Systematic Review of Comparative Patient Reported Outcomes and Health-Related Quality of Life after Management of Localized Renal Masses or Renal Cell Carcinomas
Abstract
:Introduction
Materials and Methods
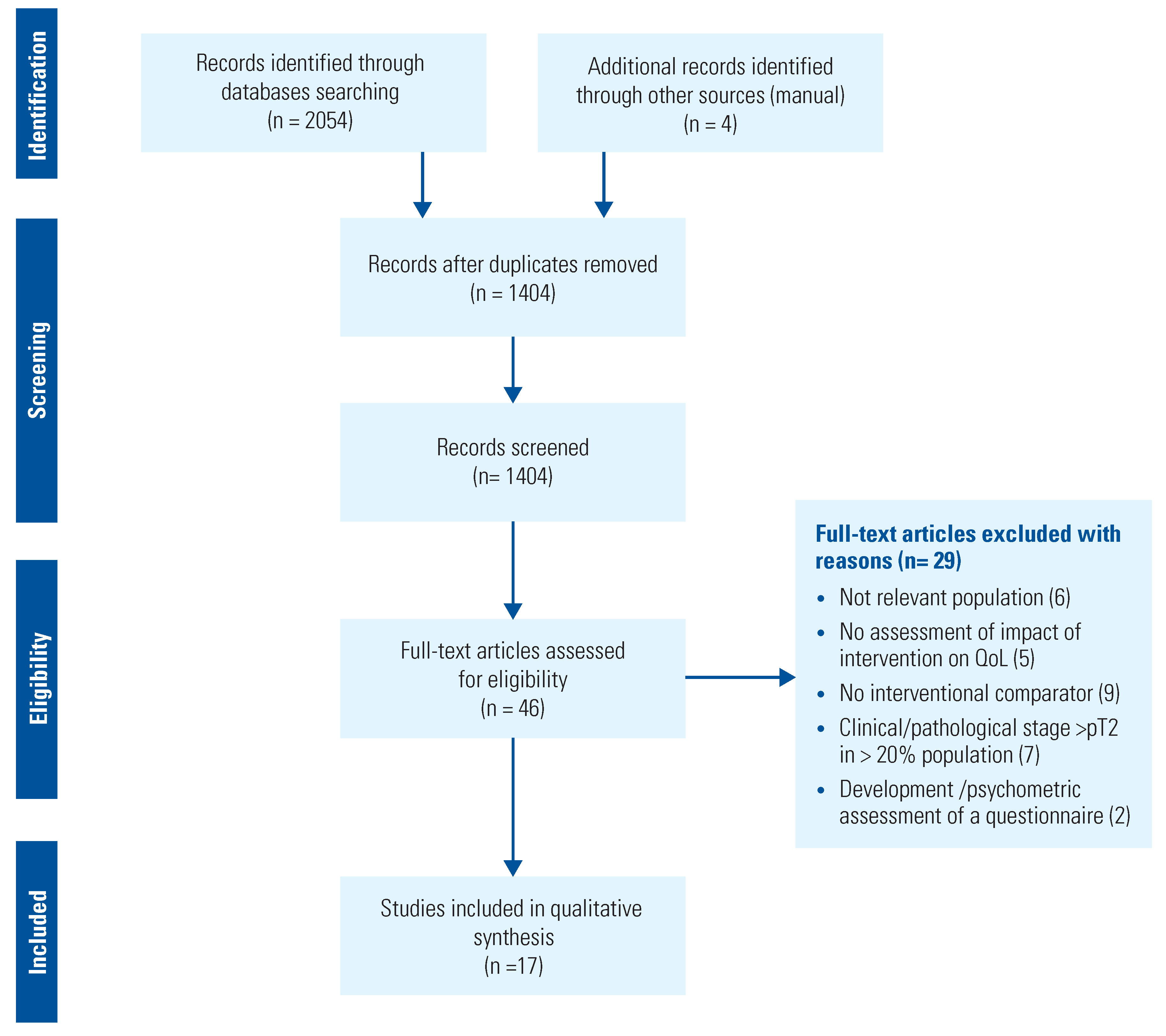
Data Sources and Searches
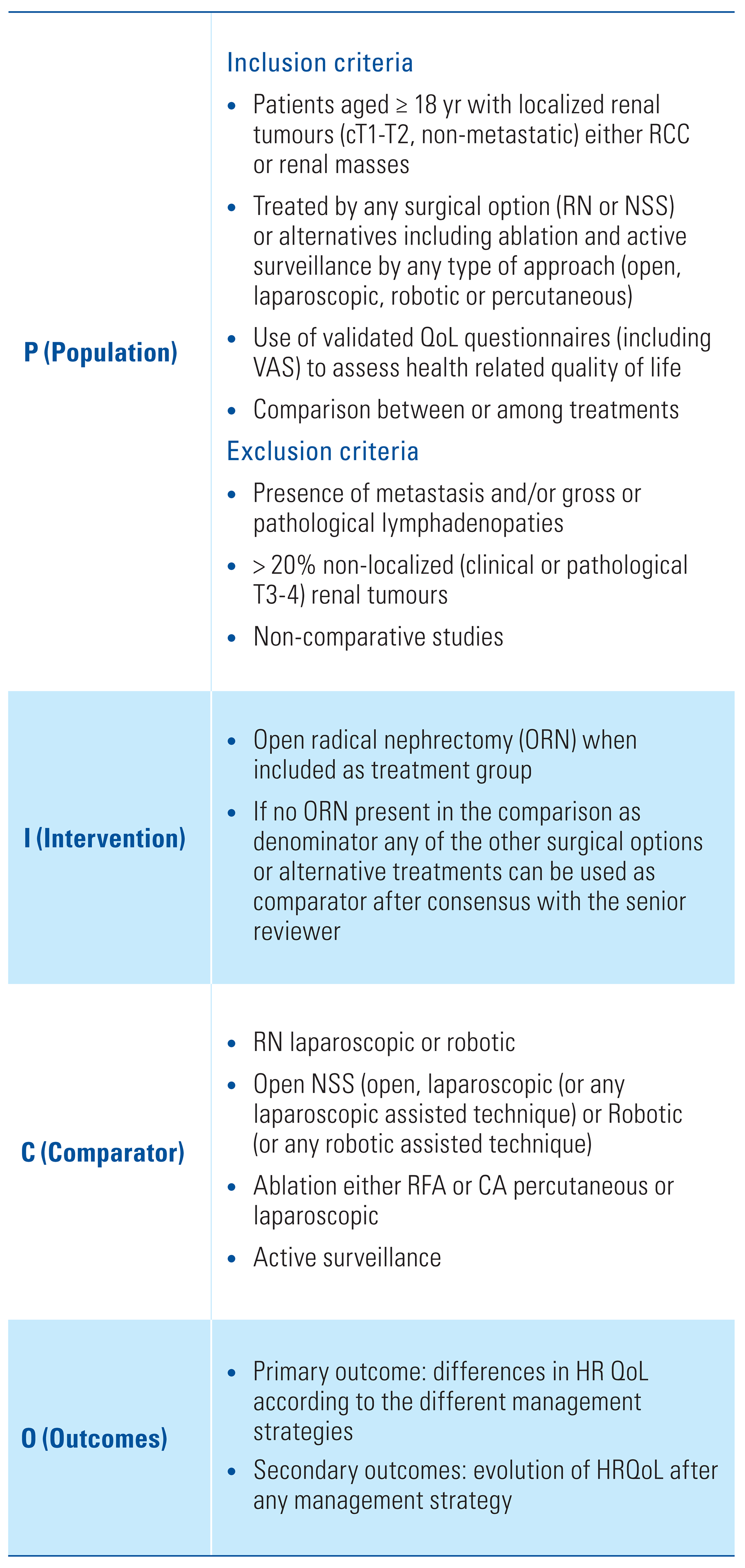
Study Selection, Data Extraction and Analysis
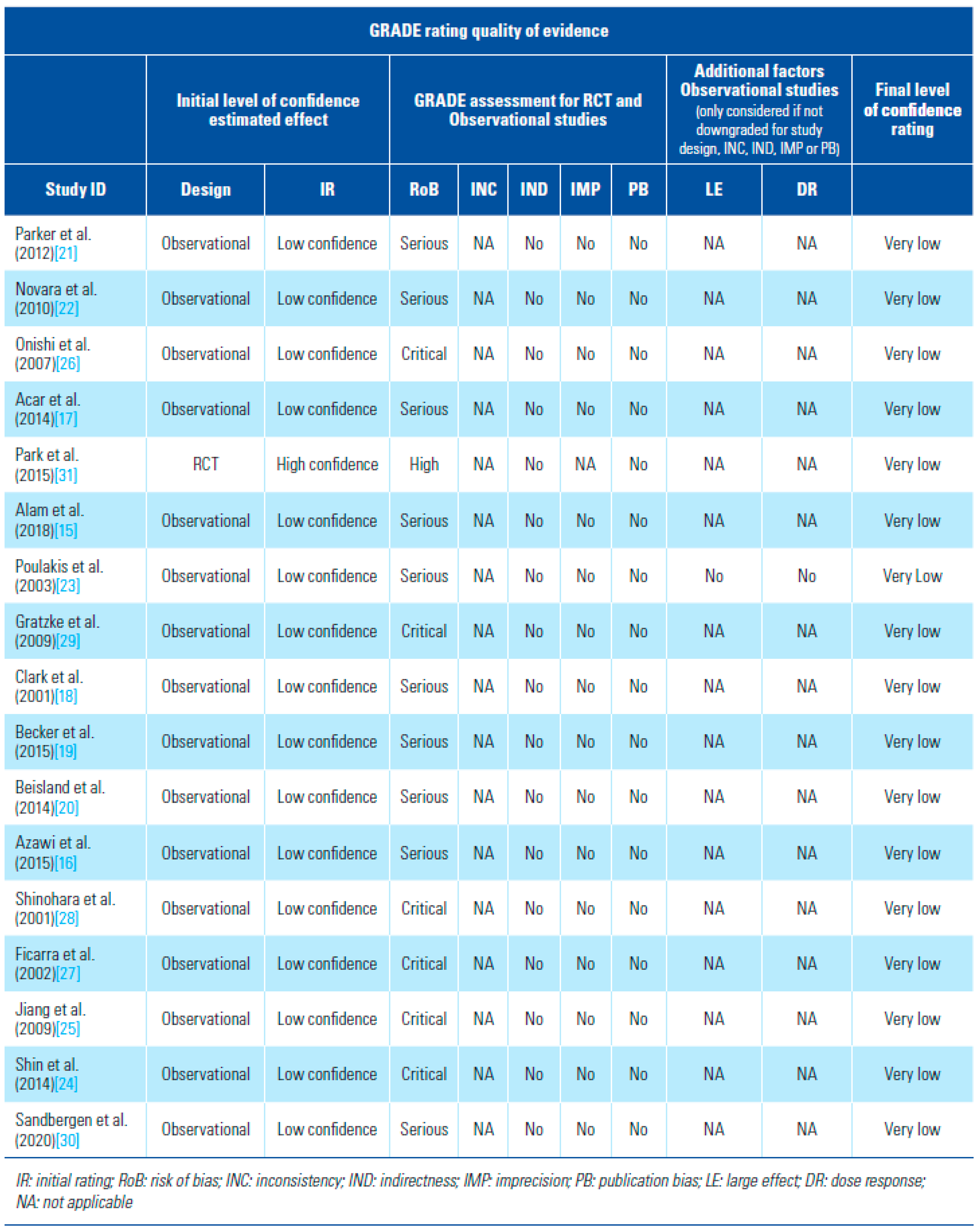
Risk of Bias Assessment and Strength of Body of Evidence
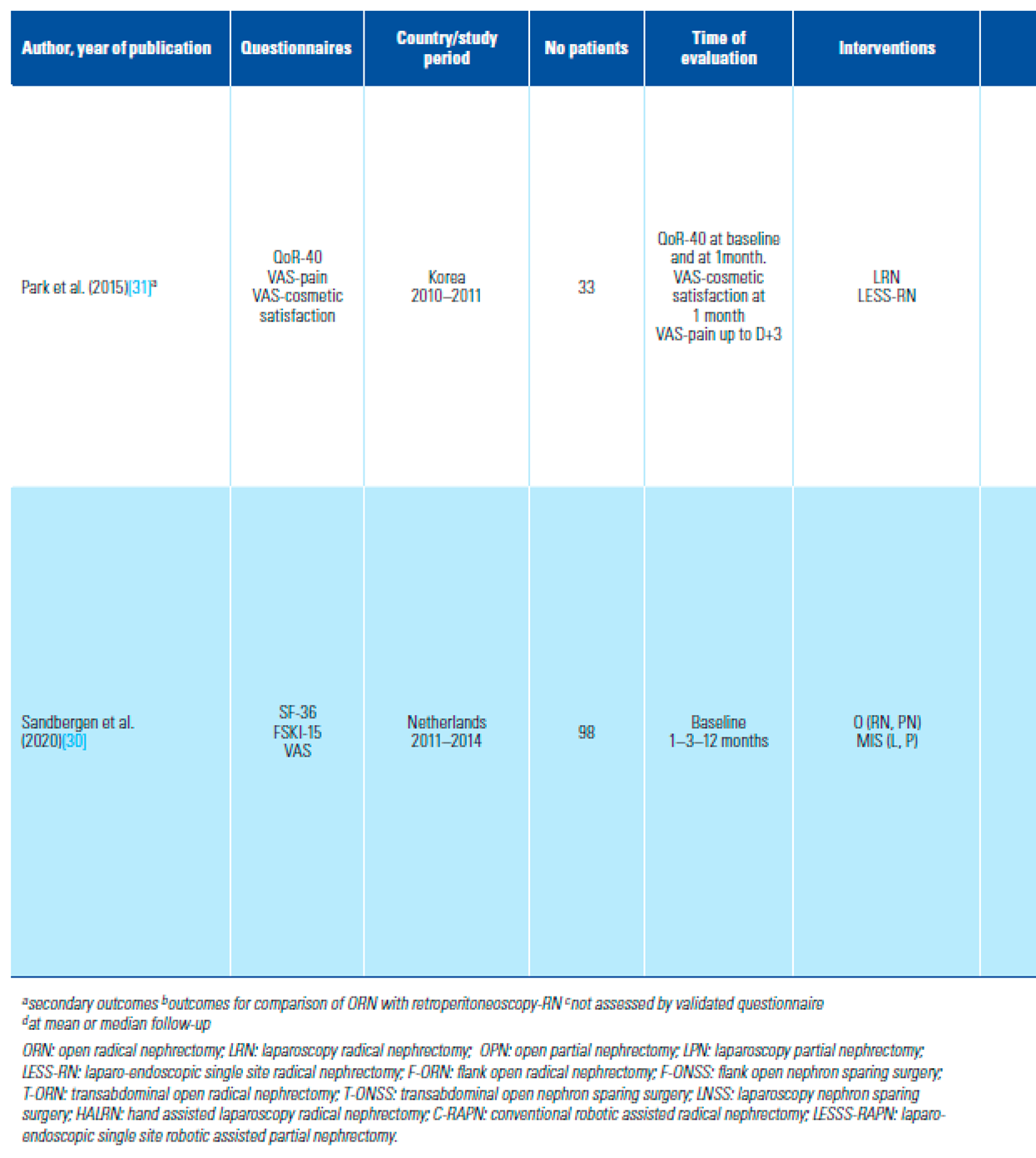
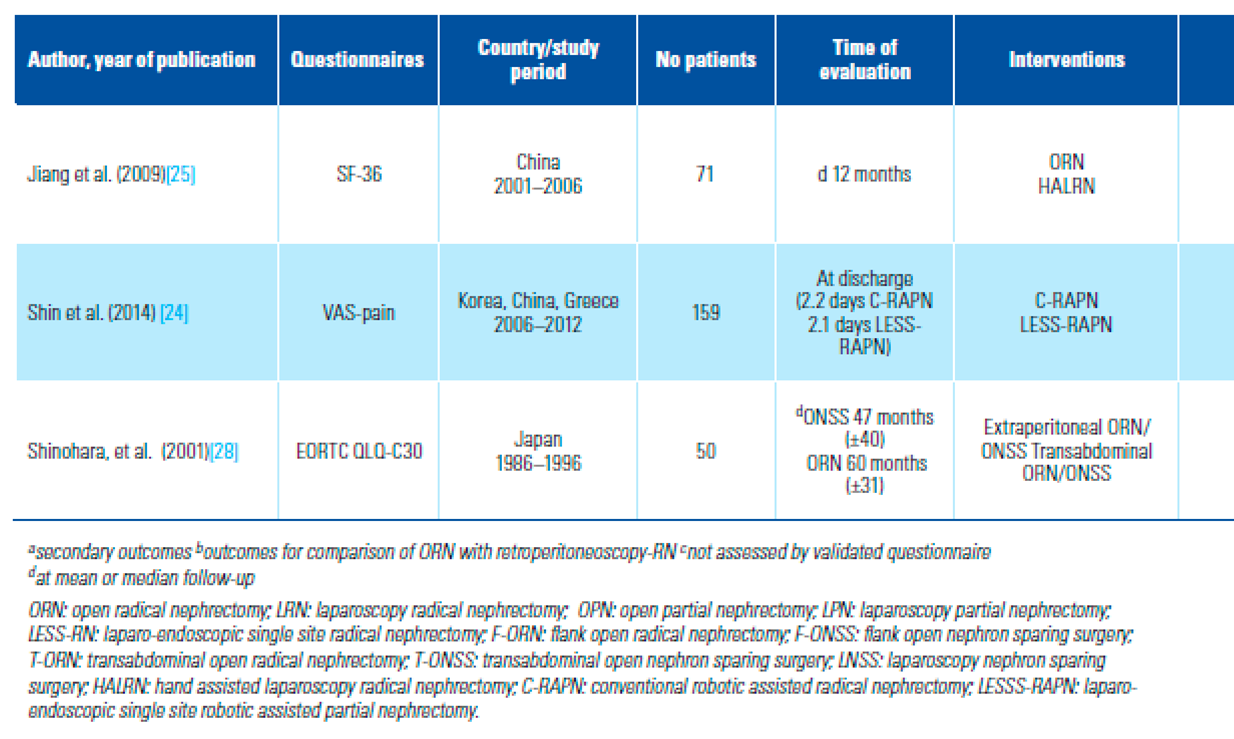
PROMs Used to Assess QoL and Distribution
Standards for Comparison
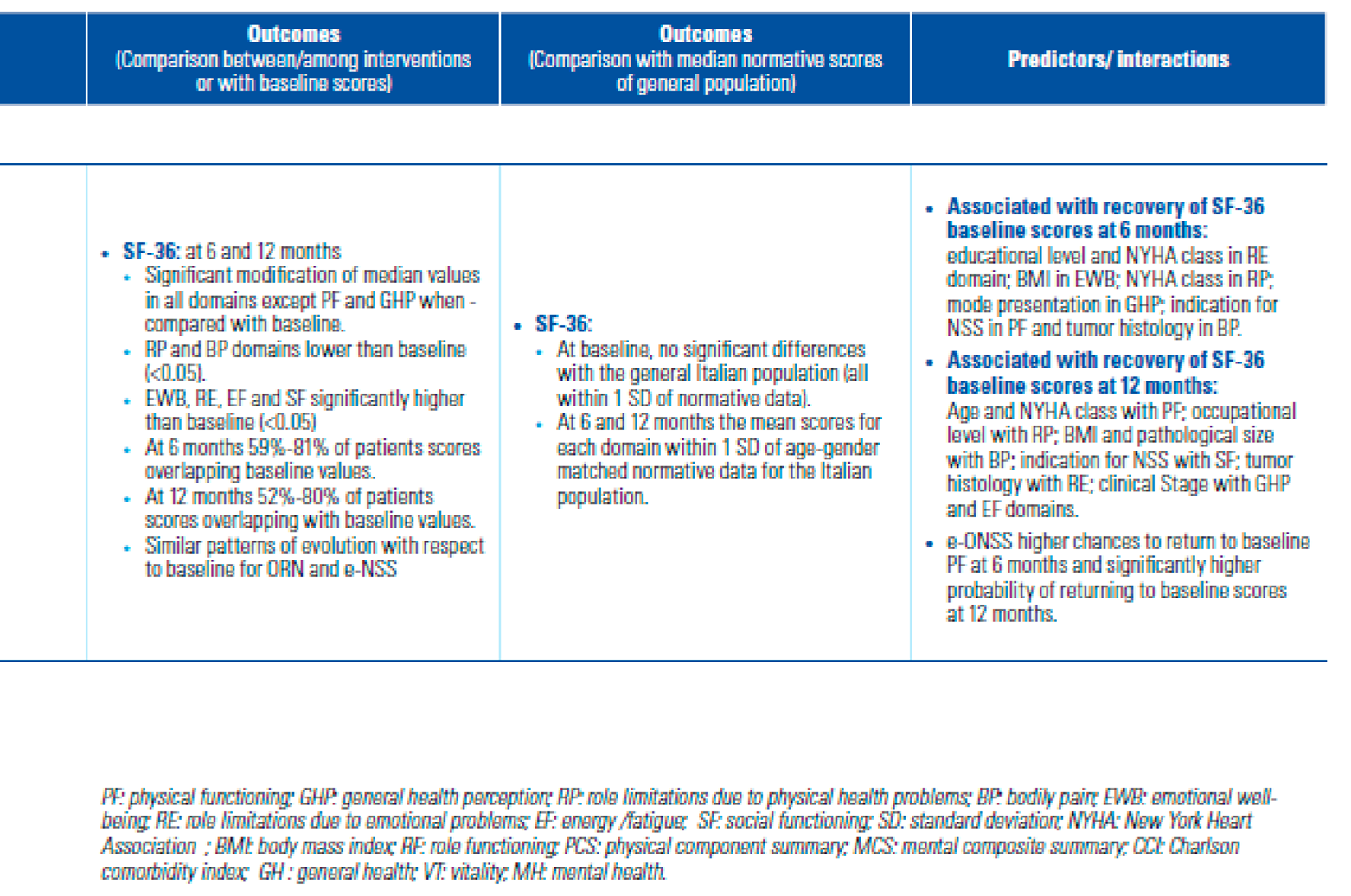
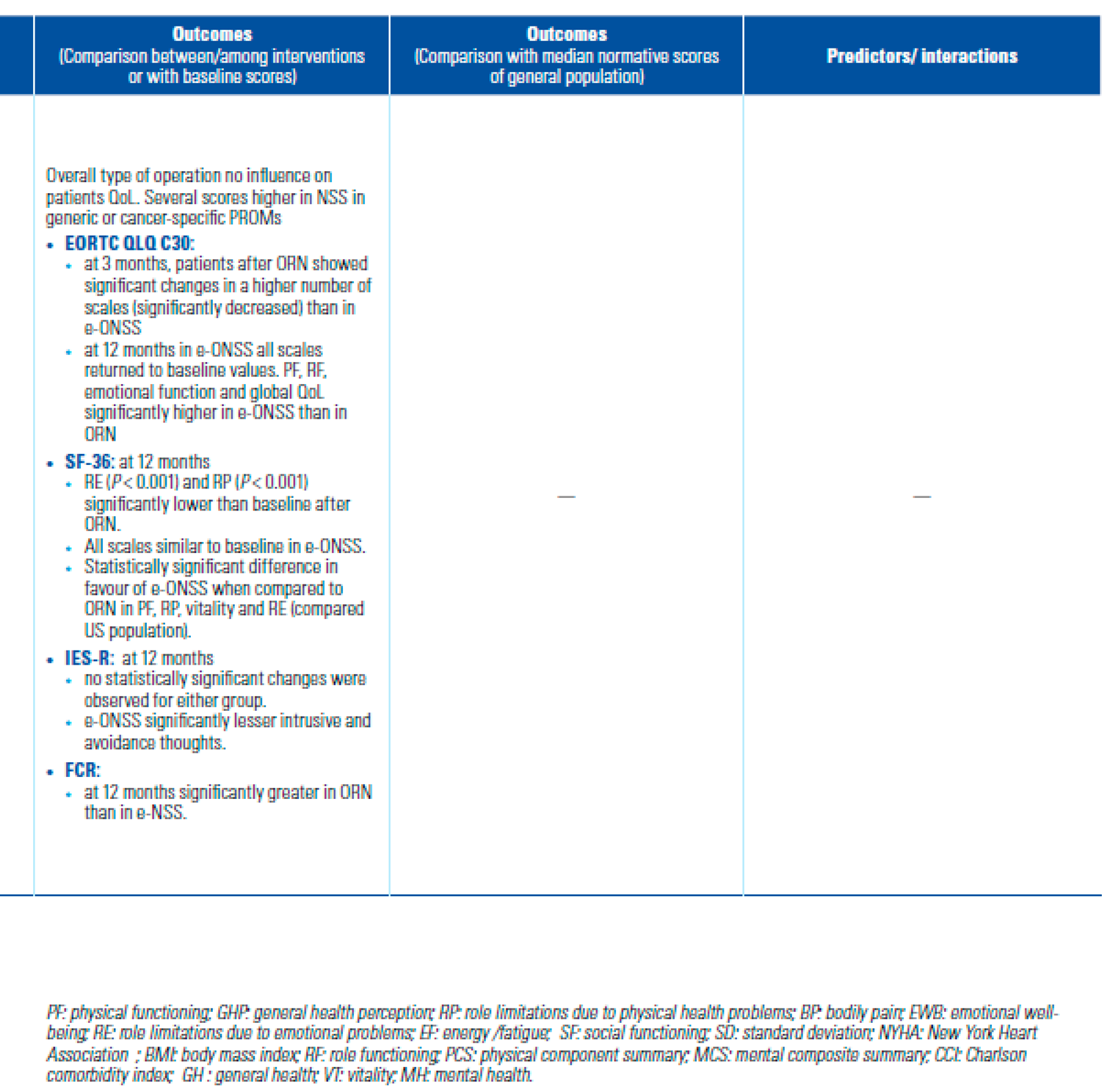
Outcomes
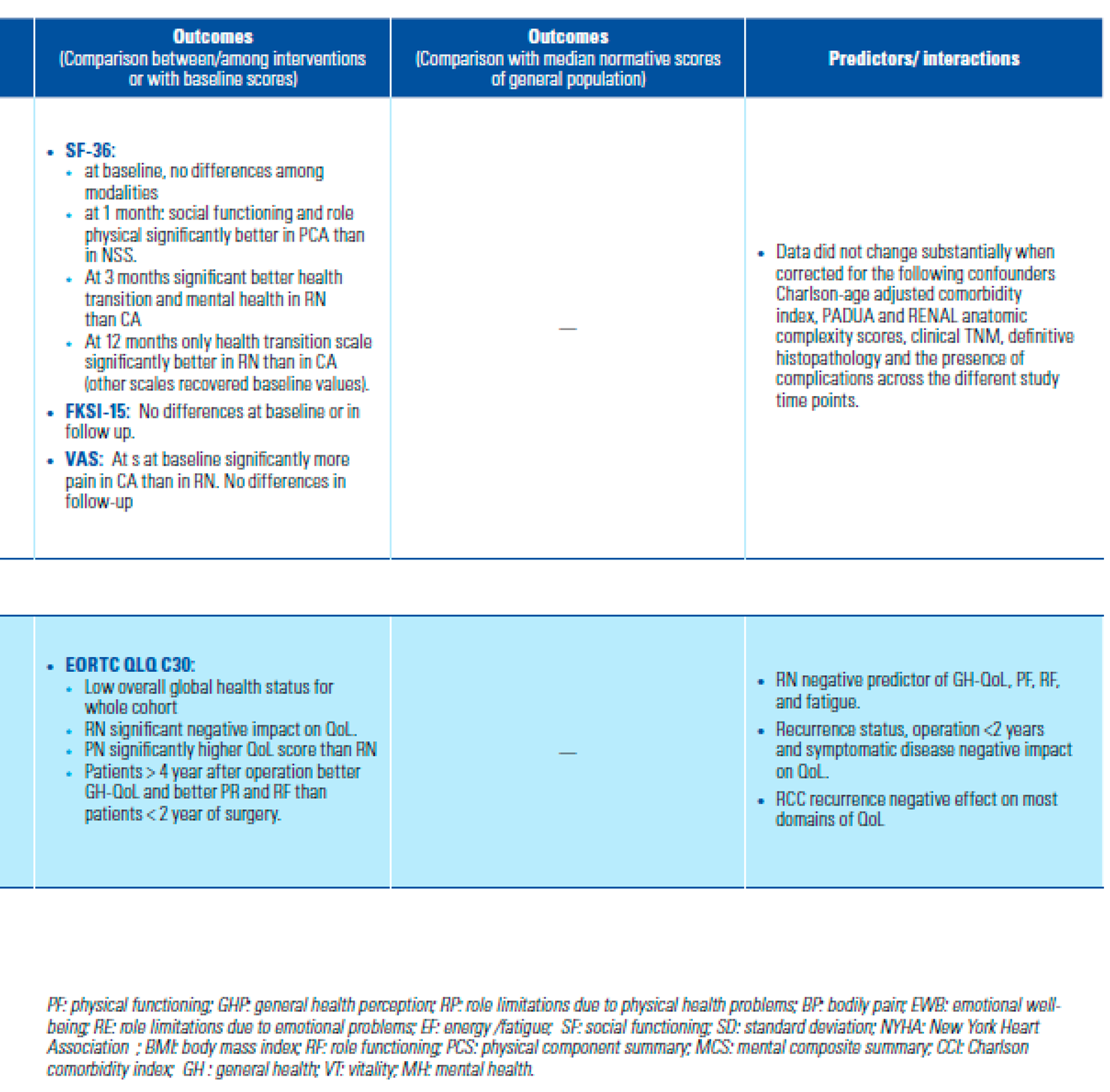
- Is HRQoL after NSS better than after RN?
