Insect Marks on Bones from La Guillerma Archaeological Locality (Salado River Depression, Buenos Aires, Argentina)
Abstract
:1. Introduction
2. Background
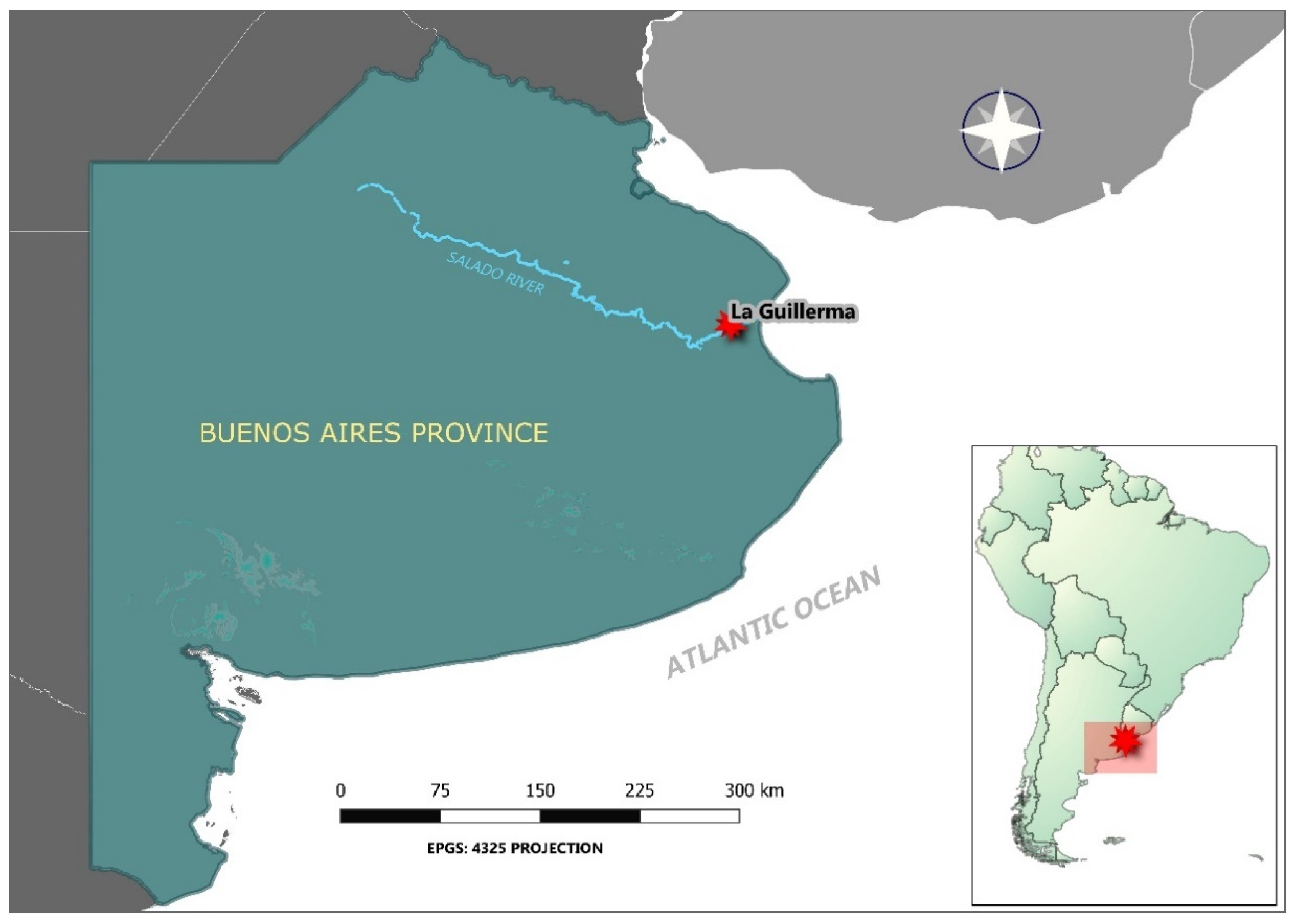
2.1. Research Area
2.2. Insects as Taphonomic Agents
3. Materials and Methods
4. Results
5. Discussion
Assessing Different Tracemakers
6. Final Remarks
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Corron, L.; Huchet, J.B.; Santos, F.; Dutour, O. Using Classifications to Identify Pathological and Taphonomic Modifications on Ancient Bones: Do “Taphognomonic” Criteria Exist? Bull. Mem. Soc. Anthropol. Paris 2017, 29, 1–18. [Google Scholar] [CrossRef]
- Fernandez-Jalvo, Y.; Andrews, P. Atlas of Taphonomic Identifications. Vertebrate Paleobiology and Paleoanthropology; Springer: Dordrecht, The Netherlands, 2016; pp. 155–166. [Google Scholar] [CrossRef]
- Lyman, R.L. Vertebrate Taphonomy; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Tomassini, R.L.; Montalvo, C.I.; Ezquiaga, M.C. The oldest record of flea/armadillos interaction as example of bioerosion on osteoderms from the late Miocene of the Argentine Pampas. Int. J. Paleopathol. 2016, 15, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Zonneveld, J.-P.; AbdelGawad, M.K.; Miller, E.R. Ectoparasite borings, mesoparasite borings, and scavenging traces in early Miocene turtle and tortoise shell: Moghra Formation, Wadi Moghra, Egypt. J. Paléontol. 2021, 1–19. [Google Scholar] [CrossRef]
- Backwell, L.R.; Parkinson, A.H.; Roberts, E.M.; D’Errico, F.; Huchet, J.-B. Criteria for identifying bone modification by termites in the fossil record. Palaeogeogr. Palaeoclim. Palaeoecol. 2012, 337–338, 72–87. [Google Scholar] [CrossRef]
- Britt, B.B.; Scheetz, R.D.; Dangerfield, A. A Suite of Dermestid Beetle Traces on Dinosaur Bone from the Upper Jurassic Morrison Formation, Wyoming, USA. Ichnos 2008, 15, 59–71. [Google Scholar] [CrossRef]
