The Bench-to-Bedside Uro-Oncology GU Cancer Triad Meeting was organized by the Société Internationale d’Urologie and was held online on May 21st and 22nd, 2021. The session on prostate cancer (PCa) took place on the morning of Saturday, May 22nd, and was chaired by Dr. Stacy Loeb (United States) and Dr. Peter C. Black (Canada). This session covered advances in the diagnosis and management of localized, locally advanced, and metastatic PCa, as well as three case-based panel discussions on biomarkers, focal therapy, and systemic therapy in PCa. Additionally, the programme included presentations on the state of the art of liquid biopsy, COVID-19 impact, and digital health in urologic oncology.
The first talk was led by Dr. Alexander Wyatt, who presented the state of the art of liquid biopsy in uro- logic oncology. In particular, he discussed the use of plasma circulating tumour DNA (ctDNA) as a biomarker in PCa. Plasma ctDNA comprises short fragments of post-apoptotic tumour DNA in the blood that are mixed with cell-free DNA from noncancer cells, such as leukocytes. Generally, ctDNA abundance is associated with tumour burden and more advanced disease[1]. While several non-genomic biomarkers, such as serum prostate-specific antigen (PSA), are already well-estab- lished in PCa management, ctDNA-based biomarkers may be particularly useful for predicting treatment efficacy, especially in the metastatic setting.
The fraction of ctDNA (as a proportion of total cell- free DNA in plasma) is a strong prognostic factor for disease outcomes. In two randomized phase 2 trials, ctDNA fraction correlated with overall survival (OS), with patients exhibiting poorer survival outcomes with increasing ctDNA fraction, independent of clin- ical prognostic factors[2,3]. In addition, changes in ctDNA fraction during treatment may indicate not only response but also pending relapse in the met- astatic castration-sensitive PCa (mCSPC) and meta- static castration-resistant PCa (mCRPC) settings[3,4]. Understanding the prognostic value of ctDNA frac- tion may also help to guide treatment decisions. The ongoing open-label phase 2 PROTRACT trial aims to provide insights on whether ctDNA fraction can assist treatment selection for second-line androgen receptor pathway inhibitor (ARPI) compared to taxane-based chemotherapy[5].
Dr. Wyatt emphasized the key differences between tumour tissue and plasma ctDNA sequencing. Sequencing of tumour tissue is only performed in samples with high tumour content (>20%) to lower the chance of false negatives. By contrast, the tumour content (ctDNA fraction) of plasma cell-free DNA sam- ples cannot be discerned prior to sequencing and must instead be determined via bioinformatics after sequencing. Importantly, the precise ctDNA fraction dictates which tumour alterations can be identified. For example, practical limits of detection are around 1% ctDNA for most somatic mutations, but can be as high as 20% for certain changes in copy number[4,6]. In a phase 2 clinical trial of the poly(ADP-ribose) pol- ymerase (PARP) inhibitor rucaparib in mCRPC, BRCA alterations were identified in patient-matched tissue and plasma, with a relatively high concordance of 75%[7]. Several homozygous BRCA2 copy number losses were not identified by plasma testing, likely due to insufficient ctDNA fraction. Dr. Wyatt highlighted that around 25% of patients with mCRPC can have very low ctDNA fraction, which means that testing results must be carefully interpreted to understand if low ctDNA may prevent the detection of certain somatic alterations.
Just as tissue samples with low tumour content are triaged prior to sequencing, when using ctDNA to detect the mutational status of genes of interest, it is important to triage samples that have low tumour content in the plasma. In a recently published study of 879 patients with metastatic PCa, samples of only 635 patients had sufficient ctDNA fraction to allow iden- tification of both somatic and germline mutations[8]. Importantly, however, this approach allowed the mini- mally invasive detection of homologous recombination repair (HRR) gene mutations, such as BRCA2, ATM, and CDK12, in over 15% of patients. These mutations were identified across 94% of serial ctDNA samples as well as in all available archival primary tissues, suggesting that DNA repair gene status does not change over time in patients with metastatic PCa.
Studies of ctDNA in mCRPC are also revealing emerging biomarkers, such as the independently prognostic impact of TP53 mutations[2]. Androgen receptor (AR) copy number amplification as a contin- uous variable shows promising results as a biomarker to influence treatment selection between chemother- apy and ARPI[3,9]. The next steps for development of ctDNA-based predictive biomarkers is to prove clinical utility. A prospective biomarker-driven phase 2 trial led by the Canadian Cancer Trials Group is aiming to address this need[10]. Currently, over 425 patients with mCRPC have been screened in this trial, using ctDNA to stratify treatment arms.
During the Q&A, Dr. Wyatt discussed why some cancers shed more ctDNA than others. Differences in tumour shedding are seen not only across different cancer types, but also within the same form of cancer. In PCa, higher proliferative tumour burden is associ- ated with increasing ctDNA shedding, although there are clearly some underlying biological mechanisms that are not yet understood. Next, Dr. Wyatt discussed future applications of ctDNA biomarkers in earlier dis- ease stages, which he believes is a logical approach, although challenging. As Dr. Wyatt pointed out, there are good biomarkers already available in earlier PCa settings to determine minimal residual disease or even early diagnosis. He advised caution for rushing to implement any new ctDNA-based biomarkers in this space to avoid the risk of overdiagnosing or over- treating. Dr. Wyatt then commented on differences in allelic percentages reported by commercially available tests. According to him, these differences are likely due to the sampling probability for rare variants in small volumes of blood. In general, the field is moving away from reporting very rare variants (allelic frequencies much lower than 1%) that may not be reflective of the cancer and instead represent other somatic clones in circulation. Dr. Wyatt also provided his insights on advances for ctDNA on the horizon. He highlighted the potential for analysis of serial ctDNA samples col- lected over time to understand mechanisms of disease evolution and progression[11].
The next presentation was by Dr. Yaw Nyame (United States), who discussed racial disparities in PCa and a patient-centred research framework for addressing inequities in PCa care and outcomes. A health disparity is defined as a higher burden of illness, injury, disability, or mortality experienced by one group relative to another. PCa is a profound example of a cancer health disparity, particularly in the United States. Disparities in PCa reflect interactions between social, health, and biologic factors. For instance, the worst outcomes in PCa in the United States are concen- trated in counties in the southeastern United States[12] that form the historic cotton belt—a region where the highest production of cotton occurred in the 19th century. Cotton production during this time period was a major economic trade in the United States and was associated with the slave trade. The cotton belt is also home to the highest proportion of men of African American ancestry in the country[13]. This example demonstrates the complexity of socio-geographic and economic factors.
Despite a 50% reduction in PCa mortality in the United States since the introduction of PSA screen- ing in the late 1980s, Black men have consistently demonstrated a two-fold greater risk of dying from the disease compared to men of all other races (gen- erated with data from[14]). This disparity appears to be even higher among younger males, in which 40- and 50-year-old Black men are at an up to four-fold higher risk of dying from PCa compared to white men[15]. Similar trends appear to occur worldwide in regions with not only large populations of African American ancestry but also in areas of the developing world[16]. Regarding PCa natural history, Black men are likely to develop PCa at younger ages and are at higher risk of progression to metastatic disease by the time of diagnosis compared to the general population[17].
