 2017, 58, 57–72. [Google Scholar] [CrossRef]
- Zander, P.D.; Wienhues, G.; Grosjean, M. Scanning Hyperspectral Imaging for In Situ Biogeochemical Analysis of Lake Sediment Cores: Review of Recent Developments Scanning Hyperspectral Imaging for n Situ Biogeochemical Analysis of Lake Sediment Cores: Review of Recent Developments. J. Imaging 2022, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Von Gunten, L.; D’Andrea, W.J.; Bradley, R.S.; Huang, Y. Proxy-to-proxy calibration: Increasing the temporal resolution of quantitative climate reconstructions. Sci. Rep. 2012, 2, 609. [Google Scholar] [CrossRef]
- Michelutti, N.; Smol, J.P. Visible spectroscopy reliably tracks trends in paleo-production. J. Paleolimnol. 2016, 56, 253–265. [Google Scholar] [CrossRef]
- Müller, P.J.; Suess, E. Productivity, sedimentation rate, and sedimentary organic matter in the oceans-I. Organic carbon preservation. Deep Sea Res. Part A Oceanogr. Res. Pap. 1979, 26, 1347–1362. [Google Scholar] [CrossRef]
- Middelburg, J.J.; Vlug, T.; Vandernat, F.J.W.A. Organic-Matter Mineralization in Marine Systems. Glob. Planet. Chang. 1993, 8, 47–58. [Google Scholar] [CrossRef]
- Ferland, M.E.; Prairie, Y.T.; Teodoru, C.; Del Giorgio, P.A. Linking organic carbon sedimentation, burial efficiency, and long-term accumulation in boreal lakes. J. Geophys. Res. Biogeosci. 2014, 119, 836–847. [Google Scholar] [CrossRef]
- Gudasz, C.; Bastviken, D.; Steger, K.; Premke, K.; Sobek, S.; Tranvik, L.J. Temperature-controlled organic carbon mineralization in lake sediments. Nature 2010, 466, 478–481. [Google Scholar] [CrossRef]
- Kristensen, E.; Holmer, M. Decomposition of plant materials in marine sediment exposed to different electron acceptors (O2, NO3− and SO42−), with emphasis on substrate origin, degradation kinetics, and the role of bioturbation. Geochim. Cosmochim. Acta 2001, 65, 419–433. [Google Scholar] [CrossRef]
- Moodley, L.; Middelburg, J.; Herman, P.; Soetaert, K.; de Lange, G. Oxygenation and organic-matter preservation in marine sediments: Direct experimental evidence from ancient organic carbon-rich deposits. Geology 2005, 33, 889–892. [Google Scholar] [CrossRef]
- Sobek, S.; Durisch-Kaiser, E.; Zurbrügg, R.; Wongfun, N.; Wessels, M.; Pasche, N.; Wehrli, B. Organic carbon burial efficiency in lake sediments controlled by oxygen exposure time and sediment source. Limnol. Oceanogr. 2009, 54, 2243–2254. [Google Scholar] [CrossRef]
- Kristensen, E. Organic matter diagenesis at the oxic/anoxic interface in coastal marine sediments, with emphasis on the role of burrowing animals. Hydrobiologia 2000, 426, 1–24. [Google Scholar] [CrossRef]
- Das, B.; Vinebrooke, R.D.; Sanchez-azofeifa, A.; Rivard, B.; Wolfe, A.P. Inferring sedimentary chlorophyll concentrations with reflectance spectroscopy: A novel approach to reconstructing historical changes in the trophic status of mountain lakes. Can. J. Fish. Aquat. Sci. 2005, 62, 1067–1078. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, X.; Nie, Y.; Sun, L. Using visible reflectance spectroscopy to reconstruct historical changes in chlorophyll-a concentration in East Antarctic ponds. Polar Res. 2013, 32, 19932. [Google Scholar] [CrossRef]
- Michelutti, N.; Blais, J.M.; Cumming, B.F.; Paterson, A.M.; Rühland, K.; Wolfe, A.P.; Smol, J.P. Do spectrally inferred determinations of chlorophyll-a reflect trends in lake trophic status? J. Paleolimnol. 2010, 43, 205–217. [Google Scholar] [CrossRef]