- Huchet, J.-B.; Le Mort, F.; Rabinovich, R.; Blau, S.; Coqueugniot, H.; Arensburg, B. Identification of dermestid pupal chambers on Southern Levant human bones: Inference for reconstruction of Middle Bronze Age mortuary practices. J. Archaeol. Sci. 2013, 40, 3793–3803. [Google Scholar] [CrossRef]
- Huchet, J.-B.; Greenberg, B. Flies, Mochicas and burial practices: A case study from Huaca de la Luna, Peru. J. Archaeol. Sci. 2010, 37, 2846–2856. [Google Scholar] [CrossRef]
- Matu, M.; Crevecoeur, I.; Huchet, J.-B. Taphonomy and Paleoichnology of Olduvai Hominid 1 (OH1), Tanzania. Int. J. Osteoarchaeol. 2017, 27, 785–800. [Google Scholar] [CrossRef]
- Parkinson, A.H. Traces of Insect Activity at Cooper’s D Fossil Site (Cradle of Humankind, South Africa). Ichnos 2016, 23, 322–339. [Google Scholar] [CrossRef]
- Di Donato, R.M. Taphos nomos: El potencial de la tafonomía en la interpretación de contextos funerarios. In Mamül Mapu: Pasado y Presente desde la Arqueología Pampeana; Berón, M.A., Luna, L.H., Bonomo, M., Eds.; Libros del Espinillo: Ayacucho, Argentina, 2010; pp. 137–152. [Google Scholar]
- Wrobel, G.D.; Biggs, J. Osteophageous insect damage on human bone from Je’reftheel, a Maya mortuary cave site in west-central Belize. Int. J. Osteoarchaeol. 2018, 28, 745–756. [Google Scholar] [CrossRef]
- Backwell, L.; Huchet, J.-B.; Jashashvili, T.; Dirks, P.H.; Berger, L.R. Termites and necrophagous insects associated with early Pleistocene (Gelasian) Australopithecus sediba at Malapa, South Africa. Palaeogeogr. Palaeoclim. Palaeoecol. 2020, 560, 109989. [Google Scholar] [CrossRef]
- Frère, M.M.; González, M.I.; Greco, C. Continuity in the Use of Shallow Sites of the Salado River Basin in the Pampean Region, Argentina. Radiocarbon 2016, 58, 921–933. [Google Scholar] [CrossRef]
