From Climatic to Anthropogenic Drivers: A Multi-Proxy Reconstruction of Vegetation and Peatland Development in the French Jura Mountains †
Abstract
1. Introduction
2. Materials and Methods
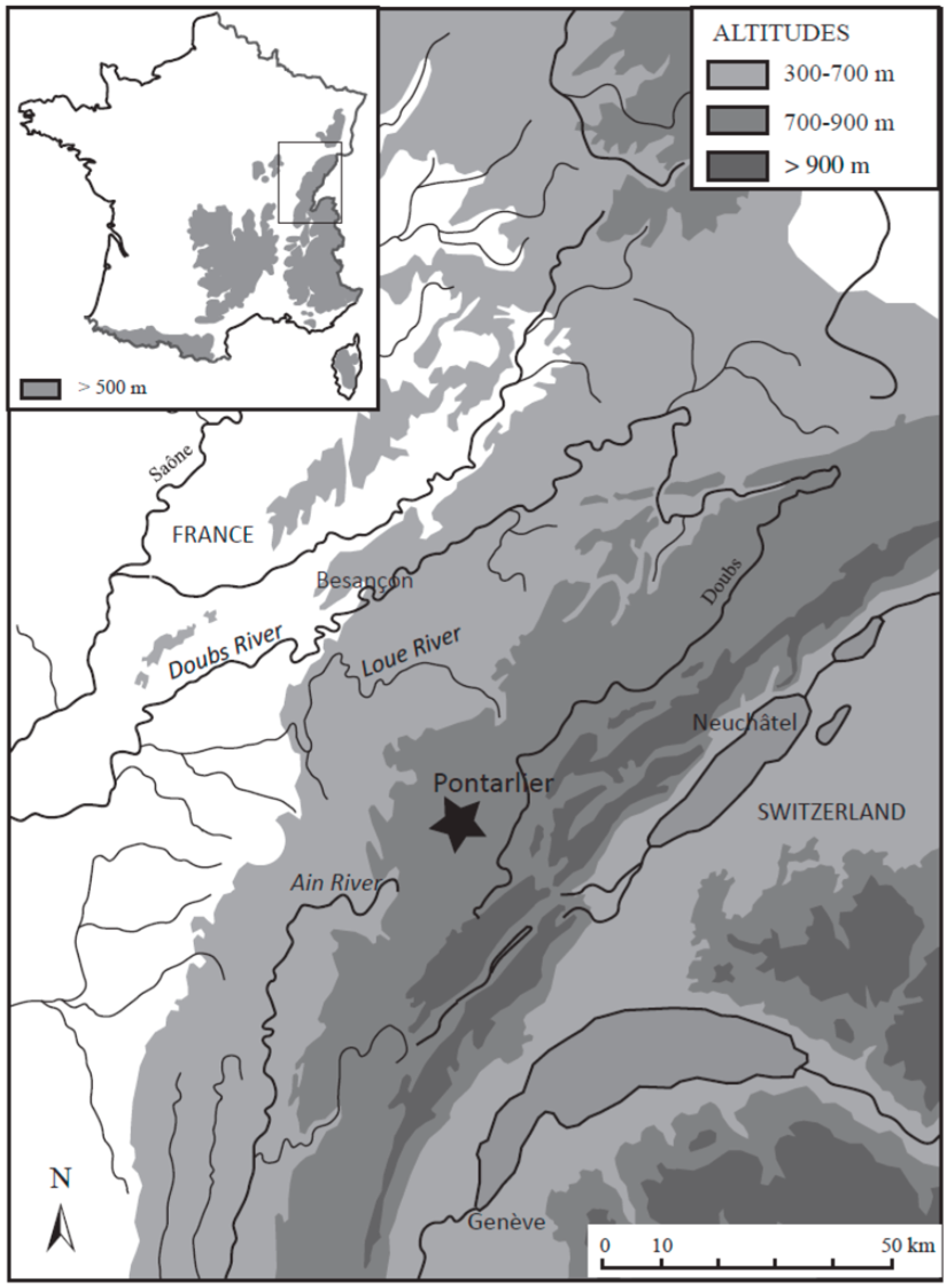
2.1. Study Area and Coring
2.2. Radiocarbon Dates and the Age–Depth Model
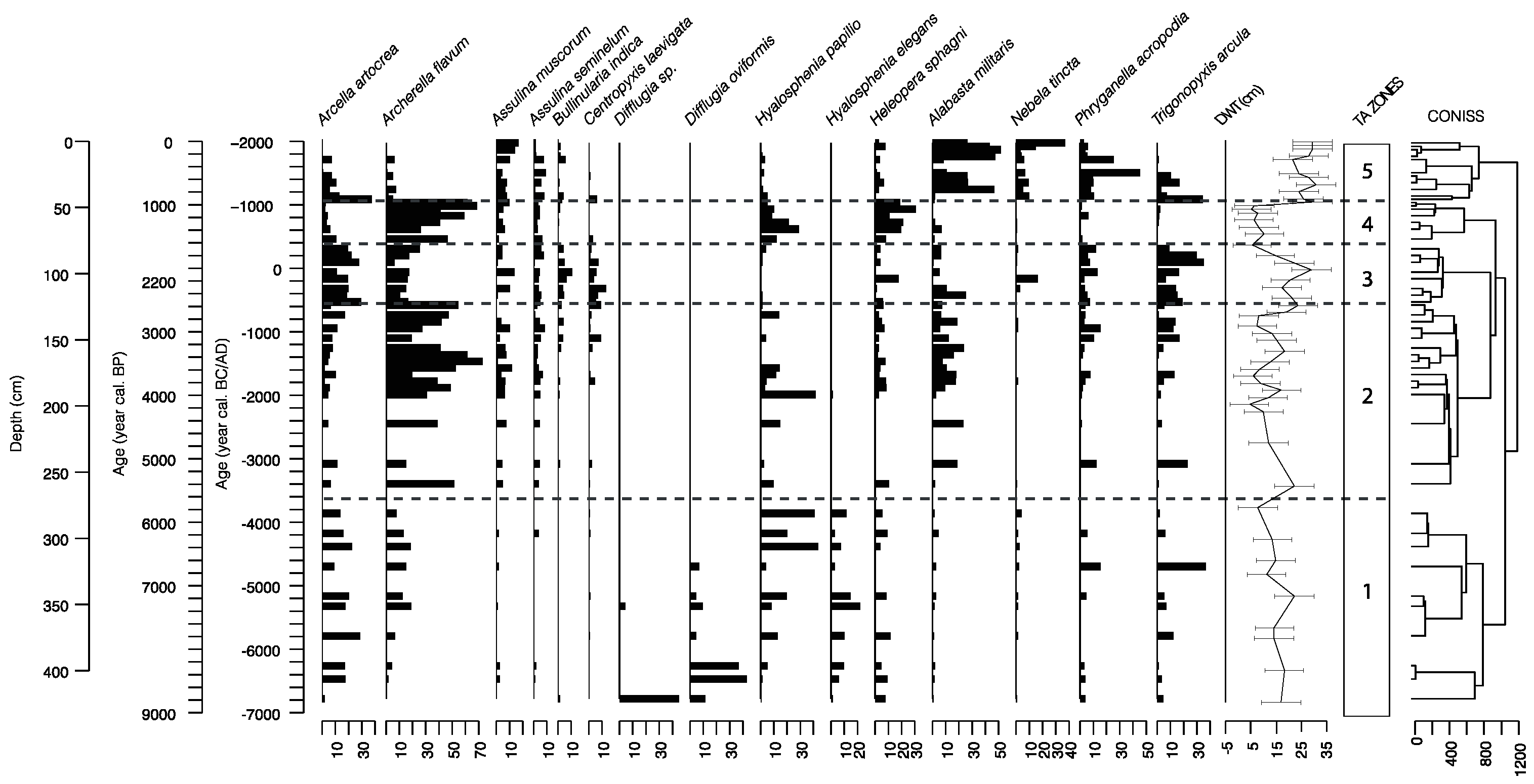
2.3. Testate Amoebae
2.4. Palynology
3. Results
3.1. Radiocarbon Dates and Age–Depth Model
3.2. Testate Amoeba Analysis
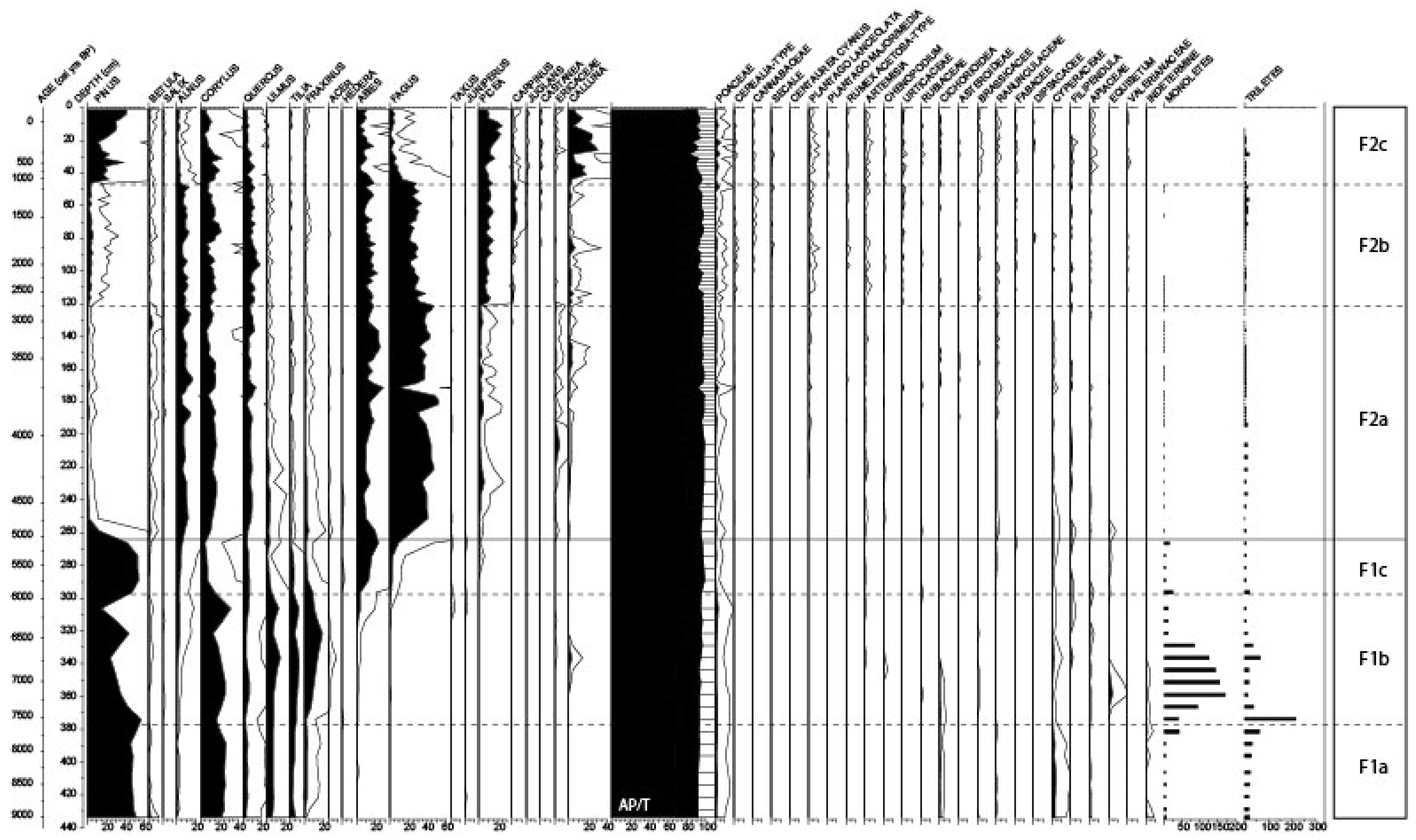
3.3. Pollen Analysis
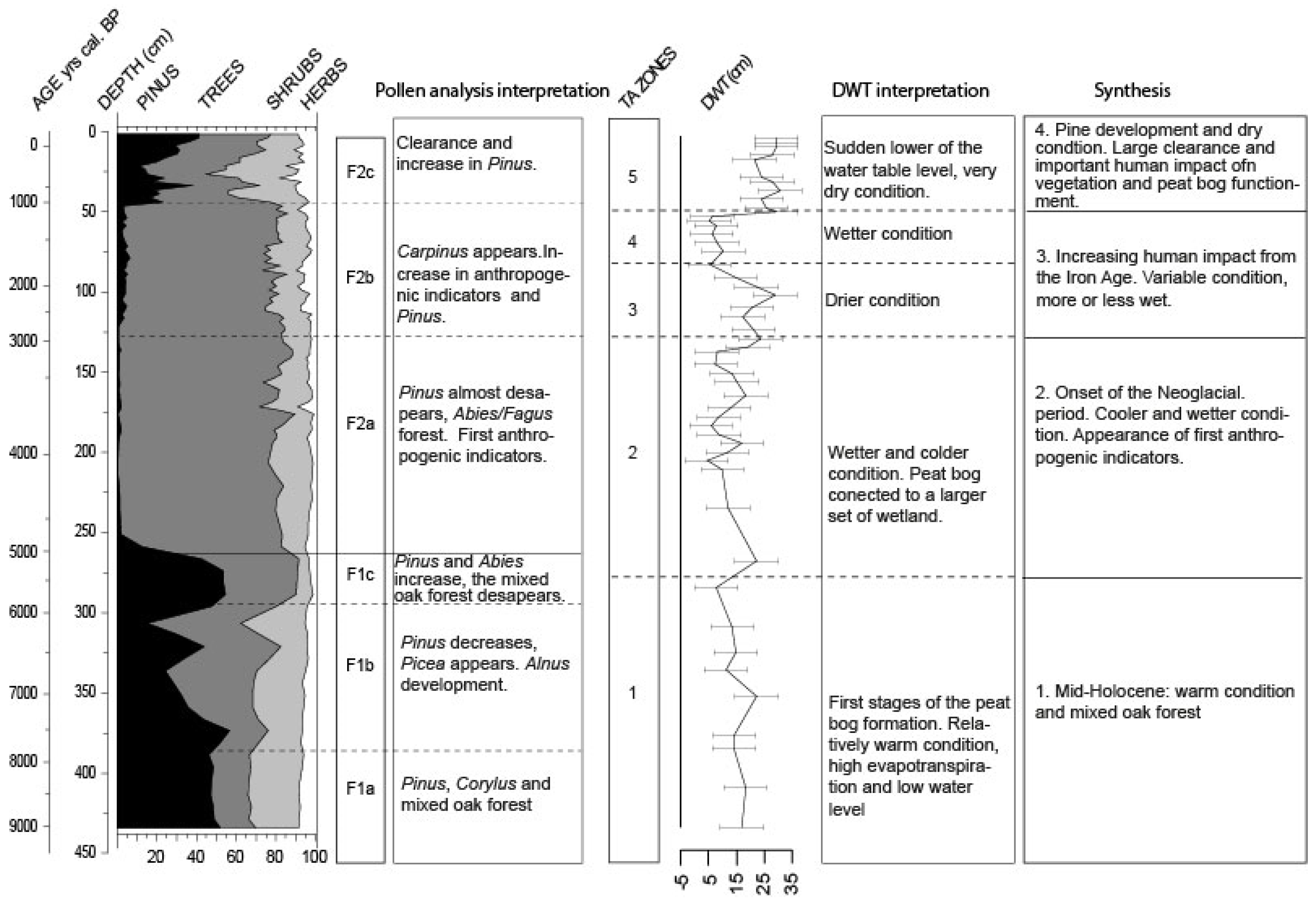
3.3.1. Local Pollen Zone F1 (ca. 9000–5000 cal BP)
3.3.2. Local Pollen zone F2 (ca. 5000 cal BP—2011 AD)
4. Discussion
4.1. Trees Migration during the First Part of the Holocene Period
4.2. Pollen Variation at 3900–3800 Cal BP
4.3. Human Impact on the Environment
4.4. The Comeback of Pinus during the Last Millennium
5. Conclusions
- (1)
- The early-middle Holocene (9000 to 5800 cal BP): peatland formation probably began 9000 years ago, first by filling a basin in which water accumulated. The relatively warm conditions during this period, which were responsible for high evapotranspiration, may have led to relatively low water levels. Dry condition enabled Pinus expansion in and around the peatland. Regionally, the mixed Quercus forest remained the main vegetation formation;
- (2)
