Spontaneous Stone Passage Rates of Ureteric Stones After Stenting for Acute Renal Colic: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
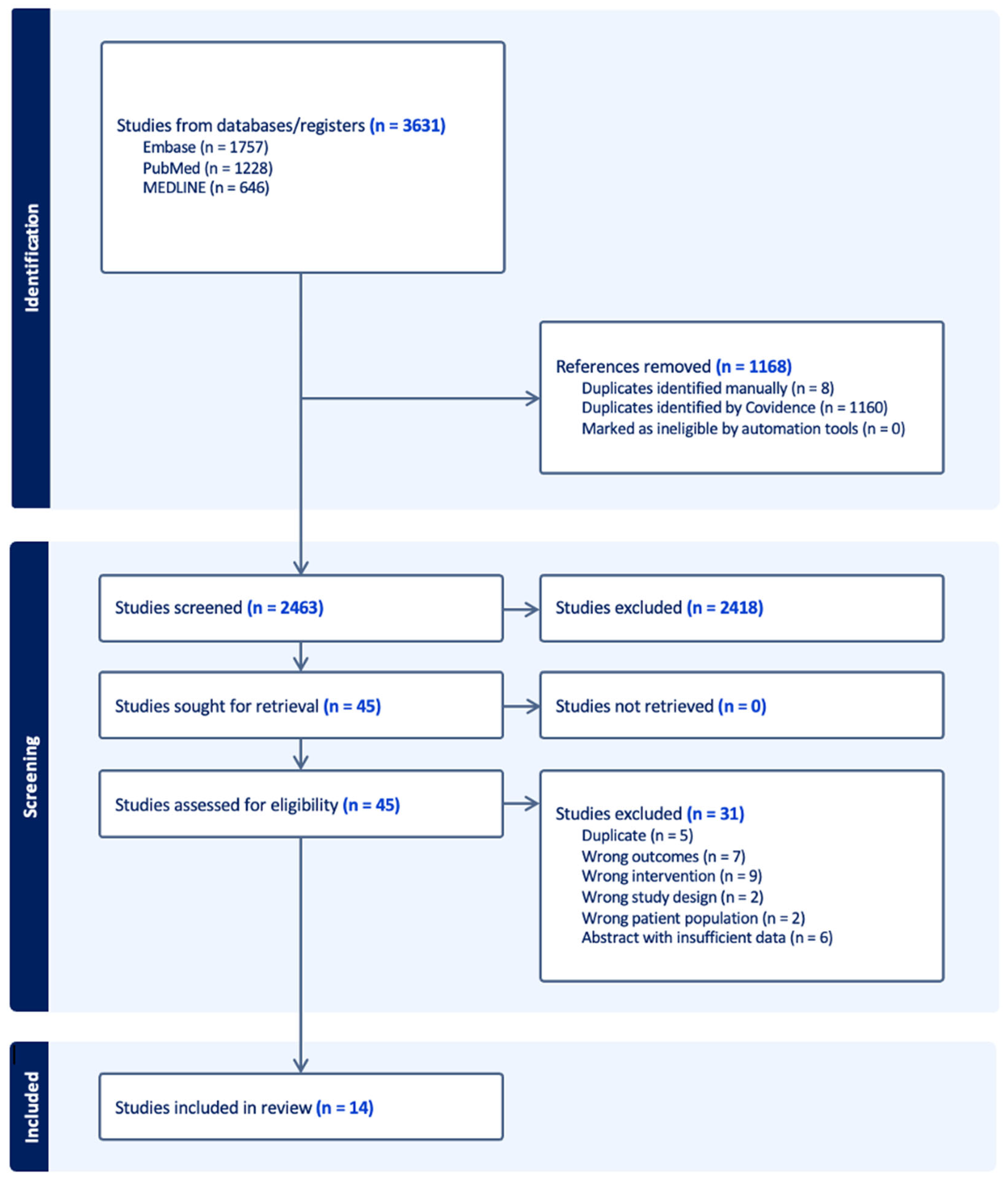
2.2. Study Selection
2.3. Data Extraction
2.4. Data Synthesis
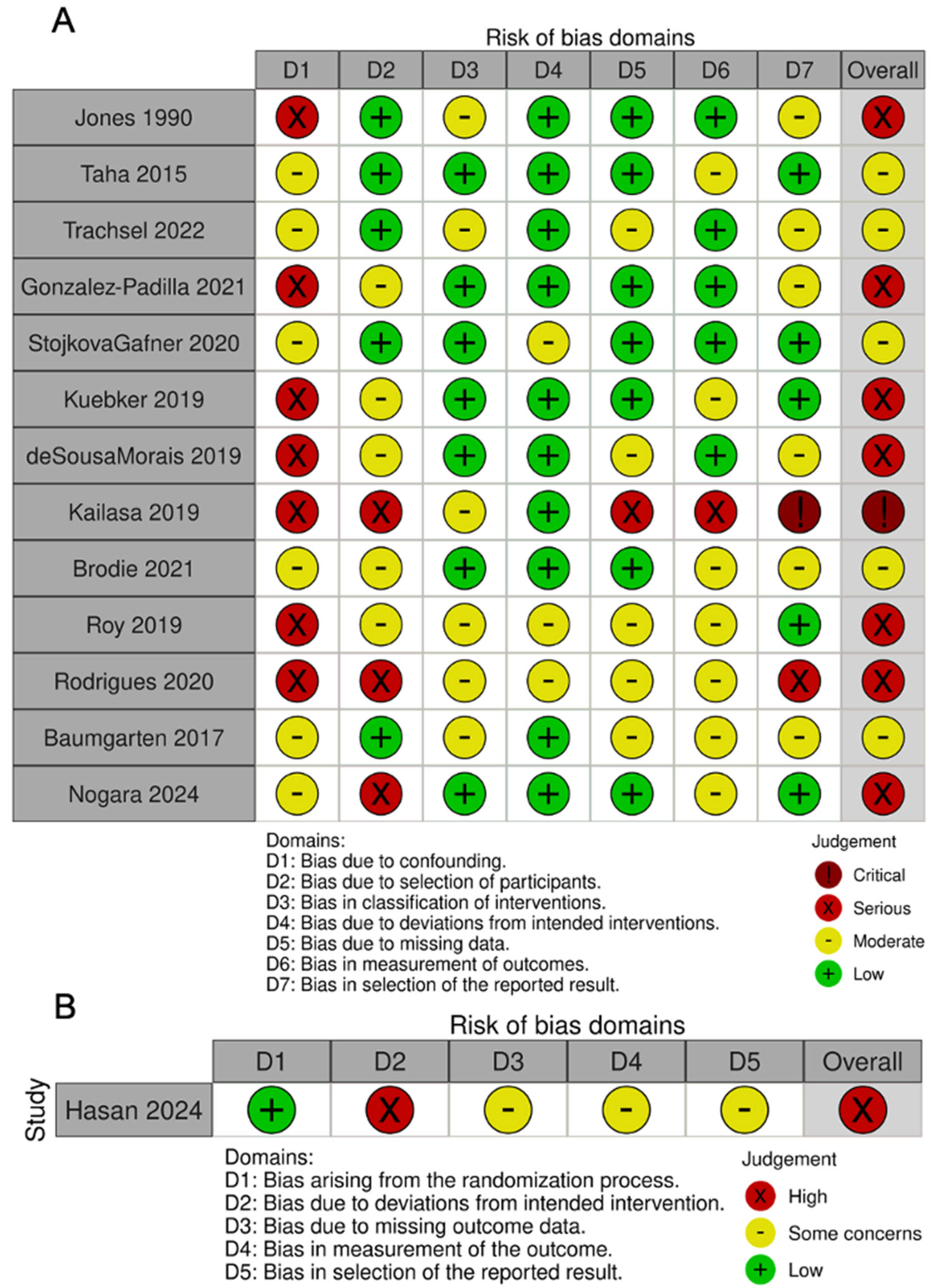
2.5. Quality Assessment
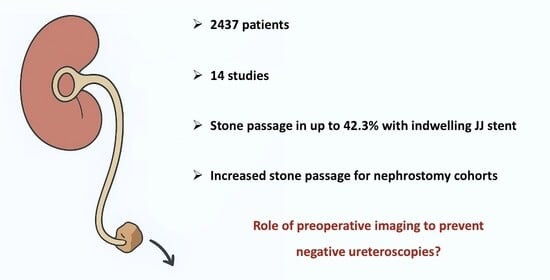
3. Results
3.1. Quality Assessment
3.2. Study Characteristics
3.3. Patient and Stone Characteristics
3.4. Spontaneous Stone Passage Rates
3.5. Comparison of Stented, Non-Stented and Nephrostomy Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pathan, S.A.; Mitra, B.; Bhutta, Z.A.; Qureshi, I.; Spencer, E.; Hameed, A.A.; Nadeem, S.; Tahir, R.; Anjum, S.; Cameron, P.A. A comparative, epidemiological study of acute renal colic presentations to emergency departments in Doha, Qatar, and Melbourne, Australia. Int. J. Emerg. Med. 2018, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Patti, L.; Leslie, S.W. Acute Renal Colic; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Rice, P.; Prattley, S.; Somani, B.K. ‘Negative Ureteroscopy’ for Stone Disease: Evidence from a Systematic Review. Curr. Urol. Rep. 2019, 20, 13. [Google Scholar] [CrossRef]
- Campbell, M.; McKenzie, J.E.; Sowden, A.; Katikireddi, S.V.; Brennan, S.E.; Ellis, S.; Hartmann-Boyce, J.; Ryan, R.; Shepperd, S.; Thomas, J.; et al. Synthesis without meta-analysis (SWiM) in systematic reviews: Reporting guideline. BMJ 2020, 368, l6890. [Google Scholar] [CrossRef] [PubMed]
- Taha, D.E.; Elshal, A.M.; Zahran, M.H.; Harraz, A.M.; El-Nahas, A.R.; Shokeir, A.A. After urgent drainage of an obstructed kidney by internal ureteric stenting; is ureteroscopic stone extraction always needed? Arab J. Urol. 2015, 13, 258–263. [Google Scholar] [CrossRef]
- Trachsel, Y.; Förster, B.; John, H.; Schregel, C. MP14-12 spontaneous passage rate of ureteral stones and predictive factors after pre-stenting. J. Urol. 2022, 207 (Suppl. 5), e236. [Google Scholar] [CrossRef]
- González-Padilla, D.A.; González-Díaz, A.; Peña-Vallejo, H.; Santos Pérez de la Blanca, R.; Teigell-Tobar, J.; Hernández-Arroyo, M.; Abad-López, P.; Rodriguez-Antolin, A.; Cabrera-Meiras, F. Long surgical waiting list times are associated with an increased rate of negative ureteroscopies. Can. Urol. Assoc. J. 2021, 15, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Stojkova Gafner, E.; Grüter, T.; Furrer, M.A.; Bosshard, P.; Kiss, B.; Vartolomei, M.D.; Roth, B. A treatment strategy to help select patients who may not need secondary intervention to remove symptomatic ureteral stones after previous stenting. World J. Urol. 2020, 38, 2955–2961. [Google Scholar] [CrossRef]
