Barriers to Introducing New Transformative Surgical Technology in Australian Healthcare: A Comprehensive Review and Guide
Abstract
1. Introduction
2. Materials and Methods
3. Barriers to Introduction of New Surgical Technology in Australian Healthcare
3.1. Medical Professionals
3.2. Government
3.3. Health Services
3.4. Patients and Consumers
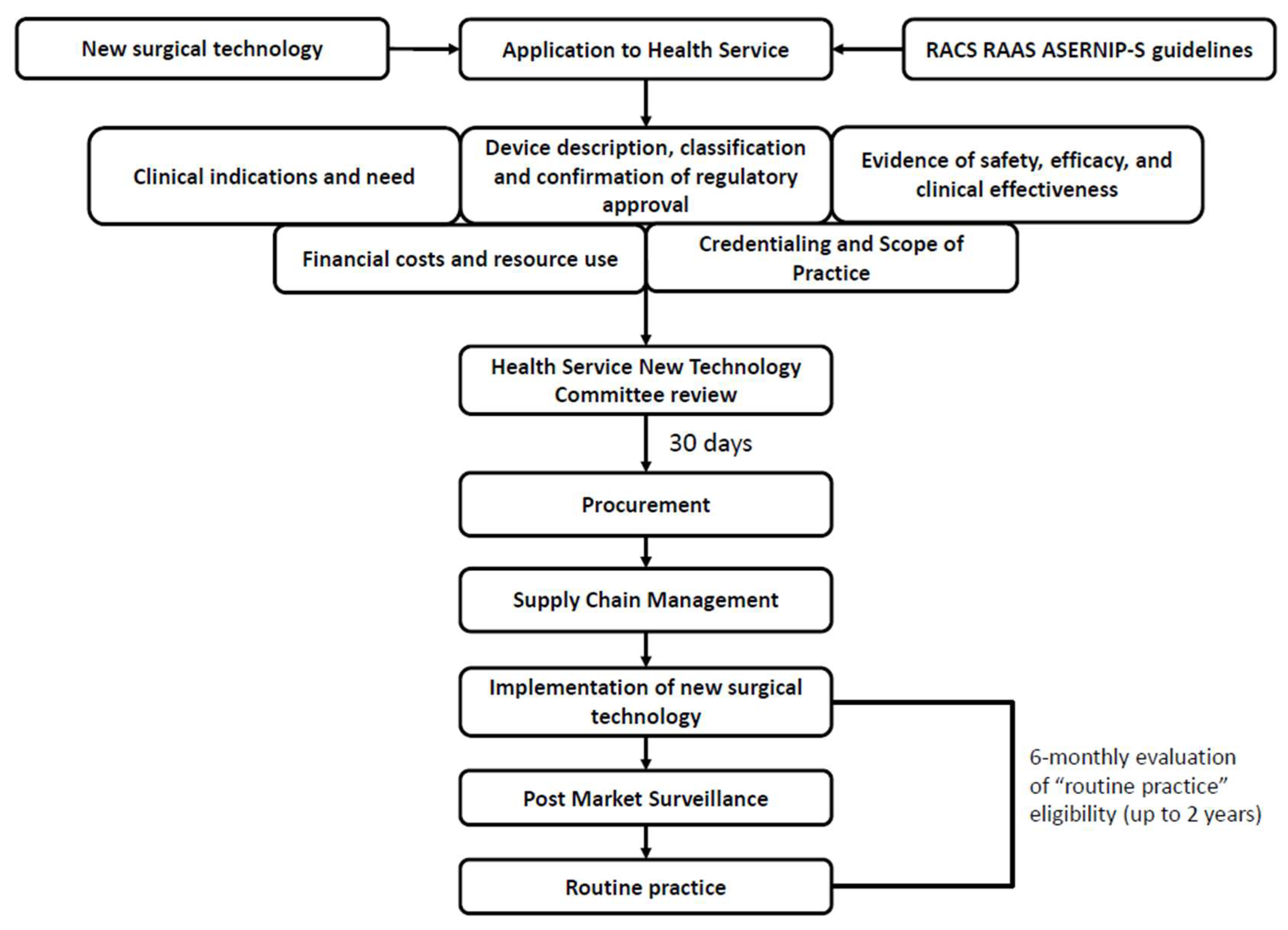
4. How to Implement New Devices in Surgical Practice
- Development of the device.