- Neoglacial period (5800 cal BP onward): around 5800/5600 cal BP, the main components of the Holocene thermal maximum mixed broad-leaves forest progressively disappeared to give way to the Fagus/Abies forest. The peatland expanded and became wetter. Testate amoebae show a long-term trend with the wetter conditions, indicating the onset of the Neoglacial period [5,29]. From this period onward, the different wetlands connected to form a vast peatland complex, with peat thickness reaching more than three meters. The near absence of anthropogenic pollen indicators is quite unusual in this region;
- (3)
- Increasing human activities (2600–1000 cal BP): it is not until 2600 cal BP (the Iron Age) that human impact seems to have played a role on peatland development, modifying the water table. The recorded changes are potentially linked to farmers’ settlements in the surroundings, increasing or decreasing land use (migration period), and the reduction or extension of the forested area at the periphery of the peatland;
- (4)
- Last millennium: the development of agricultural practices regionally on this Jura plateau over thousands of years is not well known. If human impact seems locally weak, the consequences on the peatland are clearly visible. Land use development and human impact (e.g., deforestation, drainage systems, and peat cutting) probably enhanced peatland dryness. Pinus development appears as a consequence of these massive impacts, and is therefore not a natural stage of peatland development.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jassey, V.E.J.; Chiapusio, G.; Gilbert, D.; Toussaint, M.L.; Binet, P. Phenoloxidase and peroxidase activities in Sphagnum-dominated peatland in a warming climate. Soil Biol. Biochem. 2012, 46, 49–52. [Google Scholar] [CrossRef]
- Jassey, V.E.J.; Chiapusio, G.; Binet, P.; Buttler, A.; Laggoun-Défarge, F.; Delarue, F.; Gilbert, D. Above-and belowground linkages in Sphagnum peatland: Climate warming affects plant-microbial interactions. Glob. Chang. Biol. 2013, 19, 811–823. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, E. Évolution des activités agropastorales du Haut Jura (France) au cours des trois derniers millénaires. Quaternaire 2002, 13, 137–147. [Google Scholar] [CrossRef]
- Gauthier, E. Forêts Et Agriculteurs Du Jura. Les Quatre Derniers Millénaires; Annales Littéraires de l’Université de Franche-Comté; 765, série « Environnement, sociétés et archéologie; Presses Universitaires de Franche-Comté: Besançon, France, 2004; p. 197. [Google Scholar]
- Leroux, A.; Bichet, V.; Walter-Simonnet, A.-V.; Magny, M.; Adatte, T.; Gauthier, E.; Richard, H.; Baltzer, A. Late Glacial-Holocene sequence of Lake Saint-Point (Jura Mountains, France): Detrital inputs as records of climate change and anthropic impact. C. R. Geosci. 2008, 340, 883–892. [Google Scholar] [CrossRef]