What are some of the drivers of disparity in PCa outcomes? In the early 2000s, the World Health Organization (WHO) popularized the concept of social determinants of health, which include economic stability, neighbourhood and physical environment, education, access to food, the community and social context, and the healthcare system[18]. These deter- minants dictate healthcare utilization and patient outcomes. Social determinants of equity (i.e., eco- nomic and structural barriers) inform how each social determinant of health impacts PCa risk[19]. PCa dis- parities, particularly in the United States, form what Dr. Nyame calls a “perfect storm”: it is a disease with higher incidence, in which a variety of social factors, structural and societal barriers, mistrust, and health system barriers further impact outcomes.
How can racial disparities in PCa be reduced? To answer this question, Dr. Nyame and colleagues have developed a conceptual model to help identify criti- cal checkpoints in the PCa journey that may improve patient outcomes. For example, the model demon- strates that PCa screening can be a powerful tool that may lead to early detection and mortality reduction. In this setting, access to PSA testing and biopsy may lead to distinct improvements in health inequities. As a patient progresses to treatment, there are mul- tiple timepoints where differential utilization of care influences outcomes, including: access and quality of care, modifiable risks, and treatment preferences in the localized setting; quality of surveillance following definitive treatment; and access to salvage therapy and clinical trials if PCa progresses.
PCa screening has an important benefit for mor- tality rate reduction. In a recent study, the benefit of PSA screening was evaluated over a period of more than 24 years since patients had been randomized in clinical trials[20]. Results demonstrated a positive impact of PSA screening on PCa mortality beyond 16 years, highlighting a particular benefit in young and healthier men. Given that Black men present with PCa at a younger age and with more disparate out- comes, earlier screening may be particularly valuable in this patient population[21,22]. By contrast, the 2012 U.S. Preventive Services Task Force recommenda- tion against routine PSA testing led to a decrease in PCa screening that was markedly seen among Black men[23].
Regarding definitive treatment, a recent multi- ple-cohort study analyzed data from the Surveillance, Epidemiology, and End Results (SEER), the Veterans Affairs health system, and the National Cancer Institute-sponsored Radiation Therapy Oncology Group[24]. Results demonstrated that access to care and standardized treatment, as seen in clinical trials, are associated with decreased disparities in PCa outcomes among Black men. In a cohort of men with high-risk PCa, another study evaluating data from the Cleveland Clinic, Johns Hopkins Institute, and MD Anderson Cancer Center showed no differences in outcomes with standardized treatment, regardless of ethnicity[25]. Nevertheless, the rate of definitive treatment for PCa is lower in Black men compared to other ethnicities[26]. As highlighted by Dr. Nyame, even more troubling is the fact the Black men are underrepresented in clinical trials, at roughly 3% of current practice-informing studies[27].
How can barriers to research participation and clini- cal care among Black men be overcome? In the United States, people of African American ancestry have been the subject of medical experimentation without their consent throughout history[28]. The trauma in this population is transgenerational and shapes medical mistrust and cultural health beliefs. In addition, poor and minority populations were long considered “med- ical material” for training in the clinical practice, which poses a barrier to the participation of these individuals in contemporary clinical trials.
In such a challenging context, what can be done to close the gap in PCa racial disparities? Dr. Nyame believes the focus must be in building communities and empowering patients. A patient-centred research approach is paramount and should be founded on the principles of reciprocal relationships, coopera- tive learning, partnership, transparency, honesty, and trust[29]. This novel approach is crucial to reduce racial disparities in PCa and other diseases.
In the Q&A session, Dr. Nyame expressed the need for appropriate representation to mediate effective communication between the medical community and patients. This is deeply affected by the under- representation of several populations in practice-in- forming clinical trials. Dr. Nyame discussed the role of the WHO and artificial intelligence (AI) in correcting health disparities. According to Dr. Nyame, the WHO has mobilized funds to help flight health disparities, particularly in developing countries where even access to PSA testing may be scarce. With regards to AI, he advised caution, as many platforms use real-world data that may lead to biased results and increased disparity. Next, Dr. Nyame explained how to assess the effec- tiveness of community-based research. In this case, Dr. Nyame relies on SEER data and emphasized that the ultimate goal is to produce significant improvements in the mortality curves. Lastly, Dr. Nyame discussed whether single-nucleotide polymorphism (SNP) approaches are a legitimate tool for inferring race from genetic data. He believes that these approaches are beneficial and may ultimately help to demystify the association of PCa risk and ethnicity.
Next, Dr. Neal Shore (United States) presented the five PCa practice-changing advances on the horizon, As emphasized by Dr. Shore, these advances require an understanding of genetic profiling not only to guide treatment decision-making, but also to better counsel patients regarding their own and their family’s risk of developing cancers. First, Dr. Shore discussed advances in overcoming AR resistance in PCa. Despite rapid and dramatic responses to androgen depriva- tion therapy (ADT) as monotherapy or in combination with an ARPI, all patients with PCa eventually develop castration resistance[30]. Some of the mechanisms underlying this process include AR alterations, such as AR gene or gene enhancer amplification, AR point mutations, and autocrine tumour androgen produc- tion[30]. Currently, there are several strategies under investigation to overcome AR resistance and improve treatment outcomes. One of these strategies involves the use of proteolysis targeting chimera (PROTAC) protein degraders to target ARs by engaging the ubiquitin proteasome system (UPS), which is used by cells to degrade proteins and maintain homeostasis. This approach was recently investigated in a phase 1 clinical trial and demonstrated promising results in patients with mCRPC for treatment with ARV-110, a PROTAC with high AR-degradation activity in preclin- ical models[31]. Another strategy under investigation is targeting of the DNA damage response (DDR) system with ATR inhibitors to prevent replication of defective cells with tumourigenic potential[32]. This approach may prove particularly useful in patients with mCRPC who harbour ATM mutations and are likely more susceptible to ATR inhibition. The EZH2 pathway may also offer a novel therapeutic target in mCRPC, given its involvement in important aspects of cancer genetics, acquired drug resistance, and regulation of anti-tumour immune response through gene silenc- ing[33]. Inhibition of EZH2 leads to the re-expression of silenced genes and shows potential for combination with ARPIs to overcome AR resistance. Finally, another strategy under investigation is targeting the PI3K/AKT pathway, which shows crosstalk with AR pathways. Targeting both pathways with an AKT inhibitor and ARPI shows potential to increase antitumour activity, particularly in PCa tumours with PTEN loss[34].
Then, Dr. Shore detailed the potential of bispe- cific T-cell engager (BiTE®) immunotherapy in PCa. BiTE is a novel class of therapeutic molecules that are able to activate an anti-tumour immune response by engaging the patient’s own T cells[35]. AMG 160 is a BiTE molecule that selectively targets prostate-specific membrane antigen (PSMA), which is highly expressed in PCa cells. In an ongoing phase 1 clinical trial, AMG 160 showed tolerable efficacy in patients with mCRPC[36]. Cytokine release syndrome (CRS) was the most common adverse event (AE) observed, which is commonly associated with the mechanism of action of BiTE immunotherapy.
The next practice-changing advance in PCa relates to the use of PSMA positron emission tomography (PET) imaging, which has profound diagnostic and therapeutic implications due to its high expression in PCa tumours and metastases. In the end of 2020, the U.S. Food and Drug Administration (FDA) approved 68Ga PSMA-11 for PET imaging[37]. More recently, the positive results of the phase 3 CONDOR trial[38] and the phase 2/3 OSPREY trial[39] have led that to the approval of a second PSMA PET agent, 18F-DCFPyL, in the United States. As these new imaging approaches become standard of care in the United States and other countries, it is critical that urologists understand how this additional information may influence treatment decision-making.