- González, M.I.; Escosteguy, P.D.; Salemme, M.C.; Frère, M.M.; Weitzel, C.; Vecchi, R. Assessing Strategies for Coypu Hunting and Use in the Salado River Depression (Buenos Aires Province, Argentina); Springer: Singapore, 2021; pp. 59–81. [Google Scholar]
- González, M.I.; Frère, M.M. Río Salado: Espacio de interacción de cazadores-recolectores-pescadores (provincia de Buenos Aires, Argentina). Rev. Mus. La Plata 2019, 4, 611–632. [Google Scholar] [CrossRef] [Green Version]
- González, M.I. Arqueología de Alfareros, Cazadores y Pescadores Pampeanos. Colección de Tesis Doctorales; Sociedad Argentina de Antropología: Buenos Aires, Argentina, 2005. [Google Scholar]
- López, H.L.; Baigún, C.R.M.; Iwaszkiw, J.M.; Delfino, R.; Padín, O.H. La Cuenca del Salado: Uso y Posibilidades de sus Recursos Pesqueros. Portal de Libros de la Universidad Nacional de La Plata; Editorial de la Universidad Nacional de La Plata: Buenos Aires, Argentina, 2001. [Google Scholar]
- Quirós, R.; Rennella, A.M.; Boveri, M.B.; Rosso, J.J.; Sosnovsky, A. Factores que afectan la estructura y el funcionamiento de las lagunas pampeanas. Ecol. Austral. 2002, 12, 175–185. [Google Scholar]
- Zárate, M.A. El paisaje pampeano a través del tiempo. In Mamül Mapu: Pasado y Presente desde la Arqueología Pampeana; Berón, M.A., Luna, L.H., Bonomo, M., Eds.; Libros del Espinillo: Ayacucho, Argentina, 2010; pp. 19–32. [Google Scholar]
- Pommarés, N.N.; Fucks, E.E.; Pisano, M.F.; Luengo, M.S.; Ramos, N.A.; Di Lello, C.V. Late Pleistocene-Holocene paleoenvironments in the middle basin of the Salado river, province of Buenos Aires, Argentina. J. S. Am. Earth Sci. 2021, 105, 103001. [Google Scholar] [CrossRef]
- Fucks, E.E.; Pisano, F.; Carbonari, J.; Huarte, R. Aspectos geomorfologicos del sector medio e inferior de la Pampa Deprimida, provincia de Buenos Aires. Rev. Soc. Geológica España 2012, 25, 107–118. [Google Scholar]