- Kuebker, J.M.; Robles, J.; Kramer, J.J.; Miller, N.L.; Herrell, S.D.; Hsi, R.S. Predictors of spontaneous ureteral stone passage in the presence of an indwelling ureteral stent. Urolithiasis 2019, 47, 395–400. [Google Scholar] [CrossRef]
- de Sousa Morais, N.; Pereira, J.P.; Mota, P.; Carvalho-Dias, E.; Torres, J.N.; Lima, E. Percutaneous nephrostomy vs ureteral stent for hydronephrosis secondary to ureteric calculi: Impact on spontaneous stone passage and health-related quality of life—A prospective study. Urolithiasis 2019, 47, 567–573. [Google Scholar] [CrossRef]
- Kailasa, A.K.; Bhuvanagiri, A.K.; Kannan, S.; Thangavelu, M.; Ahiaku, E.K.; Alexandrou, K. Negative ureteroscopy following emergency ureteric stenting. Eur. Urol. Suppl. 2019, 18, e2475–e2476. [Google Scholar] [CrossRef]
- Brodie, A.; Johnston, T.; Lloyd, P.; Hemsworth, L.; Barabas, M.; Keoghane, S. Reducing the rate of negative ureteroscopy: Predictive factors and the role of preoperative imaging. Ann. R. Coll. Surg. Engl. 2022, 104, 588–593. [Google Scholar] [CrossRef]
- Roy, J.; Zargar-Shoshtari, K. Emergency ureteroscopy versus stenting—Does infected urine matter? BJU Int. 2019, 123 (Suppl. 2), 5–6. [Google Scholar] [CrossRef]
- Rodrigues, R.M.; Silva, B.; Morais, N.; Pereira, J.P.; Anacleto, S.; Passos, P.; Torres, J.; Dias, E.; Lima, E.; Mota, P. MP15-05 percutaneous nephrostomy, ureteral stent or primary ureteroscopy with stone removal for the treatment of hydronephrosis secondary to ureteric calculi: A prospective evaluation of the impact on complications, stone management and health-related quality of life. J. Urol. 2020, 203 (Suppl. 4), e205–e206. [Google Scholar] [CrossRef]
- Baumgarten, L.; Desai, A.; Shipman, S.; Eun, D.D.; Pontari, M.A.; Mydlo, J.H.; Reese, A.C. Spontaneous passage of ureteral stones in patients with indwelling ureteral stents. Can. J. Urol. 2017, 24, 9024–9029. [Google Scholar]
- Nogara, A.; Lucignani, G.; Turetti, M.; Silvani, C.; Marmiroli, A.; Nizzardo, M.; Gadda, F.; Zanetti, S.P.; Longo, F.; De Lorenzis, E.; et al. Prevalence and predictors of stone passage after double J stenting for symptomatic ureteral stones: A cross-sectional, real-life study. World J. Urol. 2024, 42, 8. [Google Scholar] [CrossRef]