As highlighted by Dr. Shore, one of practice-chang- ing potentials of next-generation PSMA PET imaging is visualization of disease sites that can then be targeted with radiopharmaceutical agents. To date, 223Ra is the only radiopharmaceutical approved in the mCRPC space[40], although it remains underutilized. A new generation of radiopharmaceuticals that couple the radioactive β-emitter 177Lu to a PSMA-targeted mol- ecule is underway and has showed promising results in the phase 3 VISION trial[41,42]. Important consider- ations that emerge with new theranostic approaches include not only understanding how these will fit in the current systemic therapy landscape but also how to optimally select patients for these new treatments.
Lastly, Dr. Shore discussed the potential of com- bining PARP inhibitors with other agent classes, such as ADT, to target PCa cells. Because the AR and the HRR pathways are interconnected, combining ADT and PARP inhibition may result in synthetic lethality, as suggested by preclinical studies[43]. A similar rationale may be applied not only to PARP inhibitor combination with ADT, but also with other therapies in mCRPC, such as chemotherapy, radionuclide therapies, and immune checkpoint inhibitors (ICIs), as well as AKT and ATR inhibitors (reviewed in [44]).
During the Q&A, Dr. Shore provided his insights on practice-changing advances coming to the localized PCa setting. Several patients with high-risk localized PCa who present with grade 3 to 5 disease may relapse after radical prostatectomy or radiation therapy. In this setting, Dr. Shore emphasized the upcoming results of the phase 3 ATLAS trial, which has evaluated the role of radiation therapy in combination with ADT or apalutamide in this patient population[45]. Similar strategies in neoadjuvant and adjuvant strategies are also under investigation in patients who undergo rad- ical prostatectomy.
The PCa session continued with a case-based panel on the use of germline vs. genomic biomarkers in PCa management. The discussion was moderated by Dr. Derya Tilki (Germany), with input from Dr. Elena Castro (Spain), Dr. Matthew Cooperberg (United States), and Dr. Veda Giri (United States). The first case was a 74-year-old male who underwent prostatectomy. The final pathology revealed tumour with Gleason score (GS) 3+4, pT3a stage, no lymph node involvement, and focal positive margin. At his 2-month follow-up, the patient was using less than one pad per day and had undetectable PSA levels (<0.015 ng/mL). Since the patient presented with adverse pathology, should any additional biomarkers be assessed? In the post- operative setting, genetic-based tests may provide additional insight to guide follow-up and management decisions. The Decipher® Prostate Cancer Test is an option in this space and uses the expression of 22 selected RNA markers to predict the risk of metastasis and cancer-specific mortality[46]. For the patient in this case, the Decipher score was 0.67, just above the high-risk threshold.
What is the role of genetic biomarkers in treatment decision-making in the adjuvant setting post-rad- ical prostatectomy? In 2020, results of the phase 3 RADICALS-RT, GETUG-AFU 17, and RAVES trials demonstrated no benefit between adjuvant radiother- apy and salvage radiotherapy for patients with local- ized PCa following prostatectomy[47-49]. However, Decipher score may guide management decisions in the presence of rising PSA levels. In a retrospec- tive analysis, patients with high Decipher scores and differing PSA levels had improved outcomes when treated with adjuvant radiotherapy. By contrast, no benefit was seen in patients with low postoperative Decipher scores[50]. In addition, Decipher presents a GRID report, which provides predictive responses to different treatment approaches in presence of biochemical recurrence. A novel genomic signature approach based on 24 genes called PORTOS is under investigation to also predict response to postopera- tive radiotherapy. In a matched retrospective study, men with high PORTOS had better outcomes after receiving radiotherapy compared to those who did not. Interestingly, the opposite was seen in patients with low PORTOS[51].
At 6 months following radical prostatectomy, the patient presented with rising levels of PSA at 0.13 ng/mL. In this scenario, it may be beneficial to wait until the PSA level rises to 0.2 ng/mL to proceed to any additional interventions, according to the ARTISTIC trials[52]. In addition, this patient may benefit from germline genetic testing, given the high-risk PCa features. This decision could also be influence by the patient’s family history of cancer, which could lead to investigations for hereditary cancer syndrome.
The second case was a 75-year-old male who pro- gressed to metastatic PCa. The patient was initially treated with robotic-assisted prostatectomy. Rising PSA levels were observed postoperatively, upon which a PET/computed tomography (CT) scan revealed lymph node metastasis. At this point, the patient started on ADT, which led to PSA decline. This patient meets the criteria for germline testing in the metastatic setting, according to several guidelines worldwide (summa- rized in [53]). Another important factor highlighted in the guidelines is the family history of cancer. In the case, a pedigree revealed that the patient’s family had a history of breast and colon cancers, and also uncovered Ashkenazi Jewish ancestry on both sides of the family, which is an additional criterion for germline testing.
What genes should be analyzed for germline mutations? According to the Philadelphia Consensus, a broad panel that includes genes associated with cancer predisposition syndrome is recommended for patients with advanced PCa[54]. If this is not feasible, at least the presence of mutations in BRCA1, BRCA2, and mismatch repair genes should be investigated. Alterations in these genes have implications for therapy selection, such as PARP inhibitors and immunotherapy. Additional genes may also be included, according to the patient’s personal or family history of cancers.
The patient underwent germline testing with a mul- ti-gene panel that revealed a pathogenic mutation in BRCA2. While such patients may respond to ADT, they are at increased risk of progression and should be monitored closely. Upon progression to mCRPC, patients with germline BRCA2 may experience signif- icantly improved outcomes with PARP inhibitor ther- apy, as seen in clinical trials with rucaparib[55] and olaparib[56]. In addition, the use of PARP inhibitors is under evaluation as monotherapy or in combination with other therapies, such as ADT, in earlier disease settings (reviewed in [57]). BRCA2 mutation status may also have implications for patients with early-stage PCa, given the increased molecular risk of aggressive disease[58]. However, it is still unknown how BRCA2 and other germline mutation status may influence management during active surveillance.
Germline testing in patients with PCa also pro- vides insights on hereditary cancer syndromes. In the case, the BRCA2 mutation identified in the patient is also associated with an increased risk for hereditary breast and ovarian cancer (HBOC) syndrome, as well as additional cancer risks[54]. This is an important consideration not only for PCa survivors but also their families. The patient in the case qualifies for BRCA2 cascade testing, which involves germline testing for a particular mutation in all direct relatives, such as children and siblings. Given the patient’s Ashkenazi Jewish ancestry, cascade testing could also be offered to other family members. In addition, the pathogenic BRCA2 mutation uncovered through germline testing would lead to specific screening recommendations for PCa, melanoma, breast, ovarian, and pancreatic can- cers for the patient and his relatives. It is important to highlight that genetic counselling is critical both prior to and post germline testing to inform patients about the purpose of the test, the potential for uncovering hereditary cancer syndromes or additional cancer risks, as well as how test results may influence cancer screen- ing and management for patients and their families[54].