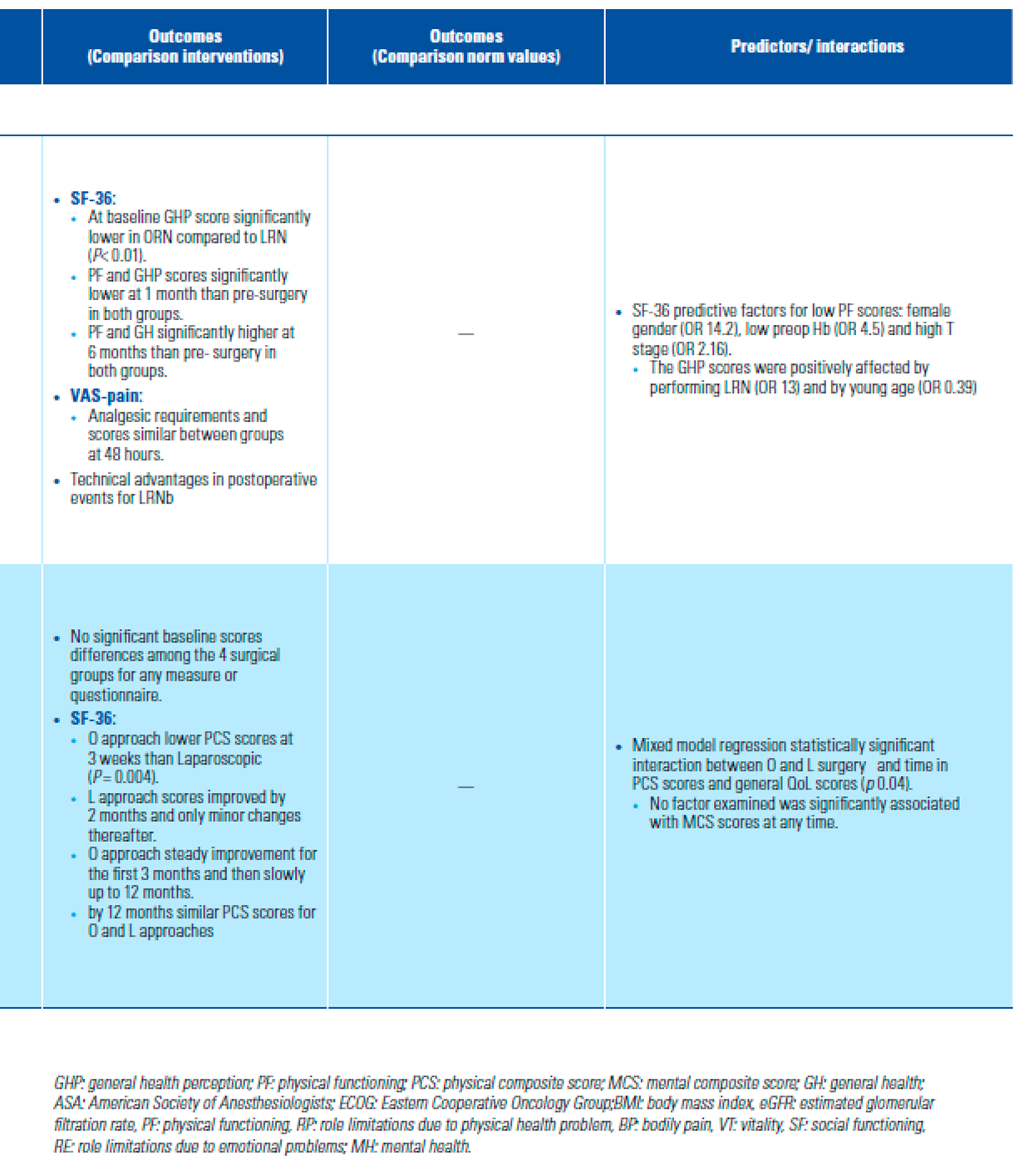
- Is HRQoL better after MIS than after open approach?
- Comparison with matched age–sex normative scores for the general population
- HRQoL outcomes in clinical or pathological T1a
- Confounders and predictive factors
- Risk of Bias assessment and certainty of evidence
Discussion
Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AS | active surveillance |
| HRQoL | health-related QoL |
| LRCC | localized renal cell carcinoma |
| LRM | localized renal mass |
| MIS | minimally invasive surgery |
| NSS | nephron-sparing surgery |
| PN | partial nephrectomy |
| PROM | patient reported outcome measures |
| RCC | renal cell carcinoma |
| RCT | randomized controlled trial |
| RN | radical nephrectomy |
| TA | thermal ablation |