- Hasan, A.M.; Abdelhamid, A.M.; Ahmed, M.A.; Reyad, A.M. Percutaneous nephrostomy tube versus double J ureteric stent for the management of non-septic calcular anuria in adults: Prospective randomized study. Arab J. Urol. 2024, 23, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.J.; Ryan, P.C.; Lyons, O.; Grainger, R.; McDermott, T.E.; Butler, M.R. Use of the double pigtail stent in stone retrieval following unsuccessful ureteroscopy. Br. J. Urol. 1990, 66, 254–256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, X.; Pu, Y.; Zhang, Y.; Fan, J. Global, Regional, and National Burden of Urolithiasis from 1990 to 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Clin. Epidemiol. 2022, 14, 971–983. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.; Lennon, G.; McLEAN, P.; Fitzpatrick, J. The effects of acute and chronic JJ stent placement on upper urinary tract motility and calculus transit. Br. J. Urol. 1994, 74, 434–439. [Google Scholar] [CrossRef]
- Lennon, G.M.; Thornhill, J.A.; Grainger, R.; McDermott, T.E.D.; Butler, M.R. Double Pigtail Ureteric Stent versus Percutaneous Nephrostomy: Effects on Stone Transit and Ureteric Motility. Eur. Urol. 1997, 31, 24–29. [Google Scholar] [CrossRef]
- Mosayyebi, A.; Manes, C.; Carugo, D.; Somani, B.K. Advances in Ureteral Stent Design and Materials. Curr. Urol. Rep. 2018, 19, 35. [Google Scholar] [CrossRef]
- Shah, T.T.; Gao, C.; Peters, M.; Manning, T.; Cashman, S.; Nambiar, A.; Cumberbatch, M.; Lamb, B.; Peacock, A.; Van Son, M.J.; et al. Factors associated with spontaneous stone passage in a contemporary cohort of patients presenting with acute ureteric colic: Results from the Multi-centre cohort study evaluating the role of Inflammatory Markers In patients presenting with acute ureteric Colic (MIMIC) study. BJU Int. 2019, 124, 504–513. [Google Scholar]
- Gupta, K.; Ricapito, A.; Lundon, D.; Khargi, R.; Connors, C.; Yaghoubian, A.J.; Gallante, B.; Atallah, W.M.; Gupta, M. Harnessing Artificial Intelligence to Predict Spontaneous Stone Passage: Development and Testing of a Machine Learning-Based Calculator. J. Endourol. 2025, 39, 738–747. [Google Scholar] [CrossRef]
- Tang, V.; Attwell-Heap, A. Computed tomography versus ureteroscopy in identification of renal tract stone with ureteral stent in situ. Ann. R. Coll. Surg. Engl. 2011, 93, 639–641. [Google Scholar] [CrossRef] [PubMed]
