Oncologic Outcomes of a Novel Mapping Biopsy Technique Before Surgical Excision in the Management of Extramammary Paget Disease
Abstract
:1. Introduction
2. Materials and Methods
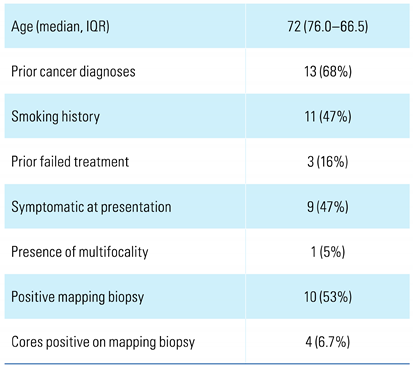
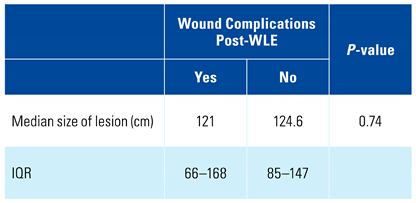
3. Results
4. Discussion
5. Conclusions
Conflicts of Interest
References
- McDaniel, B.; Brown, F.; Crane, J.S. Extramammary Paget disease. In: StatPearls. Treasure Island (FL)2022 Jan. Available online: https://pubmed.ncbi.nlm.nih.gov/29630276/ (accessed on 12 November 2022).
- Hendi, A.; Brodland, D.G.; Zitelli, J.A. Extramammary Paget’s disease: Surgical treatment with Mohs micrographic surgery. J. Am. Acad. Dermatol. 2004, 51, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Roh, M.R.; Chung, W.G.; Chung, K.Y. Comparison of Mohs micrographic surgery and wide excision for extramammary Paget’s disease: Korean experience. Dermatol. Surg. 2009, 35, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Pierie, J.P.; Choudry, U.; Muzikansky, A.; Finkelstein, D.M.; Ott, M.J. Prognosis and management of extramammary Paget’s disease and the association with secondary malignancies. J. Am. Coll. Surg. 2003, 196, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.S.-Y.; Ko, L.W.-L.; Cheung, T.-H. Extramammary Paget’s disease: Surgical control from the plastic surgery perspective. Surg. Pract. 2016, 20, 110–113. [Google Scholar] [CrossRef]
- NIH, National Center for Advancing Translational Sciences. Extramammary Paget disease. 2021. Available online: https://rarediseases.info.nih.gov/diseases/4192/extramammary-paget-disease (accessed on 17 September 2021).
- Ohara, K.; Fujisawa, Y.; Yoshino, K.; Kiyohara, Y.; Kadono, T.; Murata, Y.; et al. A proposal for a TNM staging system for extramammary Paget disease: Retrospective analysis of 301 patients with invasive primary tumors. J. Dermatol. Sci. 2016, 83, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Mitropoulos, D.; Artibani, W.; Biyani, C.S.; Bjerggaard Jensen, J.; Roupret, M.; Truss, M. Validation of the Clavien-Dindo grading system in urology by the European Association of Urology Guidelines Ad Hoc Panel. Eur. Urol. Focus 2018, 4, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Hegarty, P.K.; Suh, J.; Fisher, M.B.; Taylor, J.; Nguyen, T.H.; Ivan, D.; Prieto, V.G.; et al. Penoscrotal extramammary Paget’s disease: The University of Texas M. D. Anderson Cancer Center contemporary experience. J. Urol. 2011, 186, 97–102. [Google Scholar] [CrossRef]
- Leong, J.Y.; Chung, P.H. A primer on extramammary Paget’s disease for the urologist. Transl. Androl. Urol. 2020, 9, 93–105. [Google Scholar] [CrossRef]
- Yang, W.J.; Kim, D.S.; Im, Y.J.; Cho, K.S.; Rha, K.H.; Cho, N.H.; et al. Extramammary Paget’s disease of penis and scrotum. Urology 2005, 65, 972–975. [Google Scholar] [CrossRef] [PubMed]
- Misas, J.E.; Cold, C.J.; Hall, F.W. Vulvar Paget disease: Fluorescein-aided visualization of margins. Obstet. Gynecol. 1991, 77, 156–159. [Google Scholar]