- Zárate, M.A.; de Bonaveri, M.I.G.; Flegenheimer, N.; Bayón, C. Sitios arqueológicos someros: El concepto de sitio en estratigrafía y sitio de superficie. Cuad. Inst. Nac. Antropol. Pensam. Latinoam. 2000, 19, 635–653. [Google Scholar]
- Behrensmeyer, A.K. Taphonomic and ecologic information from bone weathering. Paleobiology 1978, 4, 150–162. [Google Scholar] [CrossRef] [Green Version]
- Escosteguy, P. Etnoarqueología de Nutrieros. Una Propuesta Metodológica Aplicada al Registro Arqueológico de la Depresión del Salado y del Noreste de la Provincia de Buenos Aires; Universidad de Buenos Aires: Buenos Aires, Argentina, 2011. [Google Scholar]
- Escosteguy, P.D.; Salemme, M.; González, I. Myocastor coypus (“coipo”, Rodentia, Mammalia) como recurso en los humedales de la Pampa bonaerense: Patrones de explotación. Rev. Mus. Antropol. 2012, 5, 13–30. [Google Scholar] [CrossRef]
- Escosteguy, P.D.; González, M.I.; Salemme, M.C. Aprovechamiento de venado de las pampas y ciervo de los pantanos en la Depresión del río Salado (región pampeana, Argentina). In Libro de Resúmenes del IV Encuentro Latinoamericano de Arqueo-Zoología (ELAZ). Homenaje al Dr. Luis A. Borrero; Cruz, I., Ed.; UNPA Edita: Río Gallegos, Argentina, 2018; pp. 133–134. [Google Scholar]
- Genise, J.F.; Mángano, M.G.; Buatois, L.A.; Laza, J.H.; Verde, M. Insect Trace Fossil Associations in Paleosols: The Coprinisphaera Ichnofacies. Palaios 2000, 15, 49–64. [Google Scholar] [CrossRef]
- Wood, W.R.; Johnson, D.L. A survey of disturbance processes in archaeological site formation. In Advances in Archaeological Method and Theory; Schiffer, M.B., Ed.; Academic Press: Cambridge, MA, USA, 1978; Volume 1, pp. 315–381. [Google Scholar]
- McBrearty, S. Consider the humble termite: Termites as agents of post-depositional disturbance at african archaeological sites. J. Archaeol. Sci. 1990, 17, 111–143. [Google Scholar] [CrossRef]