- Scarneciu, I.; Lupu, S.; Pricop, C.; Scarneciu, C. Morbidity and impact on quality of life in patients with indwelling ureteral stents: A 10-year clinical experience. Pak. J. Med. Sci. 2015, 31, 522–526. [Google Scholar] [CrossRef]
- Tomer, N.; Garden, E.; Small, A.; Palese, M. Ureteral Stent Encrustation: Epidemiology, Pathophysiology, Management and Current Technology. J. Urol. 2021, 205, 68–77. [Google Scholar] [CrossRef]
- Aghamir, S.M.K.; Mohammadi, A.; Farahmand, H.; Meysamie, A.P. Effects of Prophylactic Insertion of Double-J Stents to Decrease Episodes of Renal Colic in Patients with Recurrent Ureteral Stones. J. Endourol. 2008, 22, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Schatloff, O.; Lindner, U.; Ramon, J.; Winkler, H.Z. Randomized Trial of Stone Fragment Active Retrieval Versus Spontaneous Passage During Holmium Laser Lithotripsy for Ureteral Stones. J. Urol. 2010, 183, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Shoshany, O.; Erlich, T.; Golan, S.; Kleinmann, N.; Baniel, J.; Rosenzweig, B.; Eisner, A.; Mor, Y.; Ramon, J.; Winkler, H. Ureteric stent versus percutaneous nephrostomy for acute ureteral obstruction-clinical outcome and quality of life: A bi-center prospective study. BMC Urol. 2019, 19, 79. [Google Scholar] [CrossRef]
| Study | Study Type | Patient Population | Intervention Groups | Sample Size | Age (Mean) | Measurement of Outcome |
|---|---|---|---|---|---|---|
| Taha 2015 [5] | Prospective cohort | Ureteric colic | Single arm (stone passage with stent) | 196 | 53.7 | CT |
| Trachsel 2022 [6] | Retrospective Cohort (Abstract) | Ureteric colic | Single arm | 401 | N/A | CT or URS |
| Gonzalez-Padilla 2021 [7] | Retrospective Cohort | Single ureteral stone | Stent vs. no stent | Stent: 137 Control: 82 | Median 57.6 | CT or URS |
| Stojkova Gafner 2020 [8] | Retrospective Cohort | Single ureteral stone | Single arm | 216 | Median 54 | Presenting filtered stone or CT |
| Kuebker 2019 [9] | Retrospective Cohort | Stented for a single ureteral stone. Excluded PUJ stones. | Single arm | 209 | 51.2 | CT (n = 3) or URS (n = 206) |
| de Sousa Morais 2019 [10] | Prospective cohort | Emergency stent or PCN for ureteric colic | Stent vs. PCN | Stent: 32 PCN: 18 | 57.6 | CT |
| Kailasa 2019 [11] | Prospective cohort (Abstract) | Ureteric colic | Single arm | 44 | 54 | URS |
