Abstract
Objectives To test the hypothesis that a randomised trial of extended pelvic lymph node dissection (ePLND) can recruit at a rate acceptable for a larger scale trial. To compare the following secondary endpoints between the 2 arms: the rate of protocol violations, the intraoperative and postoperative morbidity of ePLND, and complications, and to evaluate short-term oncological outcomes comparing biochemical recurrence, clinical recurrence, and survival between arms. Patients and Methods A pilot study will be undertaken at Chris O’Brien Lifehouse and Royal Prince Alfred Hospitals for the NODE trial. Twenty patients will be randomised 1:1 to radical prostatectomy with or without ePLND. Eligible participants will have high-risk prostate cancer and will be scheduled for robotic radical prostatectomy. High-risk disease will be defined as in the 2019 NCCN guidelines (stage ≥ T3a, ISUP Grade Group ≥ 4 or PSA ≥ 20ng/mL). PSMA PET/CT staging not showing any extraprostatic disease will be required. Quality control measures to ensure consistent delivery of high-quality extended lymph node dissections are in place, and surgeons have been selected for their consistent ability to perform such procedures. Results The trial is currently underway. Conclusion On current available evidence, it is unclear if ePLND provides additional benefit over radical prostatectomy.
Introduction
Prostate cancer is one of the most commonly diagnosed cancers in men; however, the basic question of whether to perform a lymph node dissection at the time of radical prostatectomy remains unanswered. Pelvic lymph node dissection (PLND) is the most accurate test for staging and prognosis [1,2] and has substantial theoretical plausibility for survival benefit by removing micro-metastases in lymph node confined disease. There is, however, no robust evidence to support improved survival or improvement in other oncological outcomes [3].
Because of the lack of a sentinel node and the wide bilateral primary drainage area, an adequate PLND is a significant undertaking [4], and current guideline recommendations to perform the procedure are based on limited evidence [5].
The current literature is affected by multiple issues including a lack of standardisation of definitions and a preponderance of retrospective data. The extent of PLND (limited, standard, and extended) and anatomical boundaries vary between surgeons and centres, and evidence regarding both harms and benefits of PLND is conflicting [3]. Extended lymph node dissection consistently yields more lymph nodes, which would theoretically offer a larger therapeutic benefit, although there is little high-quality evidence for this either.
Risks and Benefits
PLND is associated with a higher risk of lymphocele and longer operative time [6,7]. Limited evidence exists for other purported risks including increased risk of lymphoedema, mortality, and worse functional outcomes (urinary incontinence and erectile dysfunction) [3]. A recent systematic review by Fossati et al. 2016, evaluated the benefits and harms of PLND, in which the primary outcome measures were biochemical recurrence, clinical recurrence, and survival. Overall, the quality of the evidence was low, and the risk of bias high. Three randomised control trials were available, of which one was reported as conference abstracts only [8,9], and only 4 of the remaining studies were performed prospectively. Evidence is very mixed regarding risks and benefits. Twenty-one retrospective studies compared oncological outcomes in no PLND with those in any form of PLND. None of the studies showed statistically significant differences in support of PLND for biochemical recurrence, distant metastases, or survival. Eight studies compared limited/standard PLND with extended PLND (ePLND) with conflicting results. There is some evidence of therapeutic benefit however, patients undergoing PLND showing lower biochemical recurrence rates and an additional retrospective study showing the removal of higher number of lymph nodes being associated with better cancer specific survival.
Two RCTs have been published in this area. The first [10] recruited 291 patients with intermediate- or high-risk prostate cancer, who were randomly assigned to undergo ePLND on one side of the pelvis and limited PLND on the other. After a median follow-up of 35.2 months, ePLND was associated with better tumour staging and increased morbidity. The second, more recent study [11] found no difference between the groups, though a post hoc subgroup analysis found improvement in outcomes in patients with higher Gleason grade cancers.
Currently, the European Association of Urology (EAU) guidelines recommend performing an ePLND in high-risk patients and not performing limited or standard dissection at all [5]. Limited dissection exposes the patient to additional risk of harm, but it will miss many nodal metastases and is associated with a significant risk of understaging. All patients recruited into this study, by virtue of meeting the NCCN guidelines definition of high-risk prostate cancer, also meet criteria in the EAU guidelines to recommend ePLND [12], thus the experimental arm in this trial is actually the no ePLND arm, with ePLND forming standard of care.