| VAS | visual analog scale |
References
- Saad, A.M.; Gad, M.M.; Al-Husseini MJRuhban, I.A.; Sonbol, M.B.; Ho, T.H. Trends in renal cell carcinoma incidence and mortality in the US in the last two decades; SEER-based study. Clin. Genitourin. Cancer 2019, 17, 46–57.e5. [Google Scholar] [CrossRef] [PubMed]
- Bhindi, B.; Thompson, R.H.; Lohse, C.M.; Mason, R.J.; Frank, I.; Costello, B.A.; et al. The probability of aggressive versus indolent histology based on renal tumor size: implications for surveillance and treatment. Eur Urol. 2018, 74, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.D.; Smerjian, A.; Gupta, M.; Pavlovich, C.P.; Johnson, M.H.; Gorin, M.A.; et al. Surgical removal of renal tumours with low metastatic potential based on clinical radiographic size: a systematic review of the literature. Urol Oncol. 2019, 37, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.C.; Vukina, J.; Smith, A.B.; Meyer, A.M.; Wheeler, S.B.; Kuo, T.M.; et al. Preoperatively misclassified, surgically removed benign renal masses: a systematic review of surgical series and United States population level burden estimate. J Urol. 2014, 193, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Pierorazio, P.M.; Johnson, M.H.; Patel, H.D.; Sozio, S.M.; Sharma, R.; Iyoha, E.; et al.; Management of Renal Masses and Localized Renal Cancer Effective Health Care Program. Comparative Effectiveness Review. No 167. Agency for Healthcare research and Quality (AHCQ publication No 16-EHC001-EF, February 2016). Available online: www.ahrq.gov. (accessed on 30 January 2021).