- Parkinson, A.H. Dermestes Maculatus and Periplaneta Americana: Bone Modification Criteria and Establishing Their Potential as Climatic Indicators; University of the Witwatersrand, Johannesburg: Johannesburg, South Africa, 2012. [Google Scholar]
- Cigliano, M.M.; Melo, M.C.; Montemayor, S.I.; del Río, M.G.; Dellapé, P.M. BiodAr—Biodiversidad de Insectos de la Argentina y Uruguay. Available online: https://biodar.unlp.edu.ar/ (accessed on 5 April 2021).
- Farina, J.; Cicchino, A. Una excursión entomológica por la costa atlántica bonaerense. In La Costa Atlántica de Buenos Aires. Naturaleza y Patrimonio Cultural; Athor, J., Celsi, C., Eds.; Fundación de Historia Natural Félix de Azara: Buenos Aires, Argentina, 2016; pp. 281–323. [Google Scholar]
- Holden, A.R.; Harris, J.M.; Timm, R.M. Paleoecological and Taphonomic Implications of Insect-Damaged Pleistocene Vertebrate Remains from Rancho La Brea, Southern California. PLoS ONE 2013, 8, e67119. [Google Scholar] [CrossRef] [Green Version]
- Huchet, J.; Deverly, D.; Gutierrez, B. Taphonomic evidence of a Human skeleton gnawed by termites in a Mochecivilisation grave at Huaca de la Luna, Peru. Int. J. Osteoarchaeol. 2011, 21, 92–102. [Google Scholar] [CrossRef]
- Pomi, L.H.; Tonni, E.P. Termite Traces on Bones from the Late Pleistocene of Argentina. Ichnos 2011, 18, 166–171. [Google Scholar] [CrossRef]
- Torales, G.J. Biodiversidad de Artrópodos Argentinos: Una Perspectiva Biotaxonómica; Ediciones Sur: La Plata, Argentina, 1998. [Google Scholar]
- Go, M. A Case of Human Bone Modification by Ants (Hymenoptera: Formicidae) in the Philippines. Forensic Anthr. 2018, 1, 117–123. [Google Scholar] [CrossRef]
- Pirrone, C.A.; Buatois, L.A.; Bromley, R.G. Ichnotaxobases for bioerosion trace fossils in bones. J. Paléontol. 2014, 88, 195–203. [Google Scholar] [CrossRef]