- 2.
- Compliance with regulatory processes.
- 3.
- Research clinical benefits compared to standard care.
- 4.
- Finalise device for product launch.
- 5.
- Launch device and assessment.
5. Discussion
6. Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TGA | Therapeutic Goods Administration |
| DoH | Department of Health |
| ARTG | Australian Register of Therapeutic Goods |
| FDA | Food and Drug Administration |
| RACS | Royal Australasian College of Surgeons |
| RARP | Robotic Assisted Radical Prostatectomy |
| ARGMD | Australian Regulatory Guidelines for Medical Devices |
| VHTP | Victorian Health Technology Program |
| MBS | Medicare Benefits Schedule |
| MSAC | Medical Services Advisory Committee |
| PICO | Population, Intervention, Comparator, Outcome |
| ASERNIP-S | Australian Safety and Efficacy Register of New Interventional Procedures—Surgical |
| RAAS | Research, Audit, and Academic Surgery |
| NTCP | New Technology/Clinical Practice |
| MDHTAC | Medical Devices and Human Tissue Product Advisory Committee |
| HREC | Human Research and Ethics Committee |
| ISO | International Organisation for Standardisation |
| RRP | Radical Retropubic Prostatectomy |
| LRP | Laparoscopic Radical Prostatectomy |
| CI | Confidence Interval |
| OR | Odds Ratio |
| MTAA | Medical Technology Association of Australia |
| UK | United Kingdom |
| USA | United States of America |
References
- Jarvenpaa, S.L.; Ives, B. Introducing Transformational Information Technologies: The Case of the World Wide Web Technology. Int. J. Electron. Commer. 1996, 1, 95–126. [Google Scholar] [CrossRef]
- Mytton, O.T.; Velazquez, A.; Banken, R.; Mathew, J.L.; Ikonen, T.S.; Taylor, K.; Painter, F.; Jean-Baptiste, R.; Poon, A.; Ruelas, E. Introducing new technology safely. Qual. Saf. Health Care 2010, 19 (Suppl. 2), i9–i14. [Google Scholar] [CrossRef]
- Department of Health, Disability, and Ageing AG. Understanding Processing Timeframes for Medical Device Applications. 2022; (updated 1 October 2024). Available online: https://www.tga.gov.au/resources/guidance/understanding-processing-timeframes-medical-device-applications (accessed on 18 May 2024).
- Ghosh, D.; Skinner, M.; Ferguson, L.R. The role of the Therapeutic Goods Administration and the Medicine and Medical Devices Safety Authority in evaluating complementary and alternative medicines in Australia and New Zealand. Toxicology 2006, 221, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Department of Health, Disability, and Ageing AG. Australian Register of Therapeutic Goods (ARTG). 2024. Available online: https://www.tga.gov.au/resources/artg (accessed on 18 May 2024).
- Costello, A.J.; Haxhimolla, H.; Crowe, H.; Peters, J.S. Installation of telerobotic surgery and initial experience with telerobotic radical prostatectomy. BJU Int. 2005, 96, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.; Vyas, A.; Vyas, D. The History of Robotics in Surgical Specialties. Am. J. Robot. Surg. 2014, 1, 12–20. [Google Scholar] [PubMed]
- Royal Australasian College of Surgeons (RACS). Robot-Assisted Surgery Working Party [Electronic Book]. 2023. Available online: https://www.surgeons.org/-/media/Project/RACS/surgeons-org/files/Marketing/Robot-Assisted-Surgery-Working-Party-Final-Report-and-Recommendations_June-2023.pdf?rev=de2285127fc84b038b21bde393e5f3cb&hash=78350E31C5DBF7AB1A500D59878400A0 (accessed on 11 July 2024).