- Saunders, K.M.; Kamenik, C.; Hodgson, D.A.; Hunziker, S.; Siffert, L.; Fischer, D.; Fujak, M.; Gibson, J.A.E.; Grosjean, M. Late Holocene changes in precipitation in northwest Tasmania and their potential links to shifts in the Southern Hemisphere westerly winds. Glob. Planet. Chang. 2012, 92–93, 82–91. [Google Scholar] [CrossRef]
- Meyer, I.; Van Daele, M.; Fiers, G.; Verleyen, E.; De Batist, M.; Verschuren, D. Sediment reflectance spectroscopy as a paleo-hydrological proxy in East Africa. Limnol. Oceanogr. Methods 2018, 16, 92–105. [Google Scholar] [CrossRef]
- von Gunten, L.; Grosjean, M.; Rein, B.; Urrutia, R.; Appleby, P. A quantitative high-resolution summer temperature reconstruction based on sedimentary pigments from Laguna Aculeo, central Chile, back to AD 850. Holocene 2009, 19, 873–881. [Google Scholar] [CrossRef]
- Trachsel, M.; Grosjean, M.; Schnyder, D.; Kamenik, C.; Rein, B. Scanning reflectance spectroscopy (380–730 nm): A novel method for quantitative high-resolution climate reconstructions from minerogenic lake sediments. J. Paleolimnol. 2010, 44, 979–994. [Google Scholar] [CrossRef]
- Saunders, K.M.; Grosjean, M.; Hodgson, D.A. A 950 yr temperature reconstruction from Duckhole Lake, southern Tasmania, Australia. Holocene 2013, 23, 771–783. [Google Scholar] [CrossRef]
- Amann, B.; Lobsiger, S.; Fischer, D.; Tylmann, W.; Bonk, A.; Filipiak, J.; Grosjean, M. Spring temperature variability and eutrophication history inferred from sedimentary pigments in the varved sediments of Lake Zabińskie, north-eastern Poland, AD 1907–2008. Glob. Planet. Chang. 2014, 123, 86–96. [Google Scholar] [CrossRef]
- Boldt, B.R.; Kaufman, D.S.; Mckay, N.P.; Briner, J.P. Holocene summer temperature reconstruction from sedimentary chlorophyll content, with treatment of age uncertainties, Kurupa Lake, Arctic Alaska. Holocene 2015, 25, 641–650. [Google Scholar] [CrossRef]
- Rein, B.; Sirocko, F. In-situ reflectance spectroscopy—Analysing techniques for high-resolution pigment logging in sediment cores. Int. J. Earth Sci. 2002, 91, 950–954. [Google Scholar] [CrossRef]
- Wolfe, A.P.; Vinebrooke, R.D.; Michelutti, N.; Rivard, B.; Das, B. Experimental calibration of lake-sediment spectral reflectance to chlorophyll-a concentrations: Methodology and paleolimnological validation. J. Paleolimnol. 2006, 36, 91–100. [Google Scholar] [CrossRef]
- Debret, M.; Desmet, M.; Balsam, W.; Copard, Y.; Francus, P.; Laj, C. Spectrophotometer analysis of Holocene sediments from an anoxic fjord: Saanich Inlet, British Columbia, Canada. Mar. Geol. 2006, 229, 15–28. [Google Scholar] [CrossRef]
- Barillé, L.; Méléder, V.; Combe, J.P.; Launeau, P.; Rincé, Y.; Carrère, V.; Morançais, M. Comparative analysis of field and laboratory spectral reflectances of benthic diatoms with a modified Gaussian model approach. J. Exp. Mar. Bio. Ecol. 2007, 343, 197–209. [Google Scholar] [CrossRef]
- Zander, P.D.; Żarczyński, M.; Vogel, H.; Tylmann, W.; Wacnik, A.; Sanchini, A.; Grosjean, M. A high-resolution record of Holocene primary productivity and water-column mixing from the varved sediments of Lake Żabińskie, Poland. Sci. Total Environ. 2020, 755, 143713. [Google Scholar] [CrossRef]
- Balsam, W.L.; Deaton, B.C.; Damuth, J.E. Evaluating optical lightness as a proxy for carbonate content in marine sediment cores. Mar. Geol. 1999, 161, 141–153. [Google Scholar] [CrossRef]