Extent of Dissection
Lymph node metastases from the prostate are highly variable, and the impact of the extent of dissection on outcomes reported by previous studies is unclear.
Although definitions vary, limited PLND is most commonly defined as the removal of nodal tissue from the area along the external iliac vein and above the obturator nerve; standard PLND is area covered by limited PLND plus the area below the obturator nerve and onto the internal iliac vessels; ePLND as removal of all nodes overlying the external iliac artery and vein, within the obturator fossa, and medial to the internal iliac artery.
The current definitions are somewhat arbitrary in their extent, and novel mapping study methods of lymph node drainage have shown that nodal drainage patterns are wider than previously described and extend well beyond the obturator region [4]. On the basis of these studies, a standard dissection is likely to remove only 38% of primary draining lymph nodes, but concerningly even the recommended extended lymph node dissection will remove only 63%, thus a new nodal dissection template is required.
The lack of level 1 evidence to support the therapeutic role of PLND highlights the need for a robust, adequately powered RCT. The aim of this pilot study is to determine the feasibility of the randomised trial design before the development of a full-scale RCT.
Patients and Methods
The NODE trial is a planned multicentre randomised control trial of ePLND. This protocol is for a pilot study assessing the ability of such a trial to recruit patients in a timely fashion and to determine plausibility given previous difficulties with other similar trials.
Three surgeons at 2 institutions will recruit patients over a 12-month period. The recruitment target is 20 patients, chosen without reference to any previous trial recruitment data, as none are available. Numbers of eligible patients and reasons for non-participation will be recorded.
Surgeons in this study have a documented history of performing high-quality ePLND, 2 are fellowship trained robotic surgeons and the third has > 20 years’ experience performing open and robotic radical prostatectomy.
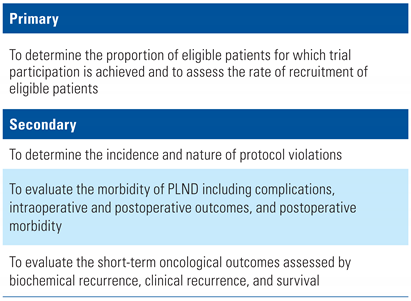
The primary and secondary objectives are listed in Table 1.

Table 1.
Primary and secondary objectives.
The study is approved (2019/ETH09765) by the Sydney Local Health District Human Research Ethics Committee (EC00113) and is registered on the Australian New Zealand Clinical Trials Registry (ACTRN12620000881932). This pilot study will require no funding. It will be conducted according to local laws and regulations, the Declaration of Helsinki, and the principles of good clinical practice. The trial schema is outlined in Figure 1.
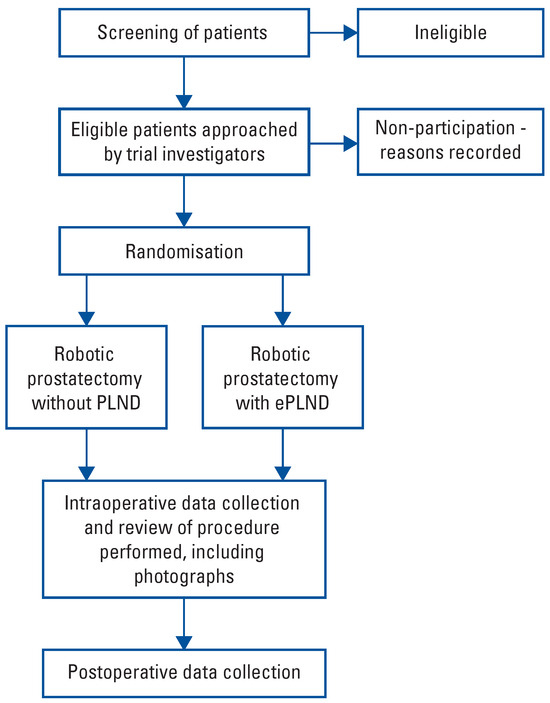
Figure 1.
Flow diagram.
Recruitment and Randomisation
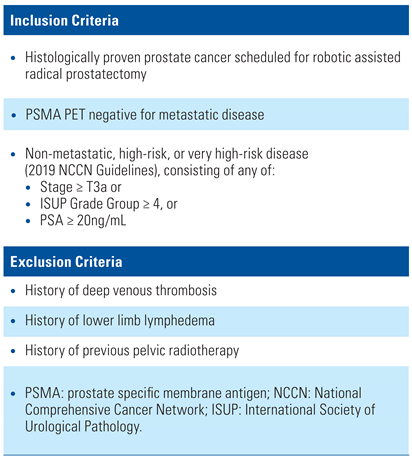
Patients will be screened for recruitment after histological diagnosis of prostate cancer has occurred. Patients will be assessed for eligibility using the inclusion and exclusion criteria listed in Table 2.