- McBride, K.; Steffens, D.; Stanislaus, C.; Solomon, M.; Anderson, T.; Thanigasalam, R.; Leslie, S.; Bannon, P.G. Detailed cost of robotic-assisted surgery in the Australian public health sector: From implementation to a multi-specialty caseload. BMC Health Serv. Res. 2021, 21, 108. [Google Scholar] [CrossRef]
- Safi, S.; Thiessen, T.; Schmailzl, K.J. Acceptance and Resistance of New Digital Technologies in Medicine: Qualitative Study. JMIR Res. Protoc. 2018, 7, e11072. [Google Scholar] [CrossRef]
- Konda, N.N.; Lewis, T.L.; Furness, H.N.; Miller, G.W.; Metcalfe, A.J.; Ellard, D.R. Surgeon views regarding the adoption of a novel surgical innovation into clinical practice: Systematic review. BJS Open 2024, 8, zrad141. [Google Scholar] [CrossRef]
- Hopper, A.N.; Jamison, M.H.; Lewis, W.G. Learning curves in surgical practice. Postgrad. Med. J. 2007, 83, 777–779. [Google Scholar] [CrossRef]
- Soomro, N.A.; Hashimoto, D.A.; Porteous, A.J.; Ridley, C.J.A.; Marsh, W.J.; Ditto, R.; Roy, S. Systematic review of learning curves in robot-assisted surgery. BJS Open 2020, 4, 27–44. [Google Scholar] [CrossRef]
- Perera, S.; Fernando, N.; O’Brien, J.; Murphy, D.; Lawrentschuk, N. Robotic-assisted radical prostatectomy: Learning curves and outcomes from an Australian perspective. Prostate Int. 2023, 11, 51–57. [Google Scholar] [CrossRef] [PubMed]
- IP Australia AG. What Are Patents? 2024. Available online: https://www.ipaustralia.gov.au/patents/what-are-patents (accessed on 15 July 2024).
- Department of Health, Disability and Ageing AG. Therapeutic Goods Act 1989. Available online: https://www.legislation.gov.au/C2004A03952/latest/text/2 (accessed on 15 July 2024).
- Department of Health, Disability and Ageing AG. Medical Devices Overview. 2022. Available online: https://www.tga.gov.au/products/medical-devices/medical-devices-overview#classification (accessed on 18 May 2024).
- Department of Health, Disability and Ageing AG. Australian Regulatory Guidelines for Medical Devices (ARGMD). 2023. Available online: https://www.tga.gov.au/resources/resource/guidance/australian-regulatory-guidelines-medical-devices-argmd#essential-principles (accessed on 18 May 2024).
- Australian Government. Fees and Charges: Summary; Department of Health and Aged Care; Therapeutic Goods Administration: Woden, ACT, Australia, 2025.
- Department of Health and Aged Care AG. Health Technology Assessment Policy and Methods Review: Australian Market Authorisation, Funding and Assessment Pathways and Timelines. 2024. Available online: https://www.health.gov.au/sites/default/files/2024-07/hta-policy-and-methods-review-australian-market-authorisation-funding-and-assessment-pathways-and-timelines.pdf (accessed on 19 February 2025).
- Department of Health VG. Health Technology Program. 2023. Available online: https://www.health.vic.gov.au/patient-care/health-technology-program (accessed on 18 May 2024).
- Department of Health VG. Victorian Health Technology Program. 2023. Available online: https://www.health.vic.gov.au/patient-care/victorian-health-technology-program (accessed on 18 May 2024).
- Department of Industry, Science and Resources AG. Industry Growth Program. 2024. Available online: https://www.industry.gov.au/science-technology-and-innovation/industry-innovation/industry-growth-program (accessed on 1 July 2024).
- Department of Industry, Science and Resources AG. Promoting and Protecting Critical Technologies. 2024. Available online: https://www.industry.gov.au/science-technology-and-innovation/technology (accessed on 1 July 2024).
- Davis, M.M.; Gunn, R.; Cifuentes, M.; Khatri, P.; Hall, J.; Gilchrist, E.; Peek, C.J.; Klowden, M.; Lazarus, J.A.; Miller, B.F.; et al. Clinical Workflows and the Associated Tasks and Behaviors to Support Delivery of Integrated Behavioral Health and Primary Care. J. Ambul. Care Manag. 2019, 42, 51–65. [Google Scholar] [CrossRef]
- Bowens, F.M.; Frye, P.A.; Jones, W.A. Health information technology: Integration of clinical workflow into meaningful use of electronic health records. Perspect. Health Inf. Manag. 2010, 7, 1d. [Google Scholar] [PubMed]
- Department of Health, Disability and Ageing AG. MBS Online. 2024. Available online: https://www.mbsonline.gov.au/internet/mbsonline/publishing.nsf/Content/Home (accessed on 18 May 2024).