- McLennan, S.; Imamura, M.; Lapitan, M.C.; Omar, M.I.; Lam, T.B.; Hilvano- Cabungcal, A.M.; et al. Systematic review of perioperative and quality of life outcomes following surgical management of localised renal cancer. Eur Urol. 2012, 62, 1097–1117. [Google Scholar] [CrossRef] [PubMed]
- Laviana, A.A.; Pannell, S.C.; Huen, K.H.Y.; Huen, K.H.Y.; Bergman, J. Engaging patients in complex clinical decision-making: successes, pitfalls, and future directions. Urol Oncol. 2017, 35, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Briffa, N. The employment of patient-reported outcome measures to communicate the likely benefits of surgery. Patient Relat Outcome Meas. 2018, 9, 263–266. [Google Scholar] [CrossRef] [PubMed]
- King, S.C.; Pollack, L.A.; Li, J.; King, J.B.; master, V.A. Continued increase in incidence of renal cell carcinoma, especially in young patients and high-grade disease: United States 2001 to 2010. J Urol. 2014, 191, 1665–1670. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA, group. Preferred reporting items for systematic reviews and metanalysis: the PRISMA statement. J Clin Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef]
- Knoll, T.; Omar, M.I.; McLennan SHernandez, V.; Canfield, S.; Yuan, Y.; et al. Key steps in conducting systematic reviews for underpinning clinical practice guidelines: methodology of the European Association of Urology. Eur Urol. 2018, 73, 290–300. [Google Scholar] [CrossRef]
- Sterne, J.; Hernan, M.A.; Reeves, B.C.; Savovic, S.; Berkman, N.D.; Viswanathan, M.; et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.O.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Johnston, B.C.; Patrick, D.L.; Busse, J.W.; Schunemann, H.J.; Agarwal, A.; Guyatt, G.H. Patient-reported outcomes in meta-analyses–Part 1, assessing risk of bias and combining outcomes. Health Qual Life Outcomes. 2013, 11, 109. [Google Scholar] [CrossRef] [PubMed]
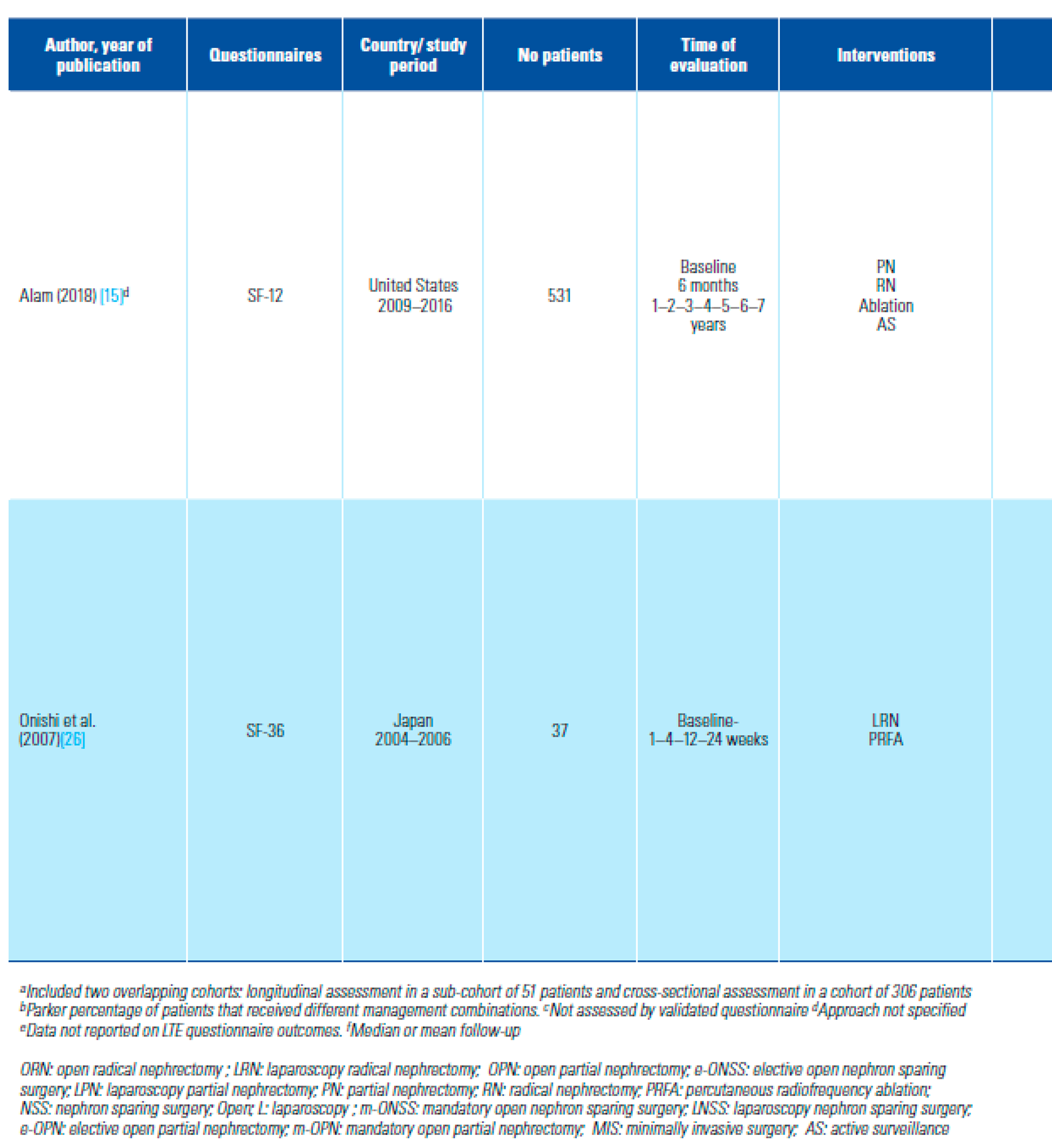
- Alam, R.; Patel, H.D.; Osumah, T.; Srivastava, A.; Gorin, M.A.; Johnson, M.A.; et al. Comparative effectiveness of management options for patients with small renal masses: a prospective cohort study. BJU Int. 2019, 123, 42–50. [Google Scholar] [CrossRef] [PubMed]
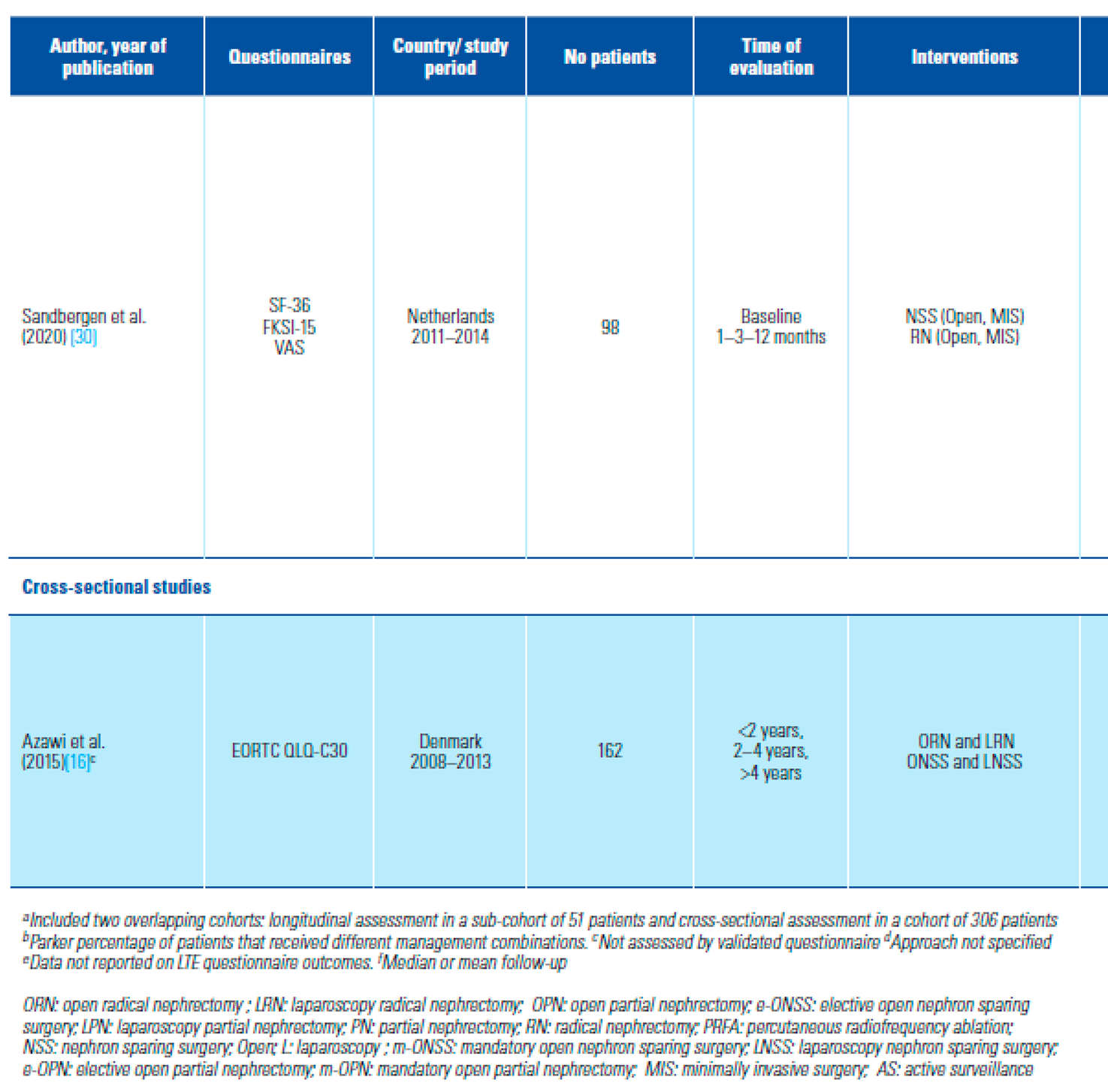
- Azawi, N.H.; Tesfalem, H.; Dahl, C. Lund L Do the different types of renal surgery impact the quality of life in the postoperative period? Int Urol Nephrol. 2015, 47, 263–269. [Google Scholar] [CrossRef] [PubMed]
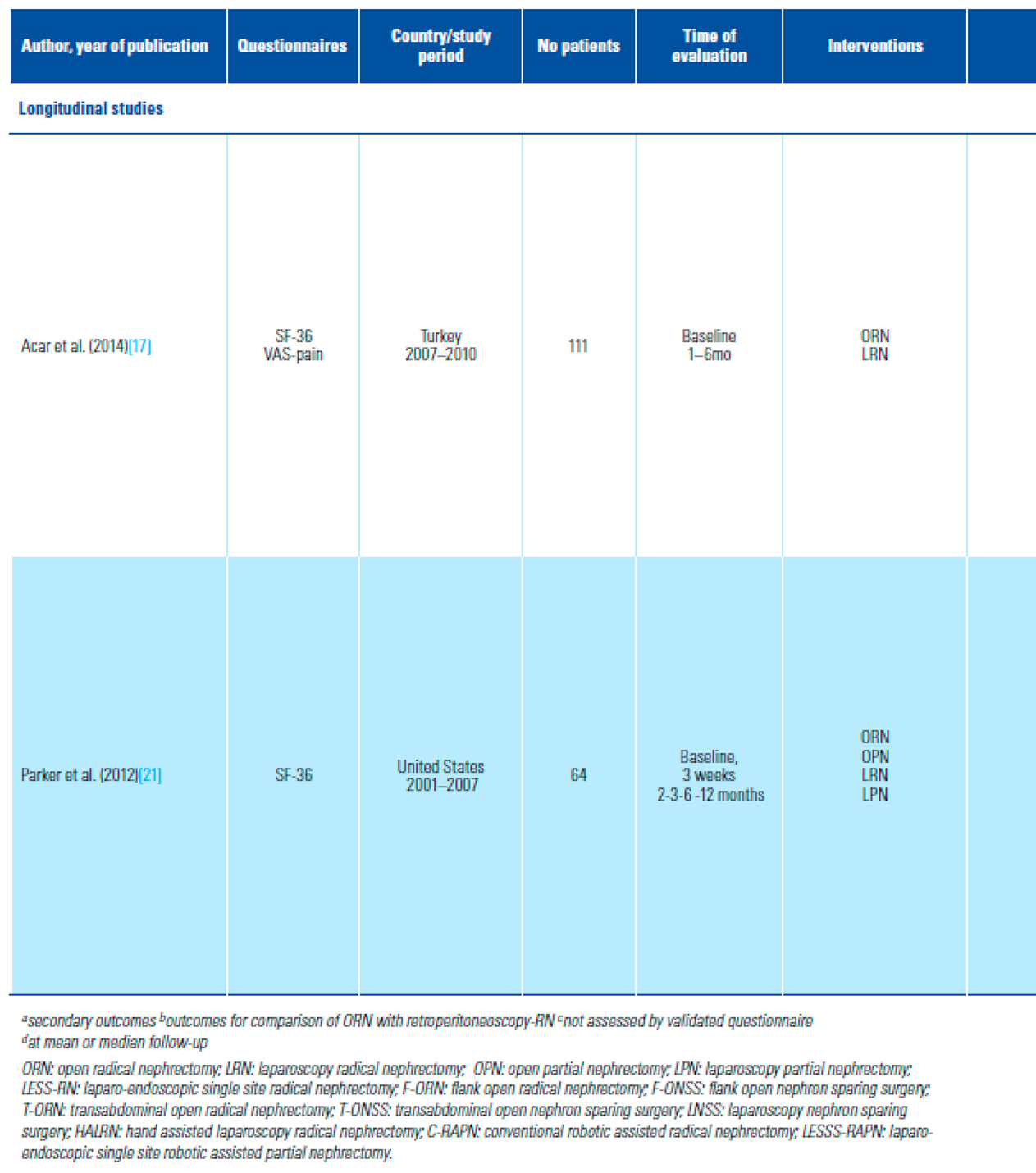
- Acar, C.; Bilen, C.; Bayazit, Y.; Aslan, G.; Koni, A.; Basok, E.; et al. Quality of life survey following laparoscopic and open radical nephrectomy. Urol J. 2014, 11, 1944–1950. [Google Scholar] [PubMed]
- Clark, P.E.; Schover, L.R.; Uzzo, R.G.; Hafez, K.S.; Rybicki, L.A.; Novick, A.C. Quality of life and psychological adaptation after surgical treatment for localized renal cell carcinoma: impact of the amount of remaining renal tissue. Urology 2001, 57, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.; Pradel, L.; Kluth, L.; Schmid, M.; Eichelberg, C.; Ahyai, S.; et al. Laparoscopic versus open partial nephrectomy for clinical T1 renal masses: no impact of surgical approach on perioperative complications and long-term postoperative quality of life. World J Urol. 2015, 33, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Beisland, C.; Beisland, E.; Hjelle, K.M.; Bostad, L.; Jensen Hedjermstad, M.; Aarstad, A.K.H.; et al. Health-related quality of life in long-term survivors after renal cancer treatment. Scand J Urol. 2014, 48, 52–64. [Google Scholar] [CrossRef]