- Debret, M.; Sebag, D.; Desmet, M.; Balsam, W.; Copard, Y.; Mourier, B.; Susperrigui, A.-S.; Arnaud, F.; Bentaleb, I.; Chapron, E.; et al. Spectrocolorimetric interpretation of sedimentary dynamics: The new “Q7/4 diagram”. Earth-Sci. Rev. 2011, 109, 1–19. [Google Scholar] [CrossRef]
- Balsam, W.L.; Deaton, B.C.; Damuth, J.E. The effects of water content on diffuse reflectance spectrophotometry studies of deep-sea sediment cores. Mar. Geol. 1998, 149, 177–189. [Google Scholar] [CrossRef]
- Balsam, W.; Ji, J.; Renock, D.; Deaton, B.C.; Williams, E. Determining hematite content from NUV/Vis/NIR spectra: Limits of detection. Am. Mineral. 2014, 99, 2280–2291. [Google Scholar] [CrossRef]
- Van Heukelem, L.; Thomas, C.S. Computer-assisted high-performance liquid chromatography method development with applications to the isolation and analysis of phytoplankton pigments. J. Chromatogr. A 2001, 910, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Jacq, K.; Martinez-Lamas, R.; Van Exem, A.; Debret, M. Hyperspectral Core-Logger Image Acquisition; Protocols.io: Berkeley, CA, USA, 2020. [Google Scholar]
- Sunshine, J.M.; Pieters, C.M.; Pratt, S.F. Deconvolution of Mineral Absorption Bands: An Improved Approach. J. Geophys. Res. 1990, 95, 6955–6966. [Google Scholar] [CrossRef]
- van der Meer, F.; Kopačková, V.; Koucká, L.; van der Werff, H.M.A.; van Ruitenbeek, F.J.A.; Bakker, W.H. Wavelength feature mapping as a proxy to mineral chemistry for investigating geologic systems: An example from the Rodalquilar epithermal system. Int. J. Appl. Earth Obs. Geoinf. 2018, 64, 237–248. [Google Scholar] [CrossRef]
- Verpoorter, C.; Carrère, V.; Combe, J.-P. Visible, near-infrared spectrometry for simultaneous assessment of geophysical sediment properties (water and grain size) using the Spectral Derivative-Modified Gaussian Model. J. Geophys. Res. Earth Surf. 2014, 119, 2098–2122. [Google Scholar] [CrossRef]
- Balsam, W.L.; Damuth, J.E.; Schneider, R.R. Comparison of shipboard vs. Shore-based spectral data from amazon fan cores: Implications for interpreting sediment composition. In Proceedings of the Ocean Drilling Program, Scientific Results; ODP Publications: College Station, TX, USA, 1997; Volume 155, pp. 193–215. [Google Scholar]
- Chang, S.; Berner, R.A. Coal weathering and the geochemical carbon cycle. Geochim. Cosmochim. Acta 1999, 63, 3301–3310. [Google Scholar] [CrossRef]
- Clark, R.N.; Swayze, G.A.; Wise, R.A.; Livo, K.E.; Hoefen, T.M.; Kokaly, R.F.; Sutley, S.J. USGS Digital Spectral Library splib06a; U.S. Geological Survey: Reston, VA, USA, 2007. [CrossRef]
- Hunt, G.R. Spectral Signatures of Particulate Minerals in the Visible and Near Infrared. Geophysics 1977, 42, 501. [Google Scholar] [CrossRef]
- Reuss, N.; Conley, D.J. Effects of sediment storage conditions on pigment analyses. Limnol. Oceanogr. Methods 2005, 3, 477–487. [Google Scholar] [CrossRef]
- Clark, R.N.; Roush, T.L. Reflectance spectroscopy: Quantitative analysis techniques for remote sensing applications. J. Geophys. Res. Solid Earth 1984, 89, 6329–6340. [Google Scholar] [CrossRef]
- Barranco, F.T.; Balsam, W.L.; Deaton, B.C. Quantitative reassessment of brick red lutites: Evidence from reflectance spectrophotometry. Mar. Geol. 1989, 89, 299–314. [Google Scholar] [CrossRef]
- Deaton, B.C.; Balsam, W.L. Visible spectroscopy—A rapid method for determining hematite and goethite concentration in geological materials. J. Sediment. Petrol. 1991, 61, 628–632. [Google Scholar] [CrossRef]
- Kaufman, D.S. An overview of late Holocene climate and environmental change inferred from Arctic lake sediment. J. Paleolimnol. 2009, 41, 1–6. [Google Scholar] [CrossRef]
- Weltje, G.J.; Tjallingii, R. Calibration of XRF core scanners for quantitative geochemical logging of sediment cores: Theory and application. Earth Planet. Sci. Lett. 2008, 274, 423–438. [Google Scholar] [CrossRef]


| Type | Continuum Removal Method | Name | Publication | Formula |
|---|---|---|---|---|
| Ratio | Divisaon by a reflectance band without influence of chlorophyll-a | 675/750 | Das et al., 2005 [34] | Ratio = |
| 645/675 | Das et al., 2005 [34] | |||
| 660/670 | Von Gunten et al., 2005 [24], Saunder et al., 2012 [37] | |||
| 590/690 | Trachsel et al., 2010 [40] | |||
| Amplitude | Subtraction by a reflectance band without infuence of chlorophyll-a | 675–750 | Das et al., 2005 [34] | Amplitude = |
| 650–675 | Das et al., 2005 [34] | |||
| First derivative | Enhance the efect of chlorophll-a on the continuum (spectral slope of the absorption feature) | d675 | Das et al., 2005 [34], Debret et al., 2006 [46], Debret et al., 2011 [50], Das et al., 2005 [34] | First derivative values at 675 nm |
| d660–d690 | Das et al., 2005 [34] | First derivative values at 660 nm | ||
| Reflectance feature depth | Theoretical continuum estimation at 670 nm and subtraction of the measured reflectance at 670 nm | RABD | Rein and Sirocko 2002 [44]; von Gunten et al., 2009 [39], von Gunten et al., 2012 [24], Trachsel et al., 2010 [40], Chen et al., 2013 [35], Saunders et al., 2012 [37], Saunders et al., 2013 [41], Amann et al., 2014 [42], Boldt et al., 2015 [43] | |
| I-band index adds a division by the mean reflectance (R mean) | Band-I | Meyer et al., 2017 [38] | I-band = | |
| Rein and Sirocko 2002 [44] | ||||
| Absorbance feature area | Area between two bands not influenced by chlorophyll-a concentration. A line draw between thoes two bans represents the theoretical continuum | Area | Wolfe et al., 2006 [45] | = ) + |
| 650–750 | ||||
| Area | Wolfe et al., 2006 [45], Michellutti 2010 [36], Saunder et al., 2012 [37], Trachsel et al., 2010 [40], Michelutti and Smol 2016 [25] | = ) + | ||
| 650–700 | ||||
| Area | Trachsel et al., 2010 [40] | |||
| 690–730 | ||||
| Area | Das et al., 2005 [34] | : Wavelength following ; : wavelength before | ||
| 600–760 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Exem, A.; Debret, M.; Copard, Y.; Jacq, K.; Verpoorter, C.; Marcotte, S.; Laignel, B.; Vannière, B. Hyperspectral Core-Logging for Past Primary Productivity Assessment. Quaternary 2022, 5, 53. https://doi.org/10.3390/quat5040053
Van Exem A, Debret M, Copard Y, Jacq K, Verpoorter C, Marcotte S, Laignel B, Vannière B. Hyperspectral Core-Logging for Past Primary Productivity Assessment. Quaternary. 2022; 5(4):53. https://doi.org/10.3390/quat5040053
Chicago/Turabian StyleVan Exem, Antonin, Maxime Debret, Yoann Copard, Kévin Jacq, Charles Verpoorter, Stéphane Marcotte, Benoit Laignel, and Boris Vannière. 2022. "Hyperspectral Core-Logging for Past Primary Productivity Assessment" Quaternary 5, no. 4: 53. https://doi.org/10.3390/quat5040053
APA StyleVan Exem, A., Debret, M., Copard, Y., Jacq, K., Verpoorter, C., Marcotte, S., Laignel, B., & Vannière, B. (2022). Hyperspectral Core-Logging for Past Primary Productivity Assessment. Quaternary, 5(4), 53. https://doi.org/10.3390/quat5040053