| Brodie 2022 [12] | Retrospective Cohort | Ureteric colic | Single arm | 257 | 56 | CT or URS |
| Roy 2019 [13] | Prospective cohort (Abstract) | Single ureteral stone with infected urine | Single arm | 47 | N/A | Unspecified |
| Rodrigues 2020 [14] | Prospective cohort (Abstract) | Ureteric colic | Stent vs. PCN | Stent: 53 PCN: 21 | N/A | Unspecified |
| Baumgarten 2017 [15] | Retrospective Cohort | Ureteric or renal colic | Stent vs. no stent | Stent: 119 Control: 75 | <30: 25 30–40: 26 40–50: 44 >50: 89 | URS |
| Nogara 2024 [16] | Retrospective Cohort | Single ureteral stone | Single arm | 249 | Median 56 | CT |
| Hasan 2024 [17] | RCT | Acute calcular anuria. Solitary and dual kidneys | Stent vs. PCN | Stent: 121 PCN: 118 | Median 48 | Unspecified |
| Jones 1990 [18] | Prospective Cohort Study | Prior failed URS for ureteric colic | Stent vs. no stent | Stent: 30 Control: 12 | Stent: 42 Control: 38 | CT or URS |
| Study | Sample Size (n) | Stone Location | Single vs. Multiple Stones | Stone Size (mm) | Stone Density (HU) |
|---|---|---|---|---|---|
| Taha 2015 [5] | 196 | Upper: 98 Middle: 69 Lower: 28 | Unspecified | Passed: 8.4 ± 2.8 Retained: 9.4 ± 2.8 | Passed: 485.6 (SD 308) Retained: 685.9 (SD 314) |
| Trachsel 2022 [6] | 401 | - | Unspecified | 4.7 (IQR 3.3–5.6) | - |
| Gonzalez-Padilla 2021 [7] | Stent: 137 Control: 82 | Proximal: 46 (21%) Mid: 46 (21%) Distal:127 (58%) | Single | Median 7 (IQR 5–8) | Median 744 (IQR 528–910) |
| Stojkova Gafner 2020 [8] | 216 | Proximal: 56 (26) Mid: 55 (25) Distal: 105 (49) | Single | Median 5 (Range 2–11) | Median 710 |
| Kuebker 2019 [9] | 209 | Proximal: 119 (57) Mid: 31 (15) Distal: 59 (28) | Single | 6.6 ± 2.5 | - |
| de Sousa Morais 2019 [10] | Stent: 32 PCN: 18 | Proximal: 27 (54.0) Distal: 23 | Stent: Single: 13 (72.2) Multiple: 5 (27.8) PCN: Single: 20 (62.5) Multiple: 12 (37.5) | Stent: Median 47.0 mm2 (IQR 21.5–84.2 mm2) PCN: Median 92.0 mm2 (IQR 55.5–128.2 mm2) | Median 500 (IQR 390–656) |
| Kailasa 2019 [11] | 44 | Proximal: 12 Mid: 14 Distal: 18 | Unspecified | - | - |
| Brodie 2022 [12] | 257 | Proximal: 160 (62) Mid: 32 (13) Distal: 64 (25) | Unspecified | 7.8 | 699 |
| Roy 2019 [13] | 47 | - | Single | 7 (Median) | - |
| Rodrigues 2020 [14] | Stent: 53 PCN: 21 | - | Unspecified | Stent: 7 PCN: 8 | - |