Table 2.
Inclusion and exclusion criteria [12].
The standard of care in Australia for staging high-risk prostate cancer is to perform a PSMA PET/CT. Patients with non-metastatic disease on PSMA PET will form the population to be evaluated in this study.
Patients will be randomised 1:1 to robotic radical prostatectomy without PLND (experimental arm) or to robotic radical prostatectomy with bilateral ePLND (standard of care). The participating surgeons in this trial share the opinion that the current evidence has not shown superiority of either approach and thus the 2 arms of the trial are at equipoise. The trial will be of open label design. Data will be analysed on an intention to treat basis.
Intraoperative Protocols
Robotic radical prostatectomy will occur at Royal Prince Alfred Hospital or Chris O’Brien Lifehouse.
The ePLND template is represented diagrammatically in Figure 2 and consists of removal of lymph nodes located along the external iliac vein, those in the obturator fossa and along the internal iliac artery up to the midcommon iliac region where the ureter crosses the iliac vessels, those that are dorsolateral to the external iliac vessels and at the bifurcation of the common iliac vessels, in the fossa of Marcille, and on the medial aspect of the internal iliac vessels.
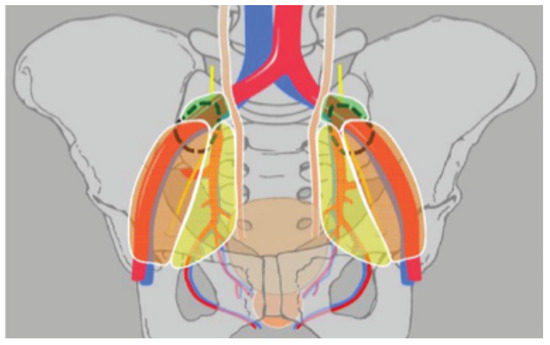
Figure 2.
Extended PLND template. The orange, yellow and green regions represent the external, internal, and common iliac regions, respectively. Dashed lines indicate the fossae of Marcille. Reprinted with permission from Maderthaner et al. “More extended lymph node dissection template at radical prostatectomy detects metastases in the common iliac region and in the fossa of Marcille.” BJU Int. 2018 May;121(5):725-731.
To ensure quality of surgical resection, 3 still photographic images will be taken for each side:
- Photo 1: medial view showing internal, external, and common iliac vessels
- Photo 2: space between external iliac vein and artery
- Photo 3: lateral view (by medial retraction of the external iliac vessel) to show the deep obturator space and fossa of Marcille.
These photographs will be reviewed by the researchers at the conclusion of the study. Failure to adequately dissect the structures or document their dissection will be considered a protocol violation.
All patients will have a pelvic drain inserted at the time of surgery.
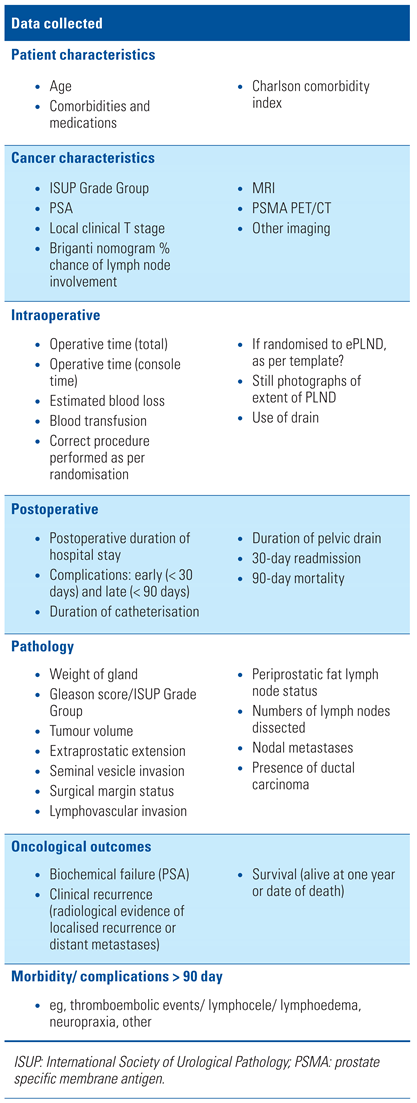
Perioperative, operative, and postoperative data will be recorded, as per fields outlined in Table 3.