The next presentation was by Dr. Maria J. Ribal (Spain), who discussed the impact of COVID-19 in urologic oncology. With nearly 153 million peo- ple affected by the pandemic worldwide, this is an unprecedented situation in modern times that has had profound effects on different aspects of uro-on- cological care. Dr. Ribal first focused on the impact on optimization of resources. At the beginning of the pandemic, all hospital resources were shifted to care for patients infected with SARS-CoV-2. This situ- ation pushed physicians to best prioritize healthcare resources, which led to the publication of guidelines specific for rapid response in the COVID-19 era. With that in mind, the European Association of Urology (EAU) published guideline recommendations in April 2020 in an effort to guide management decisions and patient prioritization during the pandemic[59]. While the clinical evidence to build these guidelines was not high level, the analysis of data collected during the past 1.5 years will provide important insights on the real impact of not only delayed therapy, but, more importantly, delayed diagnosis.
Recently, the impact of delayed treatment was systematically reviewed in a series of retrospective studies[60]. The results demonstrated an association between cancer treatment delays and increased mor- tality that may have important implications following the COVID-19 pandemic. To this end, an attractive strategy may be the establishment of COVID-19–free surgical pathways, in which major perioperative facil- ities are not shared with patients with COVID-19[61]. Another challenge is determining the appropriate timing of surgery following SARS-CoV-2 infection. In a recent prospective study, reduced mortality risk was found in patients who had a least a 7-week delay for surgery following the infection[62]. Further delays may be beneficial if COVID-19 symptoms persist for over 7 weeks.
In addition to the utilization of resources, another important aspect is the impact of COVID-19 on patients with cancer who were undergoing active treatment. This is a controversial topic, as Dr. Ribal highlighted. While early reports suggested that cancer patients with COVID-19 undergoing treatment were at an increased risk of mortality, another prospective study demonstrated that the risk was not greater compared to non-infected patients[63]. Dr. Ribal emphasized that best way to address this question is by acquiring data from clinical registries. Some exam- ples include the COVID-19 and Cancer Consortium Registry (CCC19)[64], the ASCO Survey on COVID-19 in Oncology (ASCO) Registry[65], and the ESMO-CoCare Registry[66].
The current challenge is evaluating the impact of COVID-19 vaccination on cancer. Several societies and organizations, such as the European Society of Medical Oncology (ESMO)[67], the American Society of Clinical Oncology (ASCO)[68], and the National Comprehensive Cancer Network (NCCN)[69], have provided statements encouraging the vaccination of patients with cancer, as long as components of the vaccine do not interfere with active treatments. The benefit of COVID-19 vaccination may be even greater for patients who undergo surgery, as suggested by a modelling study. Since vaccine numbers are limited, prioritizing patients undergoing surgery may not only support safe re-initiation of elective surgical services, but also reduce the incidence of postoperative pul- monary complications and the associated healthcare costs[70].
The social-distancing restrictions imposed by COVID-19 had important repercussions for the development of telemedicine. This novel modality of health care will likely remain a mainstay of health sys- tems and will require adequate training for physicians and patients alike in order to fully explore the benefits of this tool[71]. However, each patient with cancer is different and many may still require in person interac- tions with their healthcare providers[72]. Going for- ward, the benefits of in-person interactions should be balanced with the continuing need for telemedicine.
COVID-19 also imposed barriers to medical research, including patient recruitment for clinical trials and access to hospital facilities to conduct investi- gations. For several ongoing trials in genitourinary cancers, the need for repeated in-person visits has precluded accrual. Research-related personnel and resources have also been relocated to COVID-related care and research[73]. The pandemic has also posed a burden on the mental and emotional well-being of physicians[74]. Medical departments should be aware of this challenge and develop initiatives to prevent burnout among their healthcare teams.
The pandemic will have a long-lasting impact on healthcare systems, particularly because of delayed diagnoses. In a recent population-based modelling study in the United Kingdom, delayed diagnosis due to COVID-19 was shown to result in increased 5-year mortality rates for breast, colorectal, and esophageal cancers[75]. In uro-oncology, the reduction in surgeries seen since the beginning of the pandemic will also put medical units under continuous stress to be able to absorb the growing demand[76]. Dr. Ribal encourages the medical community to collaborate during these challenging times and to learn from this experience to improve patient outcomes in years to come.
In the Q&A period, Dr. Ribal provided her perspec- tive on how clinicians and researchers may overcome the obstacles in PCa research and care experienced during the pandemic. While these have been chal- lenging times, Dr. Ribal highlighted all the achieve- ments of the past year, including the development of several vaccines, and the importance of worldwide collaboration.
Next, Dr. Giovanni Lughezzani (Italy) discussed recent developments in high-resolution ultrasound (US), focusing on an update on the 29MHz micro-US. Dr. Lughezzani started by providing an overview of the PCa diagnostic pathway evolution. Conventional US-based imaging is a cheap, largely available, and easy approach that is only used for systematic biop- sies and rarely provides any information regarding the presence of PCa. Multiparametric magnetic res- onance imaging (mpMRI) represents an important improvement from conventional US for PCa diagnosis. However, the use of mpMRI-based imaging may be limited by its cost and availability, as well as the need for an expert radiologist to obtain the images and assist in targeted biopsies. High-resolution micro-US has the potential to bridge the gap between conven- tional US and mpMRI. Micro-US allows immediate vis- ualization and real-time targeting of prostatic lesions without radiologic assistance, in addition to limiting the use of mpMRI to essential cases only.
Novel micro-US operates at 29 MHz, a much higher frequency compared to conventional systems. This leads to a substantial improvement in resolution to visualize both the axial and lateral axes of the pros- tate. The micro-US technology allows the adoption of transperineal or transrectal biopsy strategies, and an MRI fusion approach can also be incorporated to allow better targeting of lesions[77]. Lesions identified by micro-US are characterized using the Prostate Risk Identification Protocol using Micro-US (PRI-MUS). This protocol classifies lesions in real time, using a scale from 1 to 5, ranging from benign to features typically associated with higher risk of PCa[78]. PRI-MUS has been validated by several studies, which have also shown that the protocol can be easily learned by urologists with previous experience with conventional US.
What are the potential applications of micro-US in the clinical practice? In addition to faster diagno- sis and no need for contrast injection, micro-US may potentially help to avoid biopsies in the presence of PRI-MUS 1 to 2 lesions. Compared to conventional US, real-time micro-US resulted in an increased detection rate of approximately 12% in an early experience at the Cleveland Clinic[79]. In a prospective study of 320 patients, similar detection rates for clinically significant PCa were found between micro-US and mpMRI[80]. More recently, in a multicentre registry of 1,040 patients, the diagnostic performance of micro-US demonstrated comparable or higher sensitivity for clinically significant PCa compared to mpMRI[81].
Micro-US may offer good visualization of target lesions. In a study of 144 prostatic lesions, 9% of lesions identified by MRI but not by micro-US were negative for clinically significant PCa at biopsy. By contrast, 11.8% of lesions identified only by micro-US but not by MRI also had a positive PCa diagnosis at biopsy[82]. In addition, in a single-institution study of 222 patients comparing different diagnostic strategies, micro-US–guided biopsy of MRI targets was superior to a robotic MRI fusion approach[83]. This suggests that a substantial proportion of MRI targets may be visible and amenable for biopsy with micro-US.