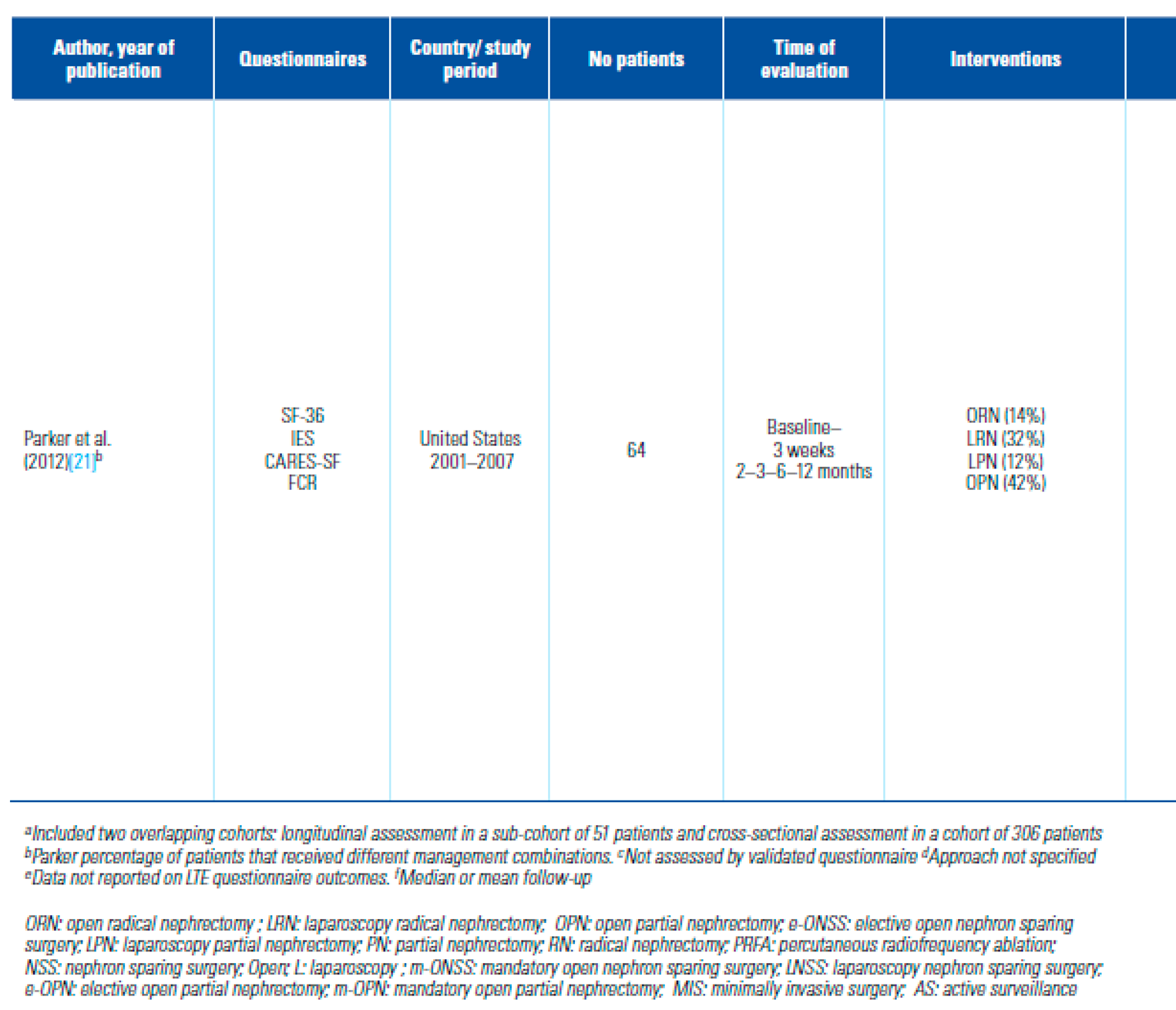
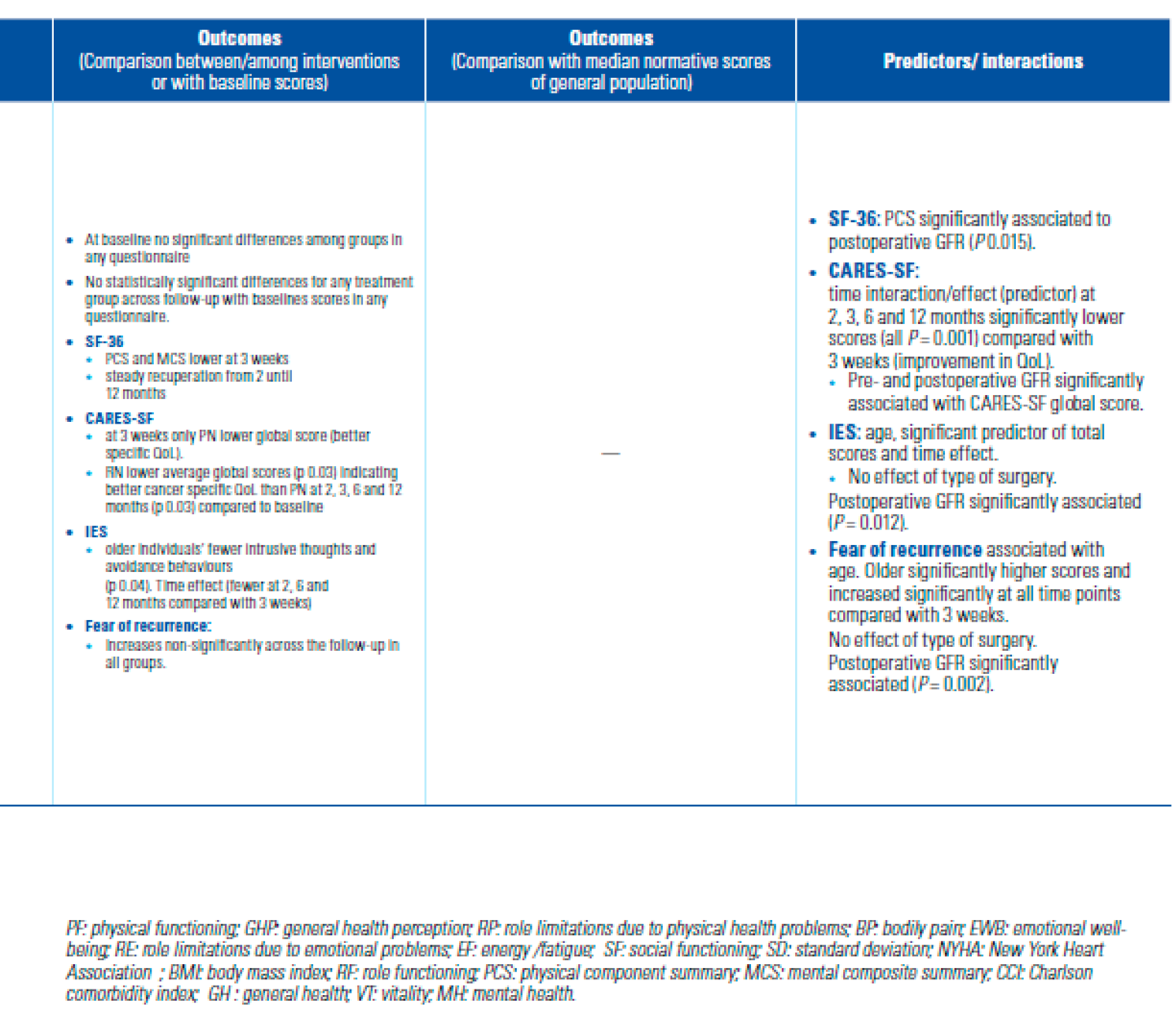
- Parker, P.A.; Swartz, R.; Fellman, B.; Urbauer, D.; Li, Y.; Pisters, L.L.; et al. Comprehensive assessment of quality of life and psychosocial adjustment in patients with renal tumors undergoing open, laparoscopic and nephron sparing surgery. J Urol. 2012, 187, 822–826. [Google Scholar] [CrossRef]
- Novara, G.; Secco, S.; Botteri, M.; De Marco, V.; Artibani, W.; Ficarra, V. Factors predicting health-related quality of life recovery in patients undergoing surgical treatment for renal tumors: prospective evaluation using the RAND SF-36 Health Survey. Eur Urol. 2010, 57, 112–120. [Google Scholar] [CrossRef] [PubMed]
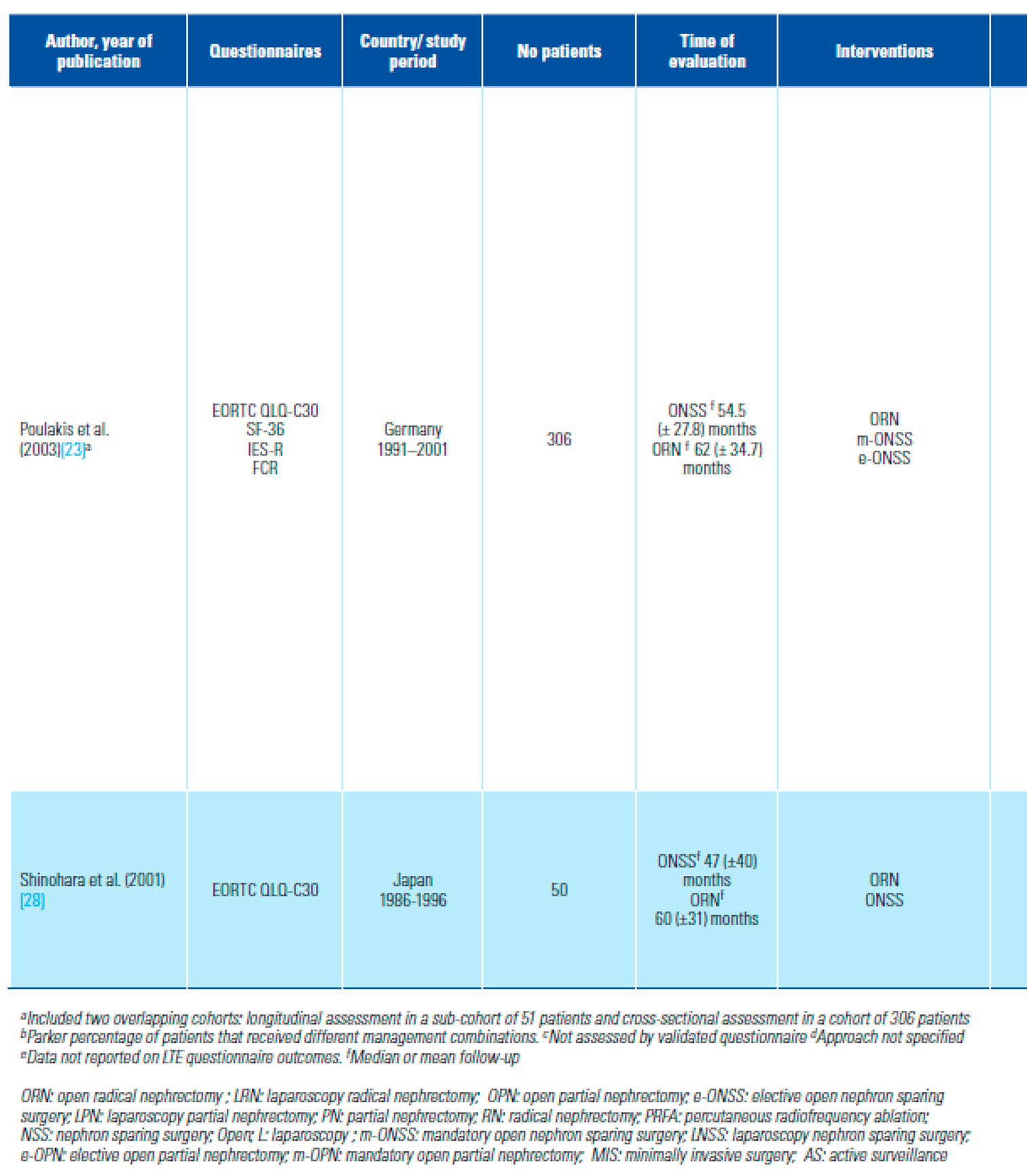
- Poulakis, V.; Witzsch, U.; de Vries, R.; Moeckel, M.; Becht, E. Quality of life after surgery for localized renal cell carcinoma: comparison between radical nephrectomy and nephron-sparing surgery. Urology 2003, 62, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Shin, T.Y.; Lim, S.K.; Komninos, C.; Kim, D.W.; Han, W.K.; Hong, S.J.; et al. Laparoendoscopic single-site (LESS) robot-assisted partial nephrectomy (RAPN) reduces postoperative wound pain without a rise in complication rates. BJU Int. 2014, 114, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zheng, X.; Qin, J.; Zheng, M.; Mao, Q.; Zhang, Z.; et al. Health-related quality of life after hand-assisted laparoscopic and open radical nephrectomies of renal cell carcinoma. Int Urol Nephrol. 2009, 41, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Onishi, T.; Nishikawa, K.; Hasegawa, Y.; Yamada y Soga, N.; Arima, K.; et al. Assessment of health-related quality of life after radiofrequency ablation or laparoscopic surgery for small renal cell carcinoma: a prospective study with medical outcomes Study 36-Item Health Survey (SF-36). Jpn J Clin Oncol. 2007, 37, 750–754. [Google Scholar] [CrossRef] [PubMed]
- Ficarra, V.; Novella, G.; Sarti, A.; Novara, G.; Galfano, A.; Cavalleri, S.; et al. Psycho-social well-being and general health status after surgical treatment for localized renal cell carcinoma. Int Urol Nephrol. 2002, 34, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, N.; Harabayashi, T.; Sato, S.; Hioka, T.; Tsuchiya, K.; Koyanagi, T. Impact of nephron-sparing surgery on quality of life in patients with localized renal cell carcinoma. Eur Urol. 2001, 39, 114–119. [Google Scholar] [CrossRef]
- Gratzke, C.; Seitz, M.; Bayrle, F.; Schlenker, B.; Bastian, P.J.; Haseke, N.; et al. Quality of life and perioperative outcomes after retroperitoneoscopic radical nephrectomy (RN), open RN and nephron-sparing surgery in patients with renal cell carcinoma. BJU Int. 2009, 104, 470–475. [Google Scholar] [CrossRef]