Table 3.
Histopathology
Lymph node specimens will be submitted separately by drainage region for right and left side as external iliac, internal iliac, common iliac, and fossa of Marcille. The histopathology will be reviewed at the Department of Tissue Pathology and Diagnostic Oncology at Royal Prince Alfred Hospital by specialist uropathologists. All the submitted nodal tissue will be embedded for microscopic analysis.
Follow-Up
Following discharge, patients will be followed up by their urologist. They will have planned follow-ups at 4 weeks, 3 months, 6 months, and 12 months postoperatively, with a PSA test before each appointment.
Sample Size and Power Calculation
No sample size or power calculation was performed for this study given its nature as a pilot study. Twenty patients will be recruited, or if <20 patients are recruited after 12 months, recruitment will cease, and patient numbers will be capped at that level.
Results
Recruitment
The ability of this pilot study to recruit patients for a larger trial forms the primary outcome. This will be reported as a proportion of eligible patients who are successfully enrolled and randomised. Reasons for nonenrolment of eligible patients will be recorded. Failure to recruit the target number of patients will not preclude progression to a larger trial, but may influence its design.
Protocol Violations
Performing the large lymph node dissection required in this study consistently while maintaining a high standard will add significant time and complexity to the procedures. Monitoring both the intraoperative and postoperative rate of study protocol violations will therefore ensure that this approach can be successfully deployed in a larger trial. Protocol violations will be reported by procedure stage and nature.
Adverse Events
Intraoperative, immediate postoperative and post discharge adverse events will be reported in both arms using the Clavien-Dindo classification.
Oncological Outcomes
The occurrence of biochemical recurrence (serum PSA ≥ 0.2ng/mL [13,14]), clinical (radiological) recurrence, and survival time will be recorded between the 2 arms.
Discussion
There are a number of causes of the current lack of clarity regarding the therapeutic benefit of pelvic lymph node dissection.
As discussed above, no trials comparing ePLND with no PLND have been reported. Of the 2 published RCTs, one randomised patients to one side extended and one side limited dissection, limiting its ability to provide prognostic data [10]. A second RCT [11] has reported no significant difference between limited and extended PLND, but on post hoc subgroup analysis, found it to be beneficial in patients with higher Gleason scores. Multiple factors make randomised trials in this area difficult, and clear level 1 evidence is lacking as a result.
Difficulties inherent in surgical randomised trials are exacerbated for trials of PLND in prostate cancer. Despite a lack of proven therapeutic benefit, pelvic lymph node dissection has substantial biological plausibility and forms a widespread component of surgical practice. Reluctance to have patients randomised to one arm of a trial is a common impediment to surgical trials, and many surgeons would feel uncomfortable not performing a lymph node dissection on high-risk patients.
Performing large pelvic lymph node dissections is also time-consuming and difficult, and participating surgeons need to be motivated to perform an adequate dissection. A large, bilateral lymph node dissection is likely to add significantly to the length of a case, even in the hands of a surgeon experienced at performing the procedure. It requires dissection around the large blood vessels of the pelvis, and this may be outside skill set of many surgeons, especially if performed robotically. For this reason, the intraoperative photographs have been included, in an attempt to monitor and maintain dissection quality.
Trials in this area have previously had difficulty recruiting [15]; given this and the above reasons, it is prudent to perform a pilot study.
Previous trials comparing limited with ePLND may have been easier to perform, and they sidestepped the potential ethical issue of not performing a PLND in high-risk patients, but their trial design is not the right one for answering this question. The current state of the literature is that there is no level 1 evidence for the performance of lymph node dissection, and the inclusion of a limited PLND is likely only to act as a confounder. There is good evidence that ePLND provides superior staging, and the EAU guidelines thus recommend performing an ePLND if any lymph node dissection is going to be performed. Regarding the ethical issue of not performing a PLND at all compared with a limited dissection, limited PLND is a procedure with the risks but not the benefits of a larger dissection, and should thus be regarded as inferior to both ePLND and no dissection [16].
Patient selection needs to be judicious to ensure the highest likelihood of showing, or definitively disproving, a benefit of PLND. Patients with no lymph node metastasis will derive no benefit from PLND, nor are those with established distant metastatic disease likely to benefit, as their prognosis will be substantially determined by their distant disease. Patients with high risk of lymph node metastases but the lowest chance of distant metastatic disease are thus the ideal target population.