- Department of Health, Disability and Ageing AG. About Private Health Insurance. 2021. Available online: https://www.health.gov.au/topics/private-health-insurance/about-private-health-insurance (accessed on 18 May 2024).
- Department of Health, Disability and Ageing AG. What We Do 2024. Available online: https://www.msac.gov.au/about-us/what-we-do (accessed on 4 March 2025).
- Department of Health, Disability and Ageing AG. Application Timelines. 2024. Available online: https://www.msac.gov.au/apply/before-you-apply/application-timelines (accessed on 2 April 2025).
- Australian Commission on Safety and Quality in Health Care. Clinical Governance. 2024. Available online: https://www.safetyandquality.gov.au/our-work/clinical-governance#:~:text=Clinical%20governance%20is%20the%20set,to%20ensure%20good%20clinical%20outcomes (accessed on 1 July 2024).
- Royal Australasian College of Surgeons (RACS). Research & Evaluation, Inc. ASERNIP-S. 2024. Available online: https://www.surgeons.org/research-audit/research-evaluation-inc-asernips (accessed on 10 July 2024).
- Maddern, G.; Boult, M.; Ahern, E.; Babidge, W. ASERNIP-S: International trend setting. ANZ J. Surg. 2008, 78, 853–858. [Google Scholar] [CrossRef]
- Royal Australasian College of Surgeons (RACS) A-S. General Guidelines for Assessing, Approving & Introducing New Surgical Procedures into a Hospital or Health Service. 2008. Available online: https://www.surgeons.org/-/media/Project/RACS/surgeons-org/files/position-papers/rea_ase_3103_p_general_guidelines_for_assessing_approving_introducing_new_surgical_procedures_into_a.pdf?rev=40cc6edcb50c40d3baf777bb2f98ad68&hash=F81D0558402F1F7229CC070E1647FA8C (accessed on 10 July 2024).
- Austin Health. Austin 2025 Clinical Services Plan. 2024. Available online: https://www.austin.org.au/Assets/Files/Austin%202025%20Clinical%20Services%20Plan.pdf (accessed on 1 July 2024).
- Department of Finance AG. What Is Procurement? 2024. Available online: https://sellingtogov.finance.gov.au/guide/introduction-a-guide-to-government-procurement#:~:text=Procurement%20is%20a%20term%20used,buy%20to%20meet%20this%20need (accessed on 1 July 2024).
- National Academy of Engineering (US) and Institute of Medicine (US) Committee on Engineering and the Health Care System. Building a Better Delivery System: A New Engineering/Health Care Partnership [Internet]; Reid, P.P., Compton, W.D., Grossman, J.H., Fanjiang, G., Eds.; National Academies Press (US): Washington, DC, USA, 2005. Available online: https://nap.nationalacademies.org/catalog/11378/building-a-better-delivery-system-a-new-engineeringhealth-care-partnership (accessed on 3 April 2025).
- Rutala, W.A.; Weber, D.J. Disinfection and Sterilization in Health Care Facilities: An Overview and Current Issues. Infect. Dis. Clin. N. Am. 2016, 30, 609–637. [Google Scholar] [CrossRef] [PubMed]
- Department of Health, Disability and Ageing AG. Medical Devices and Human Tissue Products Covered Under Private Health Insurance. 2017. Available online: https://www.health.gov.au/topics/private-health-insurance/what-private-health-insurance-covers/medical-devices-and-human-tissue-products-covered-under-private-health-insurance?language=und (accessed on 1 July 2024).
- Department of Health, Disability and Ageing AG. About the Prescribed List. 2025. Available online: https://www.health.gov.au/our-work/the-prescribed-list/about (accessed on 3 April 2025).
- Australian Government. The Prescribed List of Medical Devices and Human Tissue Products Guide—DRAFT; Department of Health and Aged Care: Phillip, ACT, Australia, 2023.