| Baumgarten 2017 [15] | Stent: 119 Control: 75 | Stent: Renal Pelvis: 22 (18) Proximal Ureter: 42 (35) Mid: 19 (16) Distal: 32 (27) Unknown: 4 (3) Control: Renal Pelvis: 27 (36) Proximal Ureter: 17 (23) Mid: 10 (13) Distal: 21 (28) | Stent: Single: 108 (91) Multiple: 11 (9) Control: Single: 70 (93) Multiple: 5 (7) | Stent: <4 mm: 13 (11) 4–8 mm: 63 (53) >8 mm: 37 (31) Unknown: 6 (5) Control: <4 mm: 3 (4) 4–8 mm: 36 (48) >8 mm: 35 (47) Unknown: 1 (1) | - |
| Nogara 2024 [16] | 249 | Proximal: 102 (41.0) Mid: 48 (19.3) Distal: 99 (39.8) | Single | 7.1 (Median) | Median: 671 |
| Hasan 2024 [17] | 239 | Stent: 48 (Median) PCN: 52 (Median) | Unspecified | Stent: 1334 mm3 (Volume) PCN: 1326 mm3 (Volume) | Stent: 880 PCN: 893 |
| Jones 1990 [18] | Stent: 30 Control: 12 | Stent: Upper: 18 (60.0) Mid: 7 (23.3) Lower: 5 (16.7) Control: Upper: 7 (58.3) Mid: 3 (25.0) Lower: 2 (16.7) | Unspecified | Stent: 9 Control: 7.4 | - |
| Study | Follow-Up Duration | Spontaneous Stone Passage (Stented) | Stone Passage (PCN) | Stone Passage (Non-stented) | Other Pertinent Findings |
|---|---|---|---|---|---|
| Taha 2015 [5] | 3 Months (0.5–6.3) | 83 (42.3) | - | - | Opacity (p < 0.001), stone width (p = 0.038), and positive urine culture (p = 0.046) associated with greater probability of SSP. |
| Trachsel 2022 [6] | Median 26 days (IQR 22–31) | Overall: 95 (23.7) 0–2.9 mm: 75% 3–4.9 mm: 37.1% 5–6.9 mm: 19.1 7–8.9 mm: 10.3% >9 mm: 8.2% Distal: 70.8% Middle: 12.5% Proximal: 16.7% | - | - | Size (p = 0.002) and location (p < 0.001) associated with greater likelihood of SSP. |
| Gonzalez-Padilla 2021 [7] | Median 74 (IQR 45–127) | 19 * (13.9) | - | 22 * (26.83) | Lower SSP for stented patients on MVA (OR = 0.43, p = 0.019). Size, location, radioopacity associated with greater likelihood of SSP(p < 0.05). |
| Stojkova Gafner 2020 [8] | Median 4 weeks (Range 1–14) | 74 (34.3) | - | - | Smaller size (p < 0.001), distal stone location (p = 0.046) and stent dwell time (p = 0.02) independent predictors of SSP. |
| Kuebker 2019 [9] | Mean 21 (SD 15.2) | 17 (8) | - | - | Stone diameter, location, and stent duration associated with SSP on UVA (p < 0.01). Stone diameter and stent duration significantly associated on MVA |
| de Sousa Morais 2019 [10] | 30–40 days (Protocol) | 8 * (25) | 7 * (38.9) | - | Stone size (p = 0.012) associated with SSP. SSP higher for PCN (OR 6.667) adjusting for size and location. PCN was better tolerated and associated with better QoL. |