With reference to targeting this population, it is likely that multiple factors have overlapped to make previous trials inadequately powered to detect differences in outcomes. New technology in the form of PSMA scans and new research in the form of the above-mentioned technetium node mapping studies may allow this to be addressed in this trial.
Importantly, previous trials in this area have not used PSMA staging. Data from the recent proPSMA study has shown that traditional staging (CT and bone scan) fails to detect distant metastatic disease in two-thirds of cases [17]. Previous studies would have included these patients as they would have been staged as having localised disease. These patients will not benefit from local treatment, and although randomisation would ensure they were evenly distributed, their inclusion would lead to significant dilution of the effect of any lymph node dissection.
PSMA accurately predicts the presence of lymph node metastases [18]. However, in patients with lymph node metastases identified on PSMA, biochemical recurrence rates approach 50%, even in patients treated with ePLND [19], pointing to the presence of micrometastatic disease outside the pelvis. The selection of patients with no extra prostatic disease on PSMA aims to select patients who have only early nodal disease and thus use the greater accuracy from this new modality to target patients with the best likelihood of surgical cure.
The extent of dissection chosen in previous retrospective and prospective studies is also likely to have diluted previous evidence of effect. The publication of data indicating that for clearance of 75% of draining lymph nodes [4] a bilateral ePLND must be performed indicates that previous trials would have diminished effect size by missing as much as half of lymph node metastases. A trial assessing dissection matching this new mapping pattern is required.
As a pilot study, this study has many limitations. To test recruitment and the suitability of the protocol in a reasonable amount of time, this study has been kept intentionally small, with few patients, surgeons, and centres. If the study is converted to a full randomised control trial, these limitations will be addressed.
The pilot study of the NODE trial has commenced recruitment and aims to complete recruitment within 12 months. The protocol may be altered before the commencement of the NODE trial proper.
Competing Interests
None declared.
Abbreviations
| ePLND | extended PLND |
| ISUP | International Society of Urological Pathology |
| PLND | pelvic lymph node dissection |
| PSMA | prostate specific membrane antigen |
References
- Perry-Keene, J.; Ferguson, P.; Samaratunga, H.; Nacey, J.N.; Delahunt, B. total submission of pelvic lymphadenectomy tissues removed during radical prostatectomy for prostate cancer increases lymph node yield and detection of micrometastases. Histopathology 2014, 64, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Yaxley, J.W.; Raveenthiran, S.; Nouhaud, F.-X.; Samartunga, H.; Yaxley, A.J.; Coughlin, G.; Delahunt, B.; Egevad, L.; McEwan, L.; Wong, D. Outcomes of primary lymph node staging of intermediate and high risk prostate cancer with 68 Ga-PSMA positron emission tomography/computerized tomography compared to histological correlation of pelvic lymph node pathology. J. Urol. 2019, 201, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Fossati, N.; Willemse, P.-P.M.; Van den Broeck, T.; van den Bergh, R.C.N.; Yuan, C.Y.; Briers, E. The benefits and harms of different extents of lymph node dissection during radical prostatectomy for prostate cancer: A systematic review. Eur. Urol. 2017, 72, 84–109. [Google Scholar] [CrossRef] [PubMed]
- Mattei, A.; Fuechsel, F.G.; Dhar, N.B.; Warncke, S.H.; Thalmann, G.N.; Krause, T. The template of the primary lymphatic landing sites of the prostate should be revisited: Results of a multimodality mapping study. Eur. Urol. 2008, 53, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Mottet, N.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; Santis, M.D. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer—2020 Update. Part 1: Screening, diagnosis, and local treatment with curative intent. Eur. Urol. 2020, 79, 243–262. [Google Scholar] [CrossRef] [PubMed]