Micro-US may also complement MRI to improve the biopsy of target lesions. In a study of 194 patients who underwent transperineal biopsy with mpMRI- and micro-US–guided biopsy, micro-US identified 11% of additional lesions that were not detected by mpMRI. This suggests that micro-US may add significant information to current MRI approaches[84]. Similarly, in another study, the combination of micro-US and mpMRI was revealed as the best approach for the detection of clinically significant PCa[85].
Finally, micro-US also shows potential to provide local staging of PCa. In a feasibility study of 54 patients scheduled for robotic-assisted radical prostatectomy, five risk factors were evaluated to predict the presence of extraprostatic extension (EPE)[86]. This preliminary experience revealed a statistically significant associ- ation between the presence and the number of risk factors and PCa EPE.
Based on the evidence currently available, Dr. Lughezzani proposed an alternative micro-US–based pathway for the initial work-up of patients with sus- pected PCa. The micro-US findings can be used to guide subsequent decisions, which span from targeted biopsy in patients with positive findings to a tailored clinical follow-up in those with a negative micro-US result. In patients with an inconclusive micro-US image, a complementary mpMRI may be considered.
During the Q&A, Dr. Lughezzani discussed whether in the future some multiparametric features of mpMRI could be incorporated to improve the current micro-US technology. Micro-US is based on B mode images, which could potentially be improved with features such as Doppler and elastography. Dr. Lughezzani also detailed the learning curve with the micro-US technology. He explained that a urologist experienced with conventional US may need between 40 to 50 cases to become comfortable with the technology. Dr. Lughezzani also highlighted that, in his personal experience, the learning curve is continuous and there is always opportunity for growth.
Next, Dr. Rafael Sánchez-Salas (France) led a case- based panel on focal therapy advances and appli- cations in 2021. The discussion had input from Dr. Caroline Moore (United Kingdom), Dr. Art Rastinehad (United States), Dr. Linda Kerkmeijer (the Netherlands), and Dr. Hashim Ahmed (United Kingdom). Focal ther- apy offers the opportunity for achieving cancer control while preserving function in PCa patients. Some bene- fits associated with focal therapy include lower rates of incontinence requiring pads and frequent maintenance of natural erections without medication, following a day surgery procedure with short catheterization time. By contrast, patients undergoing focal therapy may need a second focal procedure (1 in 5 men) or radical treatment (1 in 15 men) after 5 years and require more stringent monitoring.
The first case was a 52-year-old male with hyper- tensive cardiac myopathy who presented with a PSA level of 6.9 ng/mL and a GS 3+4 upon biopsy. The patient underwent three treatment blocks of left focal high-intensity focused US (HIFU) treatment, with excellent Uchida changes. In his 12-month follow-up, the patient showed no signs of disease by imaging, a PSA decrease to 1.9 ng/mL, no urinary symptoms, and natural erections. In a retrospective analysis of 1,032 patients treated with HIFU, 63.4% had GS 3+4, 20% required a second focal treatment in 5 years, and 3.7% required radical treatment after 5 years[87]. When looking at predictors of need for retreatment, higher GS was associated with greater risk. In the study, a trend for reduced retreatment rates was observed over time in response to improved patient selection, imaging, and operator experience.
Patient selection is essential for treatment deci- sion-making with focal therapy. This approach is cur- rently recommended for patients with PSA levels <20 ng/mL, GS up to 7, life expectancy greater than 10 years, and any prostate volume[88]. A biopsy after 1 year is critical for evaluating treatment outcomes. In the second case, a 70-year-old male presented with GS 3+4 on transperineal MRI-US fusion biopsy 3 years after initial investigations for PCa. The patient was treated with gold nanoshell-localized photothermal ablation as part of a clinical pilot device study[89]. After infusion, the nanoshells penetrate the tumour, allowing tumour-specific ablation through heat following stim- ulation with infrared light at a specific wavelength. An MRI performed 48 hours post-ablation revealed no signs of disease in the targeted area. In a single-arm, multicentre, phase 1/2 trial of 44 patients, treatment with gold nanoshells resulted in 48% PSA decrease and 71.1% negative lesions at 12 months[90]. With improved patient selection, imaging, and treatment technologies, focal therapy may become the preferred management approach for intermediate-to-high-risk PCa in the future.
While focal therapies offer an attractive option to PCa management, it is important to also consider the limitations. PCa is a multifocal disease with several lesions that are not all detected, even with high-resolu- tion mpMRI[91]. Focal therapies are also heterogenous, both in target volume definition as well as technique, which limits comparisons[92]. In addition, the lack of prospective clinical trials comparing focal therapies to radical treatment limits inferences with respect to differences in toxicity and risk of recurrence. Currently, the EAU-EANM-ESTRO-ESUR-SIOG guidelines for local PCa treatment recommend that focal therapies are only offered in the context of a clinical trial[93].
Similar to other focal therapies, there have been promising results using focal radiotherapy approaches, such as focal brachytherapy and focal external-beam radiation therapy (EBRT)[94,95], although compara- tive trials against radical treatments are still missing. Nevertheless, radical radiotherapy has seen much improvement in the past years, with advances in MRI- guided radiotherapy and ultra-hypofractionation that lead to better targeting of the tumour[96,97]. An important consideration is whether there may be an alternative approach to whole-gland or focal radio- therapy. The focal boost strategy was investigated in the randomized phase 3 FLAME trial, which com- pared standard whole-gland EBRT to an additional focal boost to the prostatic lesion seen on mpMRI. Patients in the focal boost arm experienced signifi- cantly greater biochemical disease-free survival (DFS) at 5 years (92% vs. 85%; HR=0.45 [95% CI 0.28–0.71; P<0.001), along with similar toxicity and health-related quality of life compared to standard EBRT[98]. In the phase 2 hypo-FLAME trial, a similar approach (but with a lower whole-gland dose) was taken to investigate the role of focal boost to hypofractionated stereotactic body radiotherapy (SBRT), which resulted in no severe acute toxicities 6 months after treatment[99]. Longer follow-up is needed to evaluate the long-term effects of this novel approach.
The last case was a male in his 70s with PSA level of 6.4 ng/mL, Gleason 4+4, and metastatic lesion in the right superior public ramus as seen by MRI and subse- quent bone scan and CT. The patient started treatment with ADT and enzalutamide, which resulted in minor tumour reduction. He was then enrolled in the phase 2 ATLANTA trial, which is evaluating local treatments in men with newly diagnosed metastatic PCa[100]. In the trial, the patient was randomized to receive HIFU to the primary tumour and SBRT to treat the metastatic lesion. This ongoing trial will have a follow-up of at least 2 years and progression-free survival (PFS) as the primary endpoint.
During the Q&A, Dr. Moore discussed how tumour size may guide the decision between focal ablation and hemiablation. Based on clinical data of over 1,000 patients, no differences were seen in cancer outcomes between the two approaches. She explained that her current practice is to treat focally, with a 5- to 9-mm margin depending on tumour characteristics. Then, Dr. Rastinehad discussed the use of novel US thermometry in focal therapy. This as a new imaging technique that improves real-time assessment of tumour ablation, although it is still in early stages of development. Next, Dr. Kerkmeijer provided her insights on patient selection for focal treatment of oligometastases. She believes that there is variation in imaging and disease staging across countries that influence the classification of oligometastatic disease. This ultimately influences patient selection for focal treatment. As she pointed out, an international trial evaluating patient selection would be beneficial. Lastly, Dr. Ahmed summarized the key considerations for focal therapy. According to him, focal therapy is most advantageous for men with localized disease who require treatment. He cautioned that this approach is not an alternative to active surveil- lance and that urologists should always be cognizant of the impact of overtreatment.