| Kailasa 2019 [11] | Not provided | 17 (38.6) | - | - | Stone size significantly associated with negative URS (p = 0.02). |
| Brodie 2022 [12] | Not provided | 37 (14.4) | - | - | Logistic regression showed stone size as a predictor of negative URS (p = 0.01). |
| Roy 2019 [13] | Not provided | 5 (10.6) | - | - | - |
| Rodrigues 2020 [14] | Not provided | - | - | - | Spontaneous stone passage higher for PCN than RUS (OR = 2.31). on multivariable analysis |
| Baumgarten 2017 [15] | Stented: <30 days: 18 (15) 31–60 days: 41 (34) 61–100 days: 34 (29) >100 days: 22 (18) Unknown: 4 (3) Control: <30 days: 20 (27) 31–60 days: 26 (35) 61–100 days: 10 (13) >100 days: 16 (21) Unknown: 3 (4) | 17 (14) | 15 (20) | SSP lower in stented groups but not statistically significant (p = 0.30). Stone size significant predictor of SSP on MVA (p = 0.01). | |
| Nogara 2024 [16] | 1 month (Fixed) | 65 (26.2) | Stone diameter, density and location significantly associated with SSP (p < 0.001). | ||
| Hasan 2024 [17] | 96 h | 2 * (1.7) | 9 * (7.6) | PCN associated with higher passage rates (p = 0.028) and QoL scores (p < 0.001) compared to stent. | |
| Jones 1990 [18] | Unspecified | 3 (10.0) | 0 (0) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Société Internationale d’Urologie. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, S.; Gordon, P.; Thompson, D.; Bolton, D.; Patel, O.; Ischia, J. Spontaneous Stone Passage Rates of Ureteric Stones After Stenting for Acute Renal Colic: A Systematic Review. Soc. Int. Urol. J. 2025, 6, 65. https://doi.org/10.3390/siuj6050065
Lim S, Gordon P, Thompson D, Bolton D, Patel O, Ischia J. Spontaneous Stone Passage Rates of Ureteric Stones After Stenting for Acute Renal Colic: A Systematic Review. Société Internationale d’Urologie Journal. 2025; 6(5):65. https://doi.org/10.3390/siuj6050065
Chicago/Turabian StyleLim, Sean, Patrick Gordon, Daryl Thompson, Damien Bolton, Oneel Patel, and Joseph Ischia. 2025. "Spontaneous Stone Passage Rates of Ureteric Stones After Stenting for Acute Renal Colic: A Systematic Review" Société Internationale d’Urologie Journal 6, no. 5: 65. https://doi.org/10.3390/siuj6050065
APA StyleLim, S., Gordon, P., Thompson, D., Bolton, D., Patel, O., & Ischia, J. (2025). Spontaneous Stone Passage Rates of Ureteric Stones After Stenting for Acute Renal Colic: A Systematic Review. Société Internationale d’Urologie Journal, 6(5), 65. https://doi.org/10.3390/siuj6050065