- Fanning, J.; Lambert, H.C.; Hale, T.M.; Morris, P.C.; Schuerch, C. Paget’s disease of the vulva: Prevalence of associated vulvar adenocarcinoma, invasive Paget’s disease, and recurrence after surgical excision. Am. J. Obstet. Gynecol. 1999, 180 Pt 1, 24–27. [Google Scholar] [CrossRef]
- Marchesa, P.; Fazio, V.W.; Oliart, S.; Goldblum, J.R.; Lavery, I.C.; Milsom, J.W. Long-term outcome of patients with perianal Paget’s disease. Ann. Surg. Oncol. 1997, 4, 475–480. [Google Scholar] [CrossRef]
- Sarmiento, J.M.; Wolff, B.G.; Burgart, L.J.; Frizelle, F.A.; Ilstrup, D.M. Paget’s disease of the perianal region--an aggressive disease? Dis. Colon Rectum. 1997, 40, 1187–1194. [Google Scholar] [CrossRef]
- Yugueros, P.; Keeney, G.L.; Bite, U. Paget’s disease of the groin: Report of seven cases. Plast. Reconstr. Surg. 1997, 100, 336–339. [Google Scholar] [CrossRef]
- Zollo, J.D.; Zeitouni, N.C. The Roswell Park Cancer Institute experience with extramammary Paget’s disease. Br. J. Dermatol. 2000, 142, 59–65. [Google Scholar] [CrossRef]
- Yao, H.; Xie, M.; Fu, S.; Guo, J.; Peng, Y.; Cai, Z.; et al. Survival analysis of patients with invasive extramammary Paget disease: Implications of anatomic sites. BMC Cancer 2018, 18, 403. [Google Scholar] [CrossRef]
- Herrel, L.A.; Weiss, A.D.; Goodman, M.; Johnson, T.V.; Osunkoya, A.O.; Delman, K.A.; et al. Extramammary Paget’s disease in males: Survival outcomes in 495 patients. Ann. Surg. Oncol. 2015, 22, 1625–1630. [Google Scholar] [CrossRef] [PubMed]

 |
 |
This is an open access article under the terms of a license that permits non-commercial use, provided the original work is properly cited. © 2023 The Authors. Société Internationale d'Urologie Journal, published by the Société Internationale d'Urologie, Canada.
Share and Cite
Rose, K.M.; Zurlo, R.; Li, R.; Mosiello, G.; Spiess, P.E. Oncologic Outcomes of a Novel Mapping Biopsy Technique Before Surgical Excision in the Management of Extramammary Paget Disease. Soc. Int. Urol. J. 2023, 4, 34-38. https://doi.org/10.48083/LCME5237
Rose KM, Zurlo R, Li R, Mosiello G, Spiess PE. Oncologic Outcomes of a Novel Mapping Biopsy Technique Before Surgical Excision in the Management of Extramammary Paget Disease. Société Internationale d’Urologie Journal. 2023; 4(1):34-38. https://doi.org/10.48083/LCME5237
Chicago/Turabian StyleRose, Kyle M., Rosalie Zurlo, Roger Li, Gerard Mosiello, and Philippe E. Spiess. 2023. "Oncologic Outcomes of a Novel Mapping Biopsy Technique Before Surgical Excision in the Management of Extramammary Paget Disease" Société Internationale d’Urologie Journal 4, no. 1: 34-38. https://doi.org/10.48083/LCME5237
APA StyleRose, K. M., Zurlo, R., Li, R., Mosiello, G., & Spiess, P. E. (2023). Oncologic Outcomes of a Novel Mapping Biopsy Technique Before Surgical Excision in the Management of Extramammary Paget Disease. Société Internationale d’Urologie Journal, 4(1), 34-38. https://doi.org/10.48083/LCME5237