- Department of Health, Disability and Ageing AG. Medical Devices and Human Tissue Advisory Committee (MDHTAC). 2025. Available online: https://www.health.gov.au/committees-and-groups/medical-devices-and-human-tissue-advisory-committee-mdhtac (accessed on 3 April 2025).
- Australian Government. Cost Recovery Implementation Statement—Administration of the Prescribed List of Medical Devices and Human Tissue Products; Department of Health and Aged Care: Phillip, ACT, Australia, 2024.
- Varkey, B. Principles of Clinical Ethics and Their Application to Practice. Med. Princ. Pract. 2021, 30, 17–28. [Google Scholar] [CrossRef]
- Miller, M.E.; Siegler, M.; Angelos, P. Ethical issues in surgical innovation. World J. Surg. 2014, 38, 1638–1643. [Google Scholar] [CrossRef]
- Ravan, N.; Hasanzad, M.; Larijani, B.; Namazi, H. Ethical Concerns in Novel Medicine. In A Glimpse at Medicine in the Future; Hasanzad, M., Ed.; Springer Nature: Singapore, 2024; pp. 201–212. [Google Scholar]
- Department of Health VG. Informed Consent and Presumption of Capacity. 2022. Available online: https://www.health.vic.gov.au/mental-health-and-wellbeing-act-handbook/supported-decision-making/informed-consent-and-presumption (accessed on 3 July 2024).
- Sauchelli, S.; Pickles, T.; Voinescu, A.; Choi, H.; Sherlock, B.; Zhang, J.; Colyer, S.; Grant, S.; Sundari, S.; Lasseter, G. Public attitudes towards the use of novel technologies in their future healthcare: A UK survey. BMC Med. Inform. Decis. Mak. 2023, 23, 38. [Google Scholar] [CrossRef]
- Mejtoft, T.; Lindahl, O.; Öhberg, F.; Pommer, L.; Jonzén, K.; Andersson, B.M.; Eklund, A.; Wåhlin, A.; Hallberg, P. Medtech innovation guide: An empiric model to support medical technology innovation. Health Technol. 2022, 12, 911–922. [Google Scholar] [CrossRef]
- Lottes, A.E.; Cavanaugh, K.J.; Chan, Y.Y.; Devlin, V.J.; Goergen, C.J.; Jean, R.; Linnes, J.C.; Malone, M.; Peat, R.; Reuter, D.G.; et al. Navigating the Regulatory Pathway for Medical Devices—A Conversation with the FDA, Clinicians, Researchers, and Industry Experts. J. Cardiovasc. Transl. Res. 2022, 15, 927–943. [Google Scholar] [CrossRef]
- Touijer, K. Marketing Versus Science: A Fight Between Necessary Evil and Stern Good Over the Adoption of New Technology in Medicine. Eur. Urol. 2010, 58, 522–524. [Google Scholar] [CrossRef] [PubMed]
- Bolenz, C.; Gupta, A.; Hotze, T.; Ho, R.; Cadeddu, J.A.; Roehrborn, C.G.; Lotan, Y. Cost comparison of robotic, laparoscopic, and open radical prostatectomy for prostate cancer. Eur. Urol. 2010, 57, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Yaxley, J.W.; Coughlin, G.D.; Chambers, S.K.; Occhipinti, S.; Samaratunga, H.; Zajdlewicz, L.; Dunglison, N.; Carter, R.; Williams, S.; Payton, D.J.; et al. Robot-assisted laparoscopic prostatectomy versus open radical retropubic prostatectomy: Early outcomes from a randomised controlled phase 3 study. Lancet 2016, 388, 1057–1066. [Google Scholar] [CrossRef]
- Coughlin, G.D.; Yaxley, J.W.; Chambers, S.K.; Occhipinti, S.; Samaratunga, H.; Zajdlewicz, L.; Teloken, P.; Dunglison, N.; Williams, S.; Lavin, M.F.; et al. Robot-assisted laparoscopic prostatectomy versus open radical retropubic prostatectomy: 24-month outcomes from a randomised controlled study. Lancet Oncol. 2018, 19, 1051–1060. [Google Scholar] [CrossRef]
- Xu, M.-Y.; Zeng, N.; Ma, S.; Hua, Z.-J.; Zhang, S.-H.; Xiang, J.-C.; Xiong, Y.-F.; Xia, Z.-Y.; Sun, J.-X.; Liu, C.-Q.; et al. A clinical evaluation of robotic-assisted radical prostatectomy (RARP) in located prostate cancer: A systematic review and network meta-analysis. Crit. Rev. Oncol. Hematol. 2024, 204, 104514. [Google Scholar] [CrossRef]