- Briganti, A.; Blute, M.L.; Eastham, J.H.; Graefen, M.; Heidenreich, A.; Karnes, J.R. Pelvic lymph node dissection in prostate cancer. Eur. Urol. 2009, 55, 1251–1265. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Gao, Y.; Cheng, Y.; Qi, F.; Zou, Q. Whether extended pelvic lymph node dissection should be performed in prostate cancer: The present evidence from a systematic review and meta-analysis. Precis. Med. Sci. 2020, 9, 23–30. [Google Scholar] [CrossRef]
- Lestingi, J.F.P.; Guglielmetti, G.; Jr, J.P.; Mitre, A.I.; Sarkis, A.; Bastos, D.A.; Riechelmann, R.; Mattedi, R.L.; Cordeiro, M.; Coelho, R.; et al. Extended versus limited pelvic lymphadenectomy during radical prostatectomy for intermediate- and high-risk prostate cancer: Early outcomes from a randomized controlled phase III study. J. Clin. Oncol. 2017, 35, 5018–5018. [Google Scholar] [CrossRef]
- Schwerfeld-Bohr, J.; Kaemper, M.; Krege, S.; Heidenreich, A. 270 Prospective randomized multicenter study comparing limited vs extended pelvic lymphadenectomy in intermediate and high risk prostate cancer—Comparison of complications (SEAL, AUO AP 55/09). Eur Urol Suppl. 2014, 13, e270. [Google Scholar] [CrossRef]
- Clark, T.; Parekh, D.J.; Cookson, M.S.; Chang, S.S.; Smith, E.R., Jr.; Wells, N.; et al. Randomized prospective evaluation of extended versus limited lymph node dissection in patients with clinically localized prostate cancer. J. Urol. 2003, 169, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Lestingi, J.F.P.; Guglielmetti, G.B.; Trinh, Q.-D.; Coelho, R.F.; Pontes, J.; Bastos, D.A. Extended versus limited pelvic lymph node dissection during radical prostatectomy for intermediate- and high-risk prostate cancer: Early oncological outcomes from a randomized phase 3 trial. Eur. Urol. 2021, 79, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Mohler, J.L.; Antonarakis, E.S.; Armstrong, A.J.; D’Amico, A.V.; Davis, B.J.; Dorff, T. Prostate Cancer, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2019, 17, 479–505. [Google Scholar] [CrossRef] [PubMed]
- Cookson, M.S.; Aus, G.; Burnett, A.L.; Canby-Hagino, E.D.; D’amico, A.V.; Dmochowski, R.R.; Eton, D.T.; Forman, J.D.; Goldenberg, S.L.; Hernandez, J.; et al. Variation in the Definition of Biochemical Recurrence in Patients Treated for Localized Prostate Cancer: The American Urological Association Prostate Guidelines for Localized Prostate Cancer Update Panel Report and Recommendations for a Standard in the Reporting of Surgical Outcomes. J. Urol. 2007, 177, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Cornford, P.; van den Bergh, R.C.N.; Briers, E.; van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. Part II—2020 Update: Treatment of Relapsing and Metastatic Prostate Cancer. Eur. Urol. 2021, 79, 263–282. [Google Scholar] [CrossRef] [PubMed]
- Prospective Study to Compare a Limited Versus Extended Pelvic Lymphadenectomy During Prostatectomy (SEAL) Clinicaltrials, g.o.v.-B. 2 December. Available online: https://clinicaltrials.gov/ct2/show/NCT01555086 (accessed on 2 December 2020).
- Briganti, A.; Giannarini, G.; Karnes, R.J.; Gandaglia, G.; Ficarra, V.; Montorsi, F. What Evidence Do We Need to Support the Use of Extended Pelvic Lymph Node Dissection in Prostate Cancer? Eur. Urol. 2015, 67, 597–598. [Google Scholar] [CrossRef] [PubMed]
- Hofman, M.S.; Lawrentschuk, N.; Francis, R.J.; Tang, C.; Vela, I.; Thomas, P.; et al.; for the proPSMA Study Group Collaborators Prostate- specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (ProPSMA): A prospective, randomised, multi-centre study. Lancet 2020, 395, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Petersen, L.J.; Zacho, H.D. PSMA PET for primary lymph node staging of intermediate and high-risk prostate cancer: An expedited systematic review. Cancer Imaging 2020, 20, 10. [Google Scholar] [CrossRef] [PubMed]
- Leeuwen, P.J.; Donswijk, M.; Nandurkar, R.; Stricker, P.; Ho, B.; Heijmink, S.; et al. Gallium-68-prostate-specific membrane antigen (68Ga-PSMA) positron emission tomography (PET)/computed tomography (CT) predicts complete biochemical response from radical prostatectomy and lymph node dissection in intermediate-and high-risk prostate cancer. BJU Int. 2019, 124, 62–68. [Google Scholar] [CrossRef] [PubMed]
This is an open access article under the terms of a license that permits non-commercial use, provided the original work is properly cited. © 2021 The Authors. Société Internationale d'Urologie Journal, published by the Société Internationale d'Urologie, Canada.