- Odes, E.J.; Parkinson, A.H.; Randolph-Quinney, P.S.; Zipfel, B.; Jakata, K.; Bonney, H.; Berger, L.R. Osteopathology and insect traces in the Australopithecus africanus skeleton StW 431. S. Afr. J. Sci. 2017, 113, 7. [Google Scholar] [CrossRef] [Green Version]
- Zanetti, N.I.; Visciarelli, E.C.; Centeno, N.D. Taphonomic Marks on Pig Tissue Due to Cadaveric Coleoptera Activity Under Controlled Conditions. J. Forensic Sci. 2014, 59, 997–1001. [Google Scholar] [CrossRef]
- Zanetti, N.I.; Ferrero, A.A.; Centeno, N.D. Depressions of Dermestes maculatus (Coleoptera: Dermestidae) on Bones Could be Pupation Chambers. Am. J. Forensic Med. Pathol. 2019, 40, 122–124. [Google Scholar] [CrossRef]
- Huchet, J.B. Man-eating termites: Osteolytic lesions upon human skulls perpetrated by subterranean termites (Insecta: Isop-tera). In Proceedings of the III Meeting of the International Conference in Funerary Archaeoentomology (ICFAE), Bordeaux, France, 5 June 2019. [Google Scholar]
- Genise, J.F. A fossil termite nest from the Marplatan stage (Late Pliocene) of Argentina: Paleoclimatic indicator. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1997, 136, 139–144. [Google Scholar] [CrossRef]
- Laza, J.H. Termiteros del Plioceno y Pleistoceno de la provincia de Buenos Aires, República Argentina. Significación paleoambiental y paleozoogeográfica. Ameghiniana 2006, 43, 641–648. [Google Scholar]
- Torales, G.J.; Coronel, J.M.; Laffont, E.R.; Fontana, J.L.; Godoy, M.C. Termite associations (Insecta, Isoptera) in natural or semi-natural plant communities in Argentina. Sociobiology 2009, 54, 383–437. [Google Scholar]
- Kirkland, J.; Bader, K.; Kirkland, J.I.; Bader, K. Insect trace fossils associated with Protoceratops carcasses in the Djadokhta Formation (Upper Cretaceous), Mongolia. In New Perspectives on Horned Dinosaurs: The Royal Tyrrell Museum Ceratopsian Symposium; Ryan, M.J., Chinnery-Allgeier, B.J., Eberth, D.A., Ralrick, P.E., Eds.; Indiana University Press: Bloomington, IN, USA, 2010; pp. 509–519. [Google Scholar]