- Magny, M.; Gauthier, E.; Vannière, B.; Peyron, O. Palaeohydrological changes and human-impact history over the last millennium recorded at Lake Joux in the Jura Mountains, Switzerland. Holocene 2008, 18, 255–265. [Google Scholar] [CrossRef]
- Dubois, C.; Dubois, G.; Firtion, F. Examen micropaléontologique de tourbes du Jura français. Rev. De Sci. Nat. D’auvergne 1943, 9, 44–50. [Google Scholar]
- Firtion, F. Contribution à l’étude Paléontologique, Stratigraphique et Physicochimique des Tourbières du Jura Français; Mémoire du Service de la Carte géologique d’Alsace et Lorraine: Strasbourg, France, 1950; Volume 10, p. 92. Available online: https://www.persee.fr/doc/sgeol_0080-9020_1950_mon_10_1 (accessed on 28 November 2019).
- Reille, M. L’origine de la station de pin à crochets de la tourbière de Pinet (Aude) et de quelques stations isolées de cet arbre dans les Vosges et le Jura. Bulletin de la Société Botanique de France. Lett. Bot. 1991, 138, 123–148. [Google Scholar]
- Guiter, F.; Andrieu-Ponel, V.; Digerfeldt, G.; Reille, M.; de Beaulieu, J.L.; Ponel, P. Vegetation history and lake-level changes from the Younger Dryas to the present in Eastern Pyrenees (France): Pollen, plant macrofossils and lithostratigraphy from Lake Racou (2000 m asl). Veg. Hist. Archaeobotany 2005, 14, 99–118. [Google Scholar] [CrossRef]
- Bégeot, C.; Richard, H. L’origine récente des peuplements de Pin à crochets (Pinus uncinata Miller ex Mirbel) sur la tourbière de Frasne et exploitation de la tourbe dans le Jura. Acta Bot. Gall. 1996, 143, 47–53. [Google Scholar] [CrossRef][Green Version]