- Sandbergen, L.; Spriensma, A.S.; de la Rosette, J.J.; Laguna, M.P. Health- related quality of life in localized renal masses: a matter of sparing nephrons or minimizing the incision? Urol Oncol. 2020, 38, 43e1–43e11. [Google Scholar] [CrossRef]
- Park, Y.H.; Kim, K.T.; Ko, K.; Kim, H.H. Prospective randomized controlled trial of conventional laparoscopic versus laparoendoscopic single-site radical nephrectomy for localized renal cell carcinoma: a preliminary report regarding quality of life. World J Urol. 2015, 33, 367–72. [Google Scholar] [CrossRef]
- Chandrasekar, T.; Boorjian, S.A.; Capitanio, U.; Gershman, B.; Mir, M.C.; Kutikov, A. Eur Urol. Collaborative review: factors influencing treatment decisions for patients with a localized solid renal mass. Eur Urol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Blome, C.; Augustin, M. Measuring change in quality of life: bias in prospective and retrospective evaluation. Value Health 2015, 18, 110–115. [Google Scholar] [CrossRef]
- Bakas T, McLennon SM, Carpenter JS, Buelow JM, Otte JL, Hanna KM, et al. Systematic review of health-related quality of life models. Health Qual Life Outcomes. 2012, 10, 134. [Google Scholar] [CrossRef]
- Samsa, G.; Edelman, D.; Rothman, M.L.; Williams, J.R.; Lipscomb, J.; Matchar, D. Determining clinically important differences in health status measures. A general approach with illustration to the Health Utilities Index Mark II. Pharmacoeconomics 1999, 15, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; et al. The European Organization for research and Treatment of Cancer QLQ-C30, a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Cella, D.; Yount, S.; Du, H.; Dhanda, R.; Gondek, K.; Langefeld, K.; et al. Development and validation of the functional Assessment of Cancer Therapy-Kidney Symptoms Index (FKSI). J Support Oncol. 2006, 4, 191–199. [Google Scholar] [PubMed]
- Coscarelli Schag, C.A.; Ganz, P.A.; Heinrich, R.L. Cancer Rehabilitation Evaluation System-Short Form (CARES-SF). Cancer 1991, 68, 1406–1413. [Google Scholar] [CrossRef]
- Hyland, M.E. A brief guide to the selection of quality of life instruments. Health Qual Life Outcomes 2003, 1, 24. [Google Scholar] [CrossRef]
- Briancon, S.; Gergonne, B.; Gullemin, F.; Emepereur, F.; Klein, S. Disease- specific versus generic measurements of health related quality of life in cross-sectional and longitudinal studies: an inpatient investigation of the SF-36 and four disease-specific instruments. In Statistical Methods for Quality of Life Studies; Mesbah, M., Cole, B.F., Lee, L.T., Eds.; Springer: Boston, MA, 2002; pp. 87–99. [Google Scholar] [CrossRef]
- Efficace, F.; Bottomley, A.; Osoba, D.; Gotay, C.; Fletchner, H.; D’haese, S.; et al. Beyond the development of health-related quality-of-life (HRQOL) measure: a checklist for evaluating HRQOL outcomes in cancer clinical trials-does HRQOL evaluation in prostate cancer research inform clinical decision making? J Clin Oncol. 2003, 21, 3502–3511. [Google Scholar] [CrossRef]
- Calvert, M.; Kyte, D.; Mercieca-Bebber, R.; Slade, A.; Chan, A.W.; King, M.T.; et al. Guidelines for inclusion of patient- reported outcomes in clinical trial protocols: the SPIRIT-PRO extension. JAMA 2018, 319, 483–494. [Google Scholar] [CrossRef]