The PCa session continued with a case-based panel on the adoption of systemic therapies by urologists. The discussion was led by Dr. Shusuke Akamatsu (Japan), with input from Dr. Alexandre de la Taille (France), Dr. Martin Gleave (Canada), and Dr. Neal Shore (United States). The first case was a 67-year- old male with high-risk PCa who initially underwent radical prostatectomy and salvage radiation therapy. After starting ADT, the patient recurred and pre- sented with castration resistance. Metastases were not observed by CT or bone scan and the patient had a PSA doubling time (PSADT) of 6 months. To guide next management decisions, it is important to first evaluate whether metastases are indeed not present. PSMA PET localizes cells expressing PSMA and has better specificity and sensitivity compared to con- ventional imaging to detect metastasis. Recently, a study demonstrated that PSMA PET identified metas- tasis in 55% of patients despite negative conventional imaging[101], leading to important changes in clinical management. Another important consideration is the PSADT. Patients with PSADT <10 months are at an increased risk of developing bone metastasis[102]. If PSMA PET is negative for metastases, the patient in the case is an ideal candidate for treatment with an ARPI, such as enzalutamide, apalutamide, or darolutamide. All three agents have shown benefit in prolonging metastasis-free survival in patients with nonmetastatic CRPC (nmCRPC), with tolerable toxicity[103-105]. Radiation therapy to the primary tumour is, for some authors, questionable[106], but no prospective data have yet been reported. If PSMA PET is positive, the number of metastatic lesions has important implica- tions for treatment decision-making. In the presence of multiple metastases, ARPI (abiraterone, enzalutamide, or apalutamide) or docetaxel-based chemotherapy may be considered. By contrast, there is much debate regarding the management of oligometastatic CRPC. Current guidelines are based on conventional imag- ing and recommend ARPI as standard of care[107]. Discussion of management decisions with the tumour board is advised. After the patient has started on a sys- temic therapy, PSA levels should be monitored closely, and minor elevations may warrant subsequent imaging (either conventional imaging or PSMA PET) to assess for additional lesions.
The second case was a 59-year-old male with high-volume mCSPC with multiple bone metastases. The patient had no family history of prostate, breast, ovarian, or pancreatic cancer. He was initially treated with ADT + abiraterone but progressed to mCRPC after 5 months, with additional metastasis on conven- tional imaging. At this stage, the management plan would include the participation of a medical oncologist to start the patient on docetaxel-based chemotherapy. Enrolment in a clinical trial may also be considered. However, some biomarker advances on the hori- zon, such as the use of ctDNA, may help to identify genomic alterations to improve patient stratification and guide treatment sequencing decisions[108]. For instance, patients who progress while receiving ARPI may develop therapy-specific alterations in the AR that may alter their response to subsequent hormonal treatments[109]. These alterations may be mapped out with serial ctDNA to guide management changes during treatment resistance and progression. ctDNA may also help to identify other genomic alterations that have recently become therapeutic targets under investigation in PCa. This is the case of PI3K inhibi- tion with ipatasertib, which demonstrated prolonged radiographic PFS when combined with abiraterone in patients with PTEN loss in the IPATential150 trial[34]. Other ongoing trials are aiming to refine optimal bio- marker profiles to optimize benefit from dual AR/PI3K pathway inhibition. DNA repair alterations may also help inform therapy selection. These alterations may confer resistance to initial ADT and ARPI[2,110], but may underly good response to PARP inhibitors, as seen in the PROfound trial[111].
The third case was a 74-year-old male with node-metastatic PCa who received whole-pelvic radia- tion and adjuvant ADT for 2 years until recurrence with multiple bone metastases and local tumour extension. Subsequent treatment was composed first of ADT + enzalutamide, and then docetaxel, until the patient developed resistance, along with an additional bone metastasis and bulky primary disease. In this setting, treatment with cabazitaxel may offer benefit, which is particularly supported by the prolonged median OS observed in the CARD trial, following progression on docetaxel and an ARPI[112]. Future treatment options for patients in this disease setting may include novel radiopharmaceuticals. TheraP is a randomized phase 2 trial examining the role of 177Lu PSMA-617 vs. caba- zitaxel in patients with mCRPC who progressed after docetaxel therapy[113]. Another study is the phase 3 Vision trial, which compared 177Lu PSMA-617 + stand- ard of care to standard of care alone in patients with mCRPC and PSMA-positive metastasis[42]. Recently, it was reported that the trial had met both primary endpoints, with significant improvements in OS and radiographic PFS[41]. Other emerging therapies tar- geting PSMA are currently under investigation.
Day 2 of the B2B GU Cancers Triad Virtual Meeting concluded with Dr. Stacy Loeb (United States) pre- senting the state of the art in digital health in urologic oncology. The use of social media is expanding glob- ally, with 4.2 billion users worldwide as of January 2021[114]. There has also been an expansion in the use of health apps, which is greatest in China, India, Indonesia, and United States, according to a recent report[115]. Not surprisingly, digital health may have an important role at different stages of the patient journey with urologic cancers, such as awareness and education, screening and diagnosis, treatment selec- tion, treatment, and survivorship. At each of these stages, social media may have both a positive and a negative influence.
On the positive side, social media messaging may improve awareness and education, which may lead to increased cancer screening or lifestyle changes in response to known risk factors. At the diagnosis stage, health apps may help to guide the patient’s decision to undergo biopsy. An example is the Rotterdam Prostate Cancer Risk Calculator, a validated app that provides easy information about PCa risk based on the patient’s PSA level, prior imaging, biopsy, and other clinical data[116]. Another app, currently under devel- opment in collaboration with Dr. Veda Giri, is the HELIX Webtool. This app assists with targeted family history collection and the decision-making about germline testing. It includes interactive features, as well as a series of educational modules on PCa genetics.
With regards to treatment selection, the Virtual Tumour Board sessions promoted by the National Cancer Grid in India represent an example of a practi- cal platform where centres can come together to dis- cuss oncological management and to promote stand- ardized care to patients[117]. More recently, Twitter has become an essential platform to connect and engage the urological community worldwide. First introduced in 2018, the #UroSoMe hashtag has had a positive impact in helping the identification of urological con- tent, as well as improving community engagement on the social media platform[118]. Social networks have also developed an expanded role for the dissemination of evidence-based medicine. Some examples come from the EAU, which has been using various social platforms, such as Twitter, Facebook, and LinkedIn, to disseminate clinical practice guidelines, thereby contributing to the adherence to evidence-based medicine[119,120]. With regards to delivery of care, the use of telemedicine has shown rapid growth among urologists and uro-oncologists, particularly during the COVID-19 pandemic, when telemedicine use increased from 16% to 46%[121]. Digital tools will also have an increasing importance for the future of uro-oncology. As shown in recent studies, trainees use video content on YouTube as their preferred tool to learn surgical techniques[122] and many have reported using apps to access practice guidelines[123].