- Freléchoux, F.; Buttler, A.; Gillet, F. Dynamics of peatland-pine dominated mires in the Jura mountains, Switzerland: A tentative scheme based on synusial phytosociology. Folia Geobot. 2000, 35, 273–288. [Google Scholar] [CrossRef]
- Freléchoux, F.; Buttler, A.; Schweingruber, F.H.; Gobat, J.M. Spatio-temporal pattern of peatland pine (Pinus uncinata var. rotundata) at the interface with the Norway spruce (Picea abies) belt on the edge of a raised peatland in the Jura Mountains, Switzerland. Ann. Forest Sci. 2004, 61, 309–318. [Google Scholar]
- Mitchell, E.A.D.; Buttler, A.J.; Warner, B.G.; Gobat, J.-M. Ecology of testate amoebae (Protozoa: Rhizopoda) in Sphagnum peatlands in the Jura mountains, Switzerland and France. Ecoscience 1999, 6, 565–576. [Google Scholar] [CrossRef]
- Heuertz, M.; Teufel, J.; González-Martínez, S.C.; Soto, A.; Fady, B.; Alía, R.; Vendramin, G.G. Geography determines genetic relationships between species of mountain pine (Pinus mugo complex) in western Europe. J. Biogeogr. 2010, 37, 541–556. [Google Scholar] [CrossRef]
- Booth, R.K.; Lamentowicz, M.; Charman, D.J. Preparation and analysis of testate amoebae in peatland palaeoenvironmental studies. Mires Peat 2010, 7, 1–7. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015; Available online: http://www.R-project.org/ (accessed on 21 November 2019).
- Utermöhl, H. Zur Vervollkommnung der quantitativen Phytoplanktonmethodik. Mitt. Int. Ver. Limnol. 1958, 9, 1–38. [Google Scholar]
- Amesbury, M.J.; Swindles, G.T.; Bobrov, A.; Charman, D.J.; Holden, J.; Lamentowicz, M.; Mallon, G.; Mazei, Y.; Mitchell, E.A.D.; Payne, R.J.; et al. Development of a new pan-European testate amoeba transfer function for reconstructing peatland palaeohydrology. Quat. Sci. Rev. 2016, 152, 132–151. [Google Scholar] [CrossRef]
- Blaauw, M. Methods and code for “classical” age-modelling of radiocarbon sequences. Quat. Geochronol. 2010, 5, 512–518. [Google Scholar] [CrossRef]
- Juggins, S. C2 User Guide. Software for Ecological and Palaeoecological Data Analysis and Visualisation; University of Newcastle: Newcastle Upon Tyne, UK, 2003. [Google Scholar]
- Moore, P.D.; Webb, J.A.; Collison, M.E. Pollen Analysis; Blackwell Scientific Publications: Hoboken, NJ, USA, 1991. [Google Scholar]
- Faegri, K.; Iversen, J. Textbook of Pollen Analysis; Wiley: Chichester, UK, 1989; p. 486. [Google Scholar]
- Reille, M. Pollen et Spores d’Europe et d’Afrique du Nord. Laboratoire de Botanique Historique et Palynologie; URA CNRS: Marseille, France, 1992; p. 327. [Google Scholar]
- Beug, H.J. Leitfaden der Pollenbestimmung für Mitteleuropa und Angrenzende Gebiete; Friedrich Pfeil: München, Germany, 2004. [Google Scholar]
- Grimm, E.C. Tilia and Tiliagraph; Illinois State Museum: Springfield, IL, USA, 1991; Volume 101. [Google Scholar]
- Grimm, E.C. CONISS: A FORTRAN 77 program for stratigraphically constrained cluster analysis by the method of incremental sum of squares. Comput. Geosci. 1987, 13, 13–35. [Google Scholar] [CrossRef]
- Richard, H. Nouvelles Contributions à L’histoire de la végétation Franc-Comtoise Tardiglaciaire et Holocène à Partir des Données de la Palynologie; Université de Franche-Comté: Besançon, France, 1983; p. 155. [Google Scholar]
- Wegmüller, S. Uber die Spät- und Postglaziale Vegetationgeschichte des Südwestlichen Jura; Beitr. Geobot. Landesaufn: Schweiz, Switzerland, 1966; Volume 48, p. 142. [Google Scholar]
- Van Der Knaap, W.O.; Van Leeuwen, J.F.; Finsinger, W.; Gobet, E.; Pini, R.; Schweizer, A.; Ammann, B. Migration and population expansion of Abies, Fagus, Picea, and Quercus since 15000 years in and across the Alps, based on pollen-percentage threshold values. Quat. Sci. Rev. 2005, 24, 645–680. [Google Scholar] [CrossRef]
- Matthey, F. Contribution à l’étude de l’évolution tardi- et postglaciaire de la végétation dans le Jura central. Beitr. Geobot. Landesaufn. Schweiz 1971, 53, 86. [Google Scholar]
- Magny, M. Holocene climate variability as reflected by mid-European lake-level fluctuations and its probable impact on prehistoric human settlements. Quat. Int. 2004, 113, 65–79. [Google Scholar] [CrossRef]
- Pétrequin, P.; Magny, M.; Bailly, M. Habitat lacustre, densité de population et climat. L’exemple du Jura français. In Proceedings of the International Conference on Wetland Economies and Societies in Zürich, Switzerland, 10–13 March 2004; Della Casa, P., Trachsel, M., Eds.; Collectio Archaeologica: Zürich-Chronos, Switzerland, 2005; pp. 143–168. [Google Scholar]
- Clark, S.H.; Edwards, K.J. Elm bark beetle in Holocene peat deposits and the northwest European elm decline. J. Quat. Sci. 2004, 19, 525–528. [Google Scholar] [CrossRef]
- Bichet, P.; Millotte, J.-P. L’âge du Fer dans le Haut Jura. Les Tumulus de la région de Pontarlier (Doubs); Documents d’Archéologie Française: Paris, France, 1992; Volume 34, p. 151. [Google Scholar]
- Behre, K.E. The rôle of Man in European Vegetation History; Huntley, B., Webb, T., Eds.; Vegetation History: Dordrecht, The Netherlands, 1988; pp. 633–672. [Google Scholar]
- Rösch, M. Human impact as registred in the pollen record: Some results from the western Lake Constance region, Southern Germany. Veg. Hist. Archaeobotany 1992, 1, 101–109. [Google Scholar] [CrossRef]
- Dreßler, M.; Selig, U.; Dörfler, W.; Adler, S.; Schubert, H.; Hübener, T. Environmental changes and the Migration Period in northern Germany as reflected in the sediment of Lake Dudinghausen. Quat. Res. 2005, 66, 25–37. [Google Scholar] [CrossRef]
- Crowley, T.J.; Lowery, T.S. How warm was the medieval warm period? AMBIO A J. Hum. Environ. 2000, 29, 51–55. [Google Scholar] [CrossRef]
- Mitchell, E.A.D.; van der Knaap, W.O.; van Leeuwen, J.F.; Buttler, A.; Warner, B.G.; Gobat, J.-M. The palaeoecological history of the Praz-Rodet peatland (Swiss Jura) based on pollen, plant macrofossils and testate amoebae (Protozoa). Holocene 2001, 11, 65–80. [Google Scholar] [CrossRef]
- Gresser, P.; Robert, A.; Royer, C.; Vion-Delphin, F. Les Hommes Et La Forêt En Franche-Comté; Editions Bonneton: Paris, France, 1990. [Google Scholar]
- Chabin, J.-P. La Forêt Dans Tous Ses Etats: De La Préhistoire à Nos Jours; Presses Universitaires de Franche-Comté: Besançon, France, 2005; p. 409. [Google Scholar]
- Richard, H.; Gauthier, E. Préhistoire et histoire des forêts du Jura. Available online: https://hal.archives-ouvertes.fr/hal-01114379 (accessed on 21 November 2019).