- Prinsen, C.A.C.; Mokkink, L.B.; Bouter, L.M.; Alonso, J.; Patrick, D.L.; de Vet, H.C.W.; et al. COSMIN guidelines for systematic reviews of patient -reported outcome measures. Qual Life Res. 2018, 27, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Lapin, B.R. Considerations for reporting and reviewing studies including health-related quality of life. Chest 2020, 158, S49–S56. [Google Scholar] [CrossRef] [PubMed]
- Kutikov, A.; Egleston, B.L.; Wong, Y.N.; Uzzo, R.G. Evaluating overall survival and competing risk of death in patients with localized renal cell carcinoma using a comprehensive nomogram. J Clin Oncol. 2010, 28, 311–317. [Google Scholar] [CrossRef]
- Ciani, O.; Baldassarre Federici, C. Value lies in the eye of the patients: the why, what, and how of patient-reported outcomes measures. Clin Ther. 2020, 42, 25–33. [Google Scholar] [CrossRef]
- Unger, J.M.; Vaidya, R.; Gore, J.L. Key design and analysis principles for quality of life and patient-reported outcomes in clinical trials. Urol Oncol. 2019, 37, 324–330. [Google Scholar] [CrossRef]
- Reeve, B.B.; Wyrwich, K.W.; Wu, A.W.; Velikova, G.; Terwee, C.B.; Snyder, C.F.; et al. ISOQOL recommends minimum standards for patient-reported outcome measures used in patient centered outcomes. Qual Life Res. 2013, 22, 1889–1905. [Google Scholar] [CrossRef] [PubMed]
 |
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
 |
This is an open access article under the terms of a license that permits non-commercial use, provided the original work is properly cited. © 2022 The Authors. Société Internationale d'Urologie Journal, published by the Société Internationale d'Urologie, Canada.
Share and Cite
Sandbergen, L.; Omar, M.I.; Othman, L.; Etten-Jamaludin, F.v.; Soytas, M.; Rosette, J.J.d.l.; Laguna, M.P. Systematic Review of Comparative Patient Reported Outcomes and Health-Related Quality of Life after Management of Localized Renal Masses or Renal Cell Carcinomas. Soc. Int. Urol. J. 2022, 3, 209-239. https://doi.org/10.48083/QODE9040
Sandbergen L, Omar MI, Othman L, Etten-Jamaludin Fv, Soytas M, Rosette JJdl, Laguna MP. Systematic Review of Comparative Patient Reported Outcomes and Health-Related Quality of Life after Management of Localized Renal Masses or Renal Cell Carcinomas. Société Internationale d’Urologie Journal. 2022; 3(4):209-239. https://doi.org/10.48083/QODE9040
Chicago/Turabian StyleSandbergen, Laura, Muhammad Imran Omar, Lavin Othman, Faridi van Etten-Jamaludin, Mustafa Soytas, Jean J. de la Rosette, and M. Pilar Laguna. 2022. "Systematic Review of Comparative Patient Reported Outcomes and Health-Related Quality of Life after Management of Localized Renal Masses or Renal Cell Carcinomas" Société Internationale d’Urologie Journal 3, no. 4: 209-239. https://doi.org/10.48083/QODE9040
APA StyleSandbergen, L., Omar, M. I., Othman, L., Etten-Jamaludin, F. v., Soytas, M., Rosette, J. J. d. l., & Laguna, M. P. (2022). Systematic Review of Comparative Patient Reported Outcomes and Health-Related Quality of Life after Management of Localized Renal Masses or Renal Cell Carcinomas. Société Internationale d’Urologie Journal, 3(4), 209-239. https://doi.org/10.48083/QODE9040