Social media also has a positive role in survivorship. Patients and their families have started to make use of the GoFundMe platform to address the financial toxicity associated with cancer care. Facebook groups and online health communities may also be used by patients to obtain advice and support from others who may be going through a similar journey. Apps may be very useful during survivorship care, such as seen in patients who underwent radical prostatectomy, to help in recovery and symptom improvement[124,125]. Digital networks and apps can also be used to help identify the unmet needs of patients, such as seen through WhatsApp-based surveys[126] and social media surveillance[127] during the pandemic.
Despite the positive impact of social media and digital apps for physicians and patients, digital health may also lead to negative experiences. A particular concern is the spread of misinformative or inaccurate information through social networks. For instance, a study of the 10 most shared articles in uro-oncology on social media platforms revealed that one to seven out of those articles were misinformative or inaccu- rate[128]. Perhaps even more concerning was the fact that those articles were also 28 times more likely to be shared than factual articles. Regarding the quality of bladder cancer and PCa content on YouTube, studies have shown that many videos contain misinformative and/or poor quality content[129,130]. Moreover, poor quality content had not only more views but also generally high engagement from viewers[130]. More recently, among the limited number of TikTok posts with objective PCa information, 41% was classified as misinformative[131].
Several advances to digital health are underway. Given its increasing role in uro-oncology, it is impor- tant to consider the evolution of digital health while ensuring quality and equity. To this end, the DISCERN criteria provide a helpful guide to assist the production of consumer health information[132]. With regards to diagnosis, novel AI-based digital solutions are under development and validation. For instance, AI-based algorithms have been demonstrated to accurately assess core needle biopsies and may have important applications in pathology laboratory procedures[133]. Integration of big data may support improved risk stratification to guide treatment decision-making in PCa. An example is PIONEER, the European network of excellence for big data in PCa, which combines data from 32 partners across nine countries to improve management decisions and optimize PCa care[134]. Lastly, the role of telemedicine and remote surgery will likely expand in the future, with alternative health- care delivery models and better data transmission systems[135,136].
During the Q&A, Dr. Loeb addressed how patients may respond to engagement among physicians on social media and which best practices should be con- sidered. Dr. Loeb emphasized that physician partici- pation is key to the dissemination of evidence-based data and dilution of the misinformation spread about urologic cancers on social media. For online commu- nication, she stressed the importance of following pro- fessional guidelines, out of which she highlighted three that are specific to urology: the EAU, the American Urological Association (AUA), and the British Journal of Urology International (BJUI).
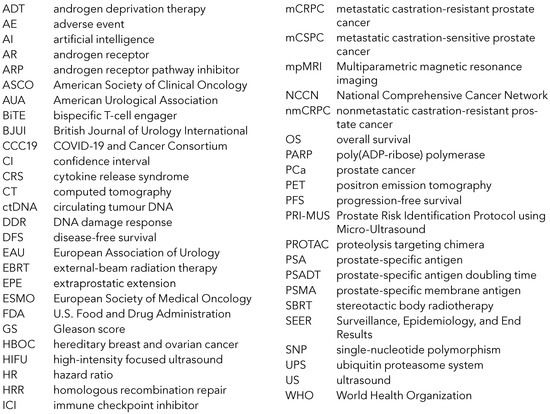
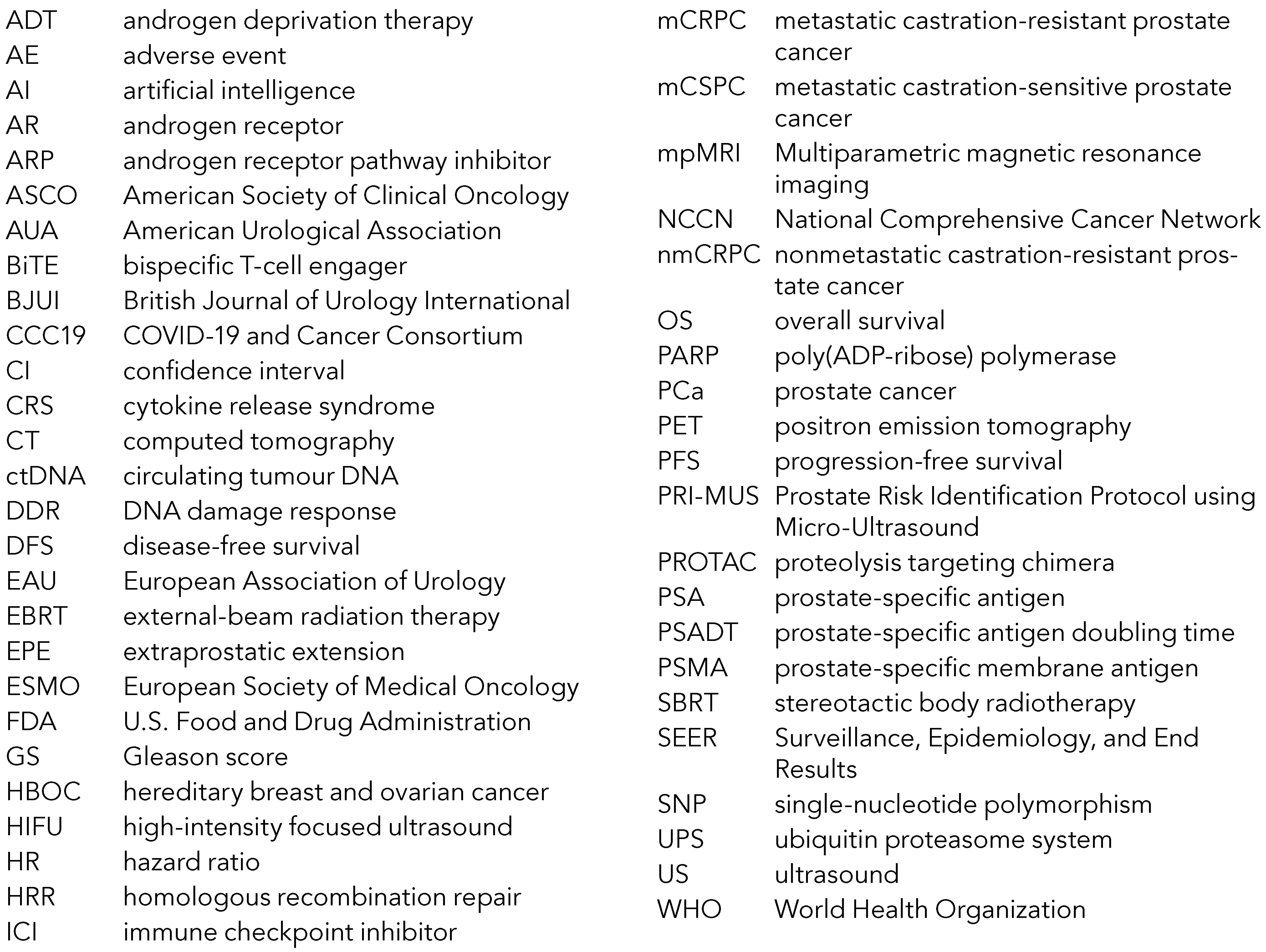
Abbreviations Used in the Text
References
- Vandekerkhove G, Wyatt A. Circulating tumour DNA as a biomarker source in metastatic prostate cancer. Société Internationale d’Urologie Journal 2020, 1, 39–48. [Google Scholar] [CrossRef]
- Annala M, Vandekerkhove G, Khalaf D, et al. Circulating tumor DNA genomics correlate with resistance to abiraterone and enzalutamide in prostate cancer. Cancer Discovery 2018, 8, 444–457. [Google Scholar] [CrossRef] [PubMed]
- Annala M, Fu S, Bacon JVW, et al. Cabazitaxel versus abiraterone or enzalutamide in poor prognosis metastatic castration-resistant prostate cancer: a multicentre, randomised, open-label, phase II trial. Annals of Oncology 2021, 32, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Vandekerkhove G, Struss WJ, Annala M, et al. Circulating tumor DNA abundance and potential utility in de novo metastatic prostate cancer. European Urology 2019, 75, 667–675. [Google Scholar] [CrossRef] [PubMed]
- PROstate Cancer TReatment optimization via analysis of circulating tumour DNA. Accessed June 6, 2021. https://clinicaltrials.gov/ct2/show/NCT04015622.