- Ballantyne, G.H. Robotic surgery, telerobotic surgery, telepresence, and telementoring. Surg. Endosc. Other Interv. Tech. 2002, 16, 1389–1402. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Rammohan, R.; Chavan, M.; Ghyar, R.; Deshpande, S.; Kulkarni, J.N.; Bhansali, H.; Ravi, B. Surgeon perception of factors affecting the efficiency of conventional and robotic laparoscopy: A Pan India study. Heliyon 2022, 8, e12561. [Google Scholar] [CrossRef]
- Almujalhem, A.; Rha, K.H. Surgical robotic systems: What we have now? A urological perspective. BJUI Compass 2020, 1, 152–159. [Google Scholar] [CrossRef]
- Medical Technology Association of Australia. Strengthening Australia’s Health System. 2024. Available online: https://www.mtaa.org.au/sites/default/files/uploaded-content/field_f_content_file/mtaa_pre-budget_submission_2024_v6_clean_final_for_submission_2.pdf (accessed on 9 April 2025).
- Australian Government. Budget 2025-26: Building Australia’s Future; Commonwealth of Australia: Canberra, ACT, Australia, 2025.
- Department of Health, Disability and Ageing AG. About the National Critical Research Infrastructure Initiative. 2025. Available online: https://www.health.gov.au/our-work/mrff-national-critical-research-infrastructure-initiative?language=en#about-the-national-critical-research-infrastructure-initiative (accessed on 9 April 2025).
- Department of Industry, Science and Resources AG. Medical Science Co-Investment Plan. 2024. Available online: https://www.industry.gov.au/publications/medical-science-co-investment-plan#introduction-2 (accessed on 9 April 2025).
- Medical Technology Association of Australia. Annual Report FY23/24; Medical Technology Association of Australia: Sydney, Australia, 2024; p. 72. [Google Scholar]
- Little, M. The fivefold root of an ethics of surgery. Bioethics 2002, 16, 183–201. [Google Scholar] [CrossRef]
- Cardenas, D. Surgical ethics: A framework for surgeons, patients, and society. Rev. Col. Bras. Cir. 2020, 47, e20202519. [Google Scholar] [CrossRef]
- Citron, P. Ethics Considerations for Medical Device R&D. Prog. Cardiovasc. Dis. 2012, 55, 307–315. [Google Scholar] [PubMed]
- Wilkinson, A. Medical Device Regulation and Litigation: A Comparative Analysis of Australia, the United Kingdom and the United States of America. Ph.D. Thesis, Queensland University of Technology, Brisbane City, Australia, 2021. [Google Scholar]
- UK Government. Consumer Protection Act 1987; Department for Digital, Culture, Media, and Sport: London, UK, 1987.
- UK Government. The Medical Devices Regulations 2002; Department for Digital, Culture, Media, and Sport: London, UK, 2002.
- UK Government. The General Product Safety Regulations 2005; Department for Digital, Culture, Media, and Sport: London, UK, 2005.
- European Union. Council Directive 93/42/EEC of 14 June 1993 Concerning Medical Devices; European Union: Brussels, Belgium, 1993. [Google Scholar]
- Federal Food. Drug, and Cosmetic Act. Cal. West. Med. 1938, 49, 172–174. [Google Scholar]
- UK Government. Medicines and Healthcare Products Regulatory Agency. 2025. Available online: https://www.gov.uk/government/organisations/medicines-and-healthcare-products-regulatory-agency/about (accessed on 9 April 2025).
- US Food and Drug Administration. About FDA. 2025. Available online: https://www.fda.gov/about-fda (accessed on 9 April 2025).