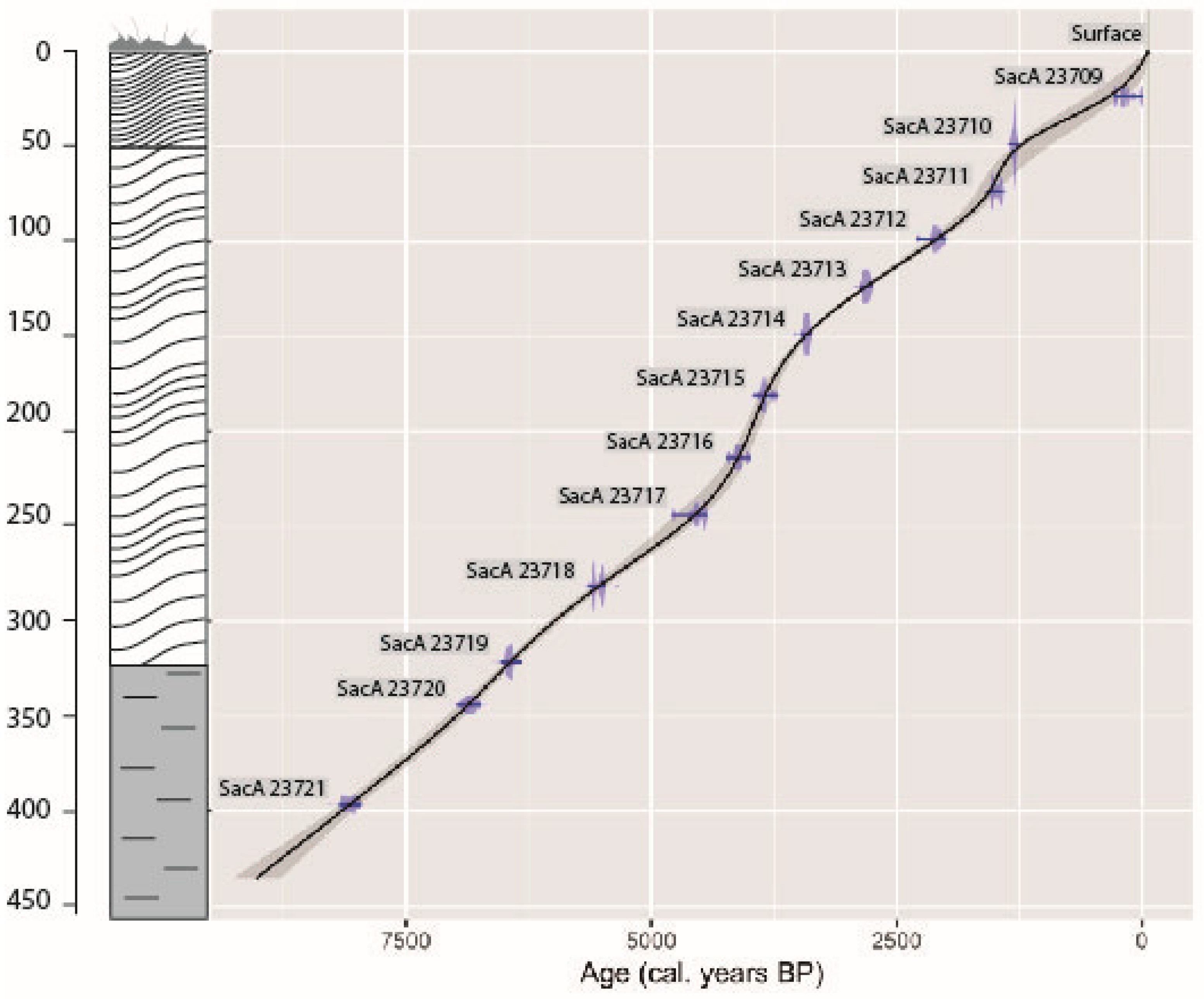
| N° | Depth (cm) | Material | d13C | AGE BP | 2σ Calibration |
|---|---|---|---|---|---|
| SacA 23709 | 22.5–25 | Peat | −25.50 | 165 ± 30 | 0–288 cal BP |
| SacA 23710 | 47.5–50 | Peat | −21.20 | 1380 ± 35 | 1194–1353 cal BP |
| SacA 23711 | 72.5–75 | Peat | −27.70 | 1610 ± 30 | 1413–1557 cal BP |
| SacA 23712 | 97.5–100 | Peat | −29.20 | 2120 ± 30 | 2000–2293 cal BP |
| SacA 23713 | 122.5–125 | Peat | −27.80 | 2715 ± 35 | 2756–2871 cal BP |
| SacA 23714 | 147.5–150 | Peat | −27.60 | 3205 ± 30 | 3368–3477 cal BP |
| SacA 23715 | 180–182.5 | Peat | −27.30 | 3555 ± 30 | 3368–3477 cal BP |
| SacA 23716 | 212.5–215 | Peat | −25.00 | 3755 ± 35 | 3988–4235 cal BP |
| SacA 23717 | 242.5–245 | Peat | −27.70 | 4055 ± 30 | 4432–4784 cal BP |
| SacA 23718 | 280–282.5 | Peat | −30.30 | 4825 ± 40 | 5471–5644 cal BP |
| SacA 23719 | 320–322.5 | Peat | −28.30 | 5665 ± 35 | 6295–6407 cal BP |
| SacA 23720 | 342.5–345 | Peat | −13.50 | 6010 ± 50 | 6733–6984 cal BP |
| SacA 23721 | 395–397.5 | Peat | −9.10 | 7250 ± 60 | 7965–8177 cal BP |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gauthier, E.; Jassey, V.E.J.; Mitchell, E.A.D.; Lamentowicz, M.; Payne, R.; Delarue, F.; Laggoun-Defarge, F.; Gilbert, D.; Richard, H. From Climatic to Anthropogenic Drivers: A Multi-Proxy Reconstruction of Vegetation and Peatland Development in the French Jura Mountains. Quaternary 2019, 2, 38. https://doi.org/10.3390/quat2040038
Gauthier E, Jassey VEJ, Mitchell EAD, Lamentowicz M, Payne R, Delarue F, Laggoun-Defarge F, Gilbert D, Richard H. From Climatic to Anthropogenic Drivers: A Multi-Proxy Reconstruction of Vegetation and Peatland Development in the French Jura Mountains. Quaternary. 2019; 2(4):38. https://doi.org/10.3390/quat2040038
Chicago/Turabian StyleGauthier, Emilie, Vincent E.J. Jassey, Edward A.D. Mitchell, Mariusz Lamentowicz, Richard Payne, Frédéric Delarue, Fatima Laggoun-Defarge, Daniel Gilbert, and Hervé Richard. 2019. "From Climatic to Anthropogenic Drivers: A Multi-Proxy Reconstruction of Vegetation and Peatland Development in the French Jura Mountains" Quaternary 2, no. 4: 38. https://doi.org/10.3390/quat2040038
APA StyleGauthier, E., Jassey, V. E. J., Mitchell, E. A. D., Lamentowicz, M., Payne, R., Delarue, F., Laggoun-Defarge, F., Gilbert, D., & Richard, H. (2019). From Climatic to Anthropogenic Drivers: A Multi-Proxy Reconstruction of Vegetation and Peatland Development in the French Jura Mountains. Quaternary, 2(4), 38. https://doi.org/10.3390/quat2040038