- Escosteguy, P.; Fernández, A.E. The Effects of Bioturbation by Earthworms. Preliminary Results of an Actualistic Taphonomy Experiment. J. Taphon. 2017, 15, 11–17. [Google Scholar]
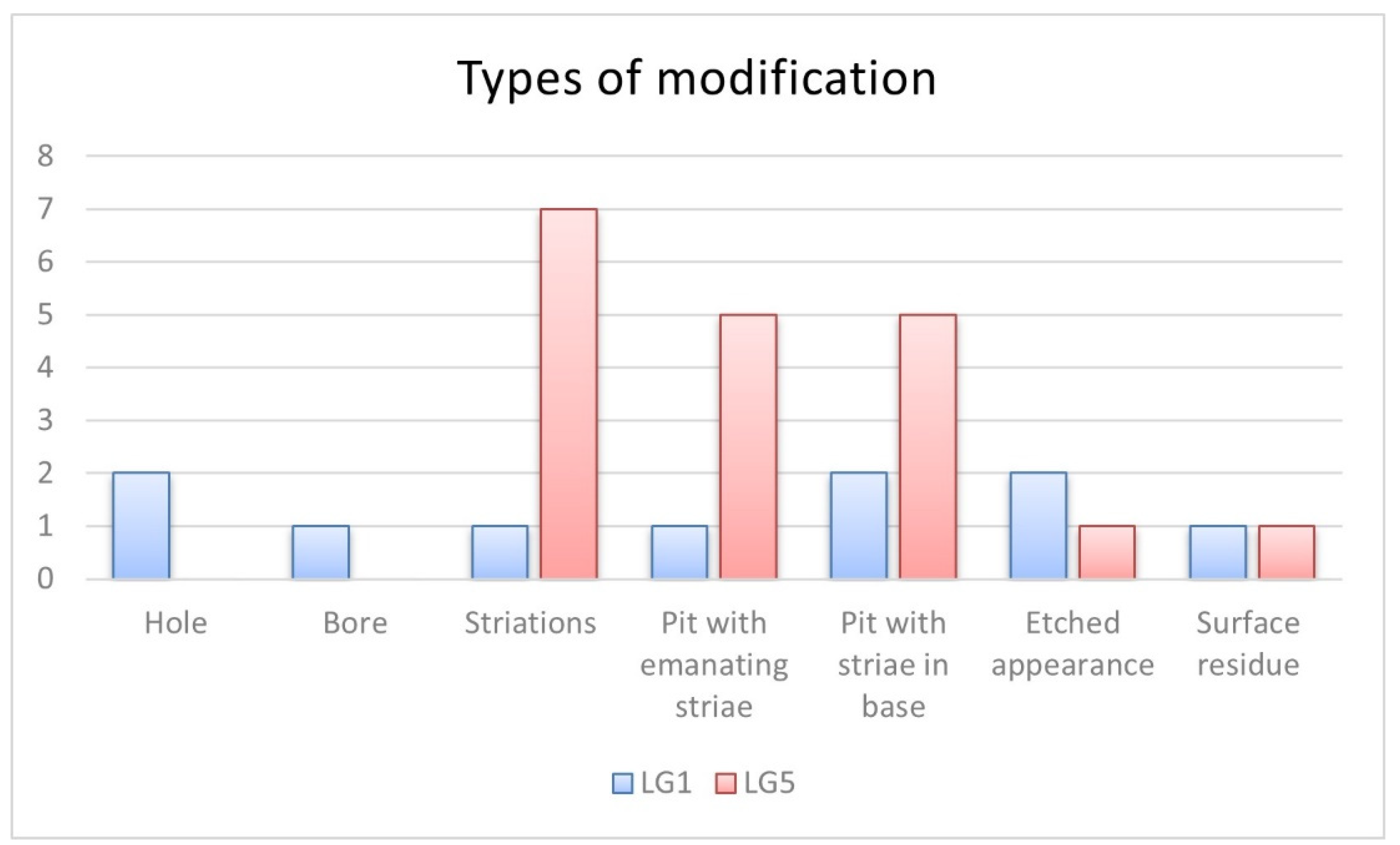


| Site | # | Taxa | Bone | Type of Modification | Measure | Observations |
|---|---|---|---|---|---|---|
| LG1 | 201 | Mammalia | Long bone | Pit with emanating striae | 4.25 mm * | Figure 10 |
| Surface residue | -- | |||||
| Etched appearance | -- | |||||
| 5 | Mammalia | Long bone | Striations | 4.89 mm ** | Edge gnawing around nutrient foramen. Figure 8B | |
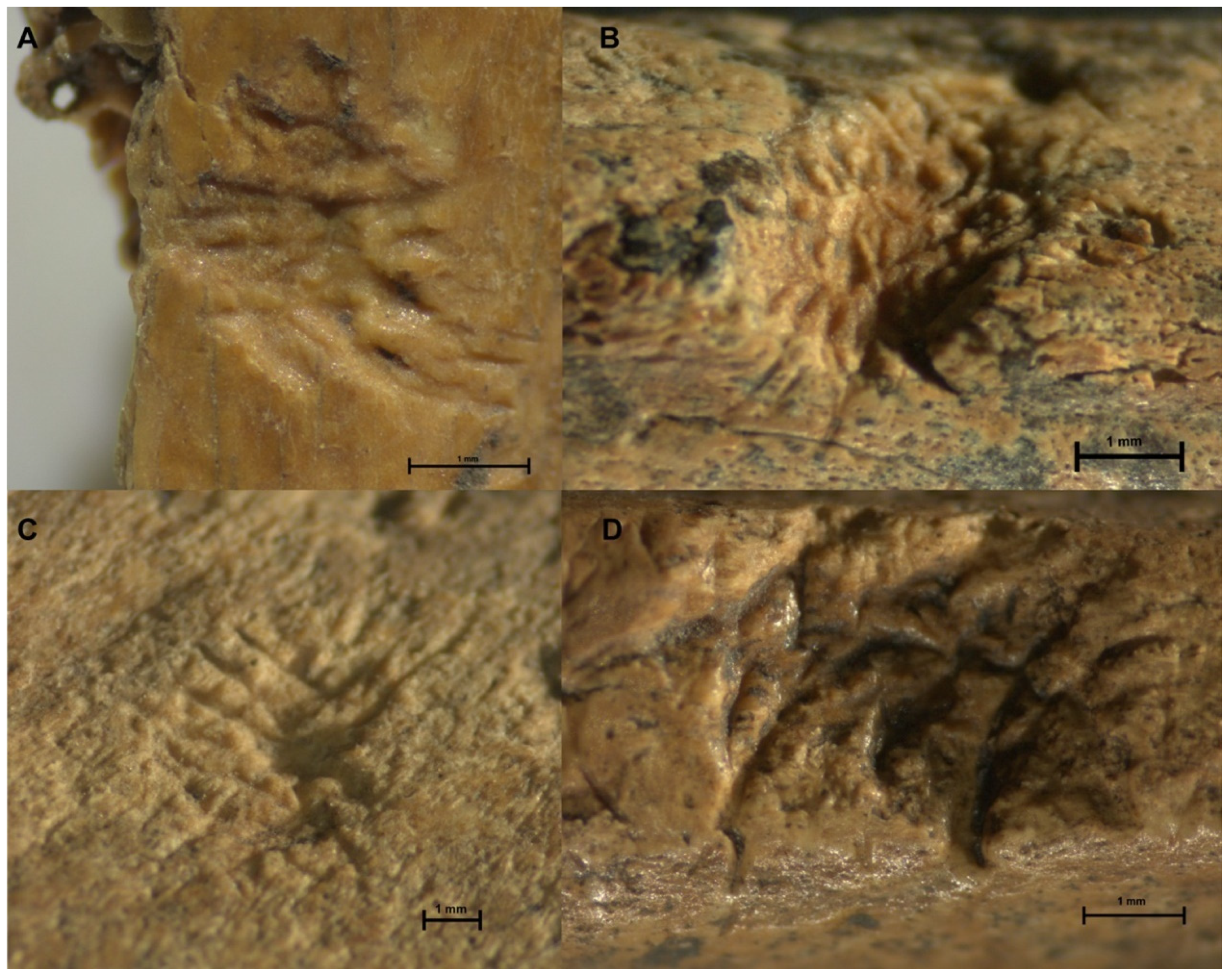
| 340 | Mammalia | Long bone | Pit with striae in base | 2.41 mm * | Figure 3C | |
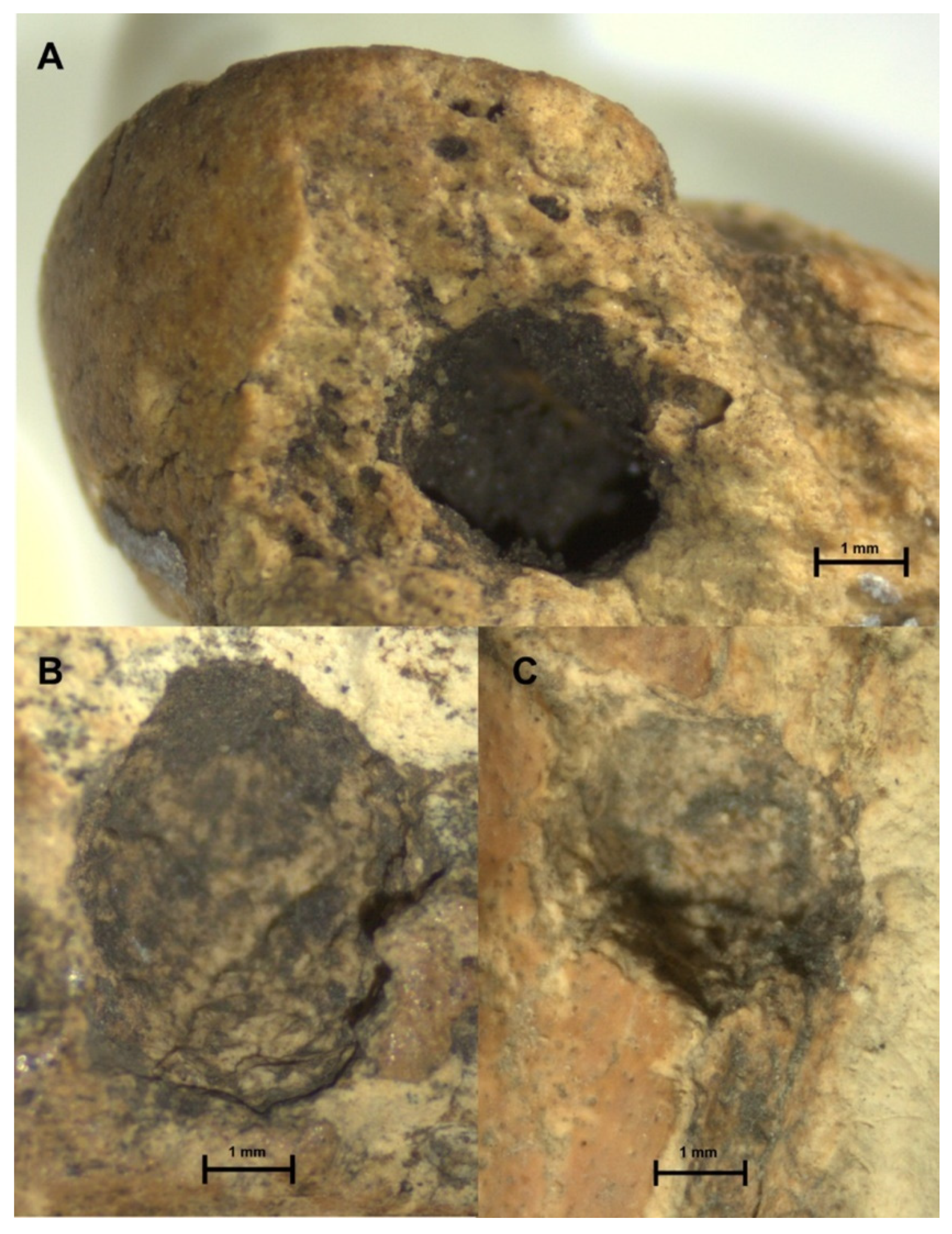
| 4 | Cervidae | Long bone | Hole | 4.06 mm * | A smooth-based boring. Figure 9B | |
| Pit with striae in base | 3.28 mm * | |||||
| 217 | Ozotocerus bezoarticus | Tibia | Hole | 2.66 mm * | A smooth-based boring. Figure 9C | |
| 362 | Ozotocerus bezoarticus | Phalange 1 | Bore | 2.93 mm * | Bore hole. Figure 9A | |
| Etched appearance | -- | |||||
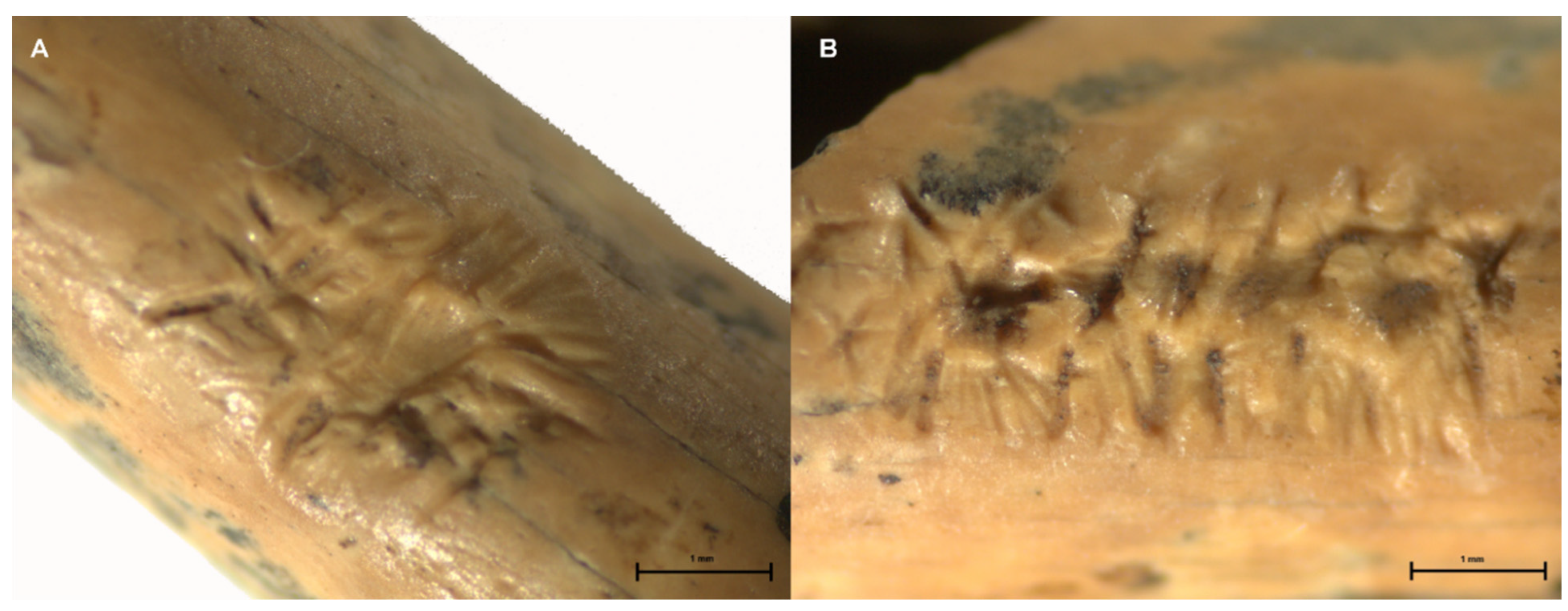
| LG5 | 205 | Aves | Long bone diaphysis | Pit with striae in base | 2.68 mm * | Foraging area. Overlapping of marks. Affected post weathering. Figure 5 |
| Pit with emanating striae | 5.12 mm ** | |||||
| Striations | -- | |||||
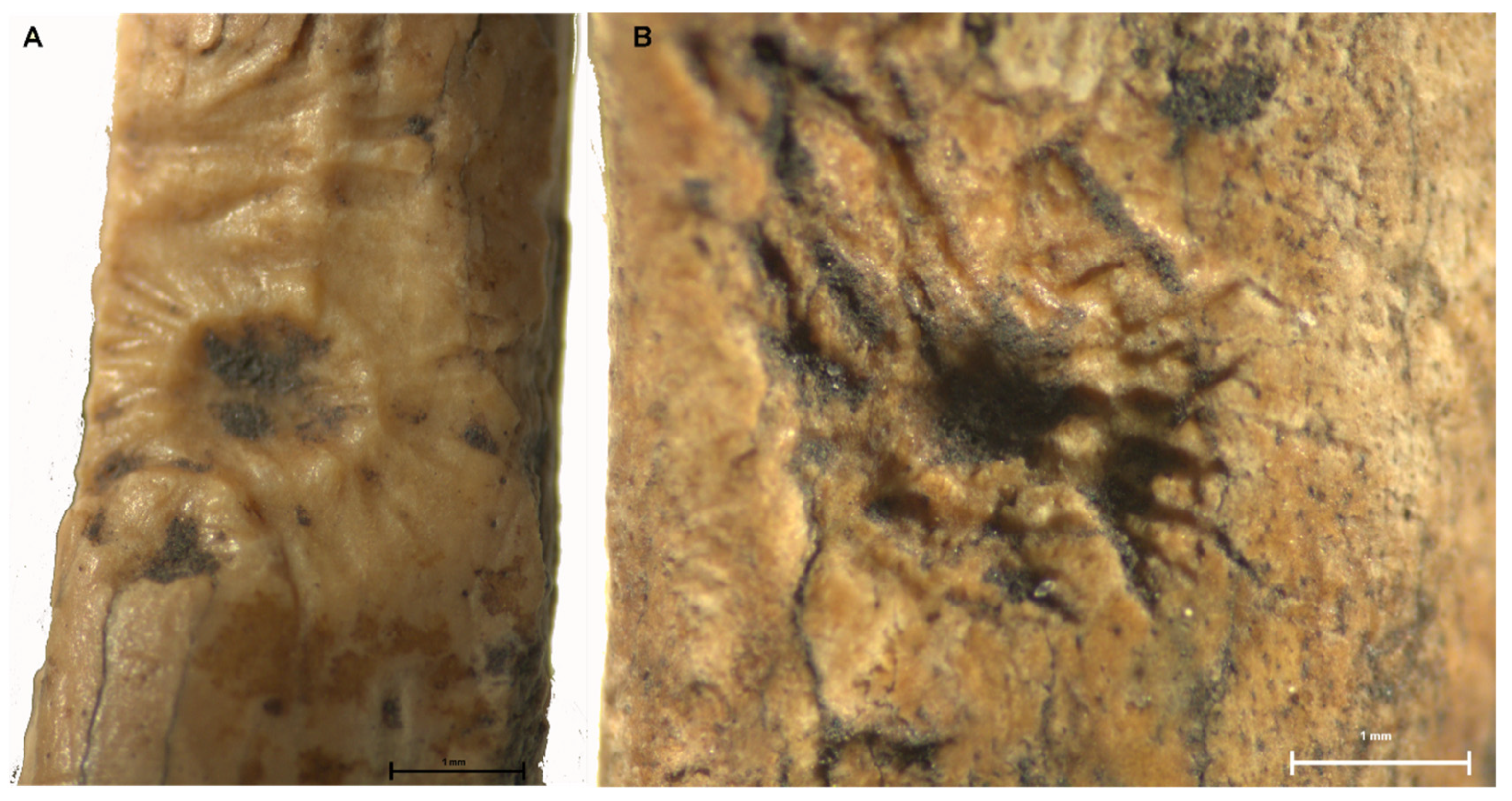
| 220 | Aves | Femur | Pit with emanating striae | 3.52 mm * | Affected post weathering. Figure 4A | |
| Surface residue | -- | |||||
| Striations | ||||||
| 2005 | Mammalia | Tibia | Pit with striae in base | 2.90 mm * | Overlapping of marks. Figure 3B | |
| Etched appearance | -- | |||||