- Therapeutic Goods Administration Department of Health, Disability and Ageing AG. Understanding Priority Applicant Determination Rules for Medical Devices Including In-Vitro Diagnostics (IVDs). 2018. Available online: https://www.tga.gov.au/resources/guidance/understanding-priority-applicant-determination-rules-medical-devices-including-vitro-diagnostics-ivds#the-two-types-of-priority-applicant-determinations (accessed on 9 April 2025).
- Kruachottikul, P.; Tea-makorn, P.; Dumrongvute, P.; Hemrungrojn, S.; Nupairoj, N.; Junchaya, O.; Vinayavekhin, S. MediGate: A MedTech product innovation development process from university research to successful commercialization within emerging markets. J. Innov. Entrep. 2024, 13, 71. [Google Scholar] [CrossRef]
- Van Norman, G.A. Drugs, Devices, and the FDA: Part 2: An Overview of Approval Processes: FDA Approval of Medical Devices. JACC Basic. Transl. Sci. 2016, 1, 277–287. [Google Scholar] [CrossRef]
- Kaplan, A.V.; Baim, D.S.; Smith, J.J.; Feigal, D.A.; Simons, M.; Jefferys, D.; Fogarty, T.J.; Kuntz, R.E.; Leon, M.B. Medical Device Development: From Prototype to Regulatory Approval. Circulation 2004, 109, 3068–3072. [Google Scholar] [CrossRef]
- Müllner, P.S.; Klinger, U. Medical Device Development. In Medical Devices and In Vitro Diagnostics: Requirements in Europe; Baumgartner, C., Harer, J., Schröttner, J., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 1–36. [Google Scholar]

| Phase One Development Surgical Device Development | Phase Two Compliance Compliance with Regulatory Process | Phase Three Research Research and Experimentation | Phase Four Finalise Finalising for Product Launch | Phase Five Launch Product Launch and Assessment |
|---|---|---|---|---|
| Identification of problem | Therapeutic Goods Administration (TGA) | Clinical trials—animal and human studies | Refining surgical device | Sales and distribution |
| Conceptualisation, design, and manufacturing | Essential Principles—general, design, and construction | Human Research and Ethics Committee (HREC) | Finalising patents | Marketing |
| Market analysis Consumer review | Australian Regulatory Guidelines for Medical Devices (ARGMD) | Comparison to standard of care | Product branding | Clinician training |
| Risk assessment | Conformity assessment certification | Identifying risks and benefits | Regulatory clearance | Supply chain management |
| International Organisation for Standardisation (ISO) Certification | Australian Register of Therapeutic Goods (ARTG) listing | Refining surgical device | Finalise reimbursement | Assessment of product quality and refine as needed (TGA/ARTG) |
| Financial evaluation | Reimbursement strategy | Consumer and patient review | Finalise target hospitals | Post-market surveillance |
| Patents and feasibility | Medical Services Advisory Committee application (post ARTG listing) | Public and private healthcare applications | Prescribed List of Medical Devices and Human Tissue Products | Audit |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Société Internationale d’Urologie. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alberto, M.; Xu, J.; Patel, O.; Bolton, D.; Ischia, J. Barriers to Introducing New Transformative Surgical Technology in Australian Healthcare: A Comprehensive Review and Guide. Soc. Int. Urol. J. 2025, 6, 49. https://doi.org/10.3390/siuj6040049
Alberto M, Xu J, Patel O, Bolton D, Ischia J. Barriers to Introducing New Transformative Surgical Technology in Australian Healthcare: A Comprehensive Review and Guide. Société Internationale d’Urologie Journal. 2025; 6(4):49. https://doi.org/10.3390/siuj6040049
Chicago/Turabian StyleAlberto, Matthew, Jennifer Xu, Oneel Patel, Damien Bolton, and Joseph Ischia. 2025. "Barriers to Introducing New Transformative Surgical Technology in Australian Healthcare: A Comprehensive Review and Guide" Société Internationale d’Urologie Journal 6, no. 4: 49. https://doi.org/10.3390/siuj6040049
APA StyleAlberto, M., Xu, J., Patel, O., Bolton, D., & Ischia, J. (2025). Barriers to Introducing New Transformative Surgical Technology in Australian Healthcare: A Comprehensive Review and Guide. Société Internationale d’Urologie Journal, 6(4), 49. https://doi.org/10.3390/siuj6040049