- Wyatt AW, Annala M, Aggarwal R, et al. Concordance of circulating tumor DNA and matched metastatic tissue biopsy in prostate cancer. Journal of the National Cancer Institute 2017, 109, djx118. [Google Scholar] [CrossRef]
- Abida W, Campbell D, Shapiro J, et al. Clinical activity of rucaparib in patients with metastatic castration- resistant prostate cancer (mCRPC) and BRCA1 or BRCA2 mutation identified by FoundationOne® Liquid CDx (F1L CDx). In: 27th Annual PCF Scientific Retreat; 2020.
- Warner E, Herberts C, Fu S, et al. BRCA2, ATM, and CDK12 defects differentially shape prostate tumor driver genomics and clinical aggression. Clinical Cancer Research 2021, 27, 1650–1662. [Google Scholar] [CrossRef]
- Conteduca V, Jayaram A, Romero-Laorden N, et al. Plasma androgen receptor and docetaxel for metastatic castration-resistant prostate cancer. European Urology 2019, 75, 368–373. [Google Scholar] [CrossRef]
- Prostate cancer biomarker enrichment and treatment selection. Accessed June 6, 2021. https://clinicaltrials. gov/ct2/show/NCT03385655.
- Annala M, Taavitsainen S, Khalaf DJ, et al. Evolution of castration-resistant prostate cancer in ctDNA during sequential androgen receptor pathway inhibition. Clinical Cancer Research. Published online June 3, 2021:clincanres.CCR-21-1625-E.2021. [CrossRef]
- Mokdad AH, Dwyer-Lindgren L, Fitzmaurice C, et al. Trends and patterns of disparities in cancer mortality among US counties, 1980-2014. JAMA 2017, 317, 388–406. [Google Scholar] [CrossRef]
- U.S. Census Bureau QuickFacts: United States. Accessed June 3, 2021. https://www.census.gov/quickfacts/fact/table/US/RHI225219#RHI225219.
- Surveillance, Epidemiology, and End Results (SEER). Prostate Cancer; 2021. Accessed June 3, 2021. https://seer.cancer.gov/statfacts/html/prost.html.
- Kelly SP, Rosenberg PS, Anderson WF, et al. Trends in the incidence of fatal prostate cancer in the United States by race. European Urology 2017, 71, 195–201. [Google Scholar] [CrossRef]
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer Journal for Clinicians 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Tsodikov A, Gulati R, de Carvalho TM, et al. Is prostate cancer different in black men? Answers from 3 natural history models. Cancer 2017, 123, 2312–2319. [Google Scholar] [CrossRef] [PubMed]
- Disparities in health and health care: 5 key questions and answers. Accessed June 3, 2021. https://www. kff.org/racial-equity-and-health-policy/issue-brief/disparities-in-health-and-health-care-5-key-question- and-answers/.
- Disparities in health and health care: 5 key questions and answers. Accessed June 3, 2021. https://www. kff.org/racial-equity-and-health-policy/issue-brief/disparities-in-health-and-health-care-5-key-question- and-answers/. [CrossRef]
- Shoag JE, Nyame YA, Gulati R, et al. Reconsidering the trade-offs of prostate cancer screening. New England Journal of Medicine 2020, 382, 2465–2468. [Google Scholar] [CrossRef] [PubMed]
- Nyame YA, Gulati R, Heijnsdijk EAM, et al. The impact of intensifying prostate cancer screening in black men: a model-based analysis. Journal of the National Cancer Institute. Published online May 8, 2021. [CrossRef]
- Etzioni R, Nyame YA. Prostate cancer screening guidelines for black men: spotlight on an empty stage. Journal of the National Cancer Institute 2021, 113, 650–651. [Google Scholar] [CrossRef] [PubMed]
- Kensler KH, Pernar CH, Mahal BA, et al. Racial and ethnic variation in PSA testing and prostate cancer incidence following the 2012 USPSTF recommendation. Journal of the National Cancer Institute 2020, 113, 719–726. [Google Scholar] [CrossRef]
- Dess RT, Hartman HE, Mahal BA, et al. Association of Black race with prostate cancer–specific and other- cause mortality. JAMA Oncology 2019, 5, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Wilkins LJ, Tosoian JJ, Reichard CA, et al. Oncologic outcomes among Black and White men with grade group 4 or 5 (Gleason score 8-10) prostate cancer treated primarily by radical prostatectomy. Cancer 2021, 127, 1425–1431. [Google Scholar] [CrossRef] [PubMed]
- Friedlander DF, Trinh Q-D, Krasnova A, et al. Racial disparity in delivering definitive therapy for intermediate/high-risk localized prostate cancer: the impact of facility features and socioeconomic characteristics. European Urology 2018, 73, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Rencsok EM, Bazzi LA, McKay RR, et al. Diversity of enrollment in prostate cancer clinical trials: current status and future directions. Cancer Epidemiology Biomarkers & Prevention 2020, 29, 1374–1380. [Google Scholar] [CrossRef]
- Washington H. Medical Apartheid: The Dark History of Medical Experimentation on Black Americans from Colonial Times to the Present. Penguin Random House; 2007.
- PCORI Engagement Rubric; 2014. Accessed June 3, 2021. http://www.pcori.org/sites/default/files/Engagement-Rubric.pdf.
- Crona DJ, Whang YE. Androgen receptor-dependent and -independent mechanisms involved in prostate cancer therapy resistance. Cancers 2017, 9, 67. [Google Scholar] [CrossRef]
- Petrylak DP, Gao X, Vogelzang NJ, et al. First-in-human phase I study of ARV-110, an androgen receptor (AR) PROTAC degrader in patients (pts) with metastatic castrate-resistant prostate cancer (mCRPC) following enzalutamide (ENZ) and/or abiraterone (ABI). Journal of Clinical Oncology 2020, 38(15_suppl):3500. [CrossRef]
- Mei L, Zhang J, He K, et al. Ataxia telangiectasia and Rad3-related inhibitors and cancer therapy: where we stand. Journal of Hematology and Oncology 2019, 12, 1–8. [Google Scholar] [CrossRef]
- Duan R, Du W, Guo W. EZH2: a novel target for cancer treatment. Journal of Hematology and Oncology 2020, 13, 1–12. [Google Scholar] [CrossRef]
- de Bono JS, Sweeney C, Bracarda S, et al. PI3K/AKT pathway biomarkers analysis from the phase III IPATential150 trial of ipatasertib plus abiraterone in metastatic castration-resistant prostate cancer. Journal of Clinical Oncology 2021, 39(6_suppl):13. [CrossRef]
- Baeuerle PA, Reinhardt C