| 3713 | Cavia aperea | Femur | Pit with striae in base | 2.81 mm * | Overlapping of marks. Figure 3A | |
| Striations | -- | |||||
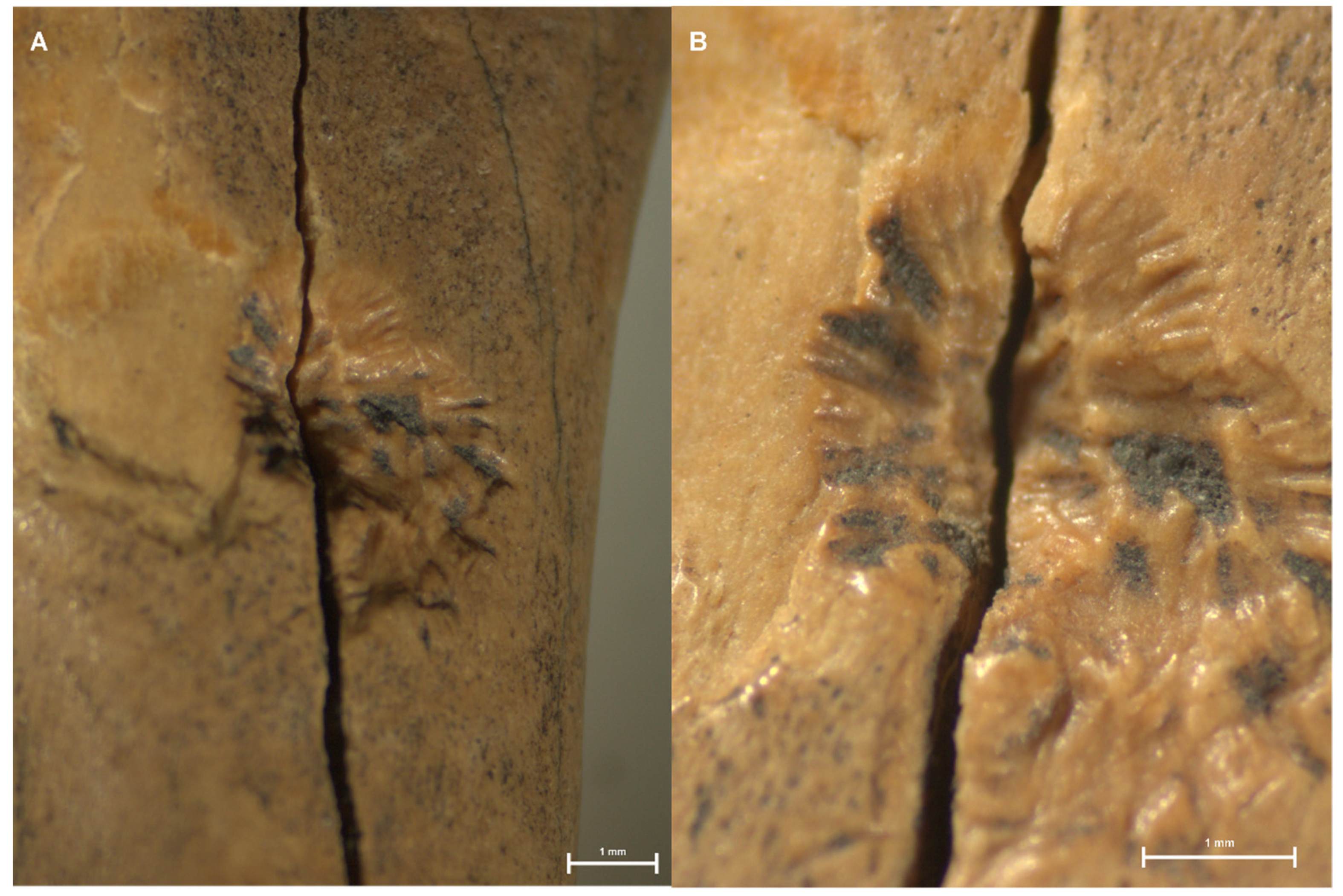
| 1623 | Myocastor coypus | Humerus | Pit with emanating striae | 4.73 mm ** | Overlapping of marks. Fissure along the bone. Figure 6 | |
| Pit with striae in base | ||||||
| Striations | ||||||
| 5332 | Myocastor coypus | Humerus | Pit with emanating striae | 3.38 mm * | Striae on base. Figure 7 | |
| 5519 | Myocastor coypus | Radius | Pit with striae in base | 3.10 mm * | Overlapping of grooves. Figure 3D | |
| Striations | -- | |||||
| 5773 | Myocastor coypus | Tibia | Pit with emanating striae | 2.67 mm * | Figure 4B | |
| Striations | -- | |||||
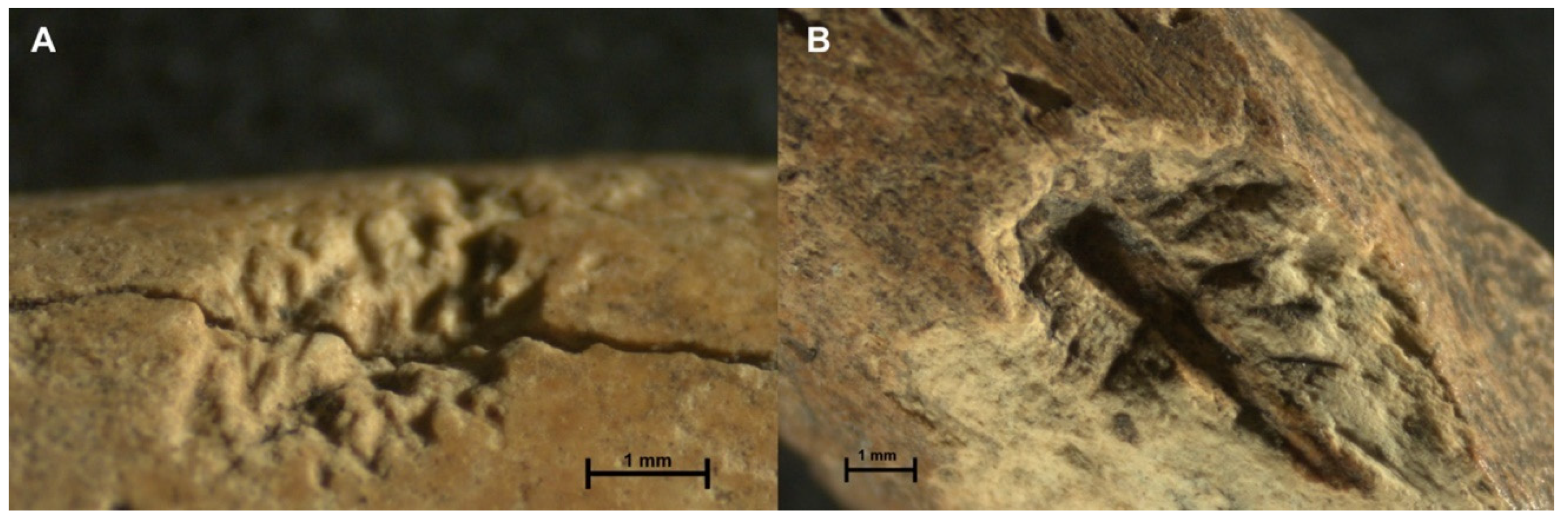
| 14 | Ozotocerus bezoarticus | Humerus | Striations | 3.35 mm ** | Overlapping of grooves. Fissure along the bone. Figure 8A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escosteguy, P.D.; Fernandez, A.E.; González, M.I. Insect Marks on Bones from La Guillerma Archaeological Locality (Salado River Depression, Buenos Aires, Argentina). Quaternary 2021, 4, 45. https://doi.org/10.3390/quat4040045
Escosteguy PD, Fernandez AE, González MI. Insect Marks on Bones from La Guillerma Archaeological Locality (Salado River Depression, Buenos Aires, Argentina). Quaternary. 2021; 4(4):45. https://doi.org/10.3390/quat4040045
Chicago/Turabian StyleEscosteguy, Paula D., Alejandro E. Fernandez, and María Isabel González. 2021. "Insect Marks on Bones from La Guillerma Archaeological Locality (Salado River Depression, Buenos Aires, Argentina)" Quaternary 4, no. 4: 45. https://doi.org/10.3390/quat4040045
APA StyleEscosteguy, P. D., Fernandez, A. E., & González, M. I. (2021). Insect Marks on Bones from La Guillerma Archaeological Locality (Salado River Depression, Buenos Aires, Argentina). Quaternary, 4(4), 45. https://doi.org/10.3390/quat4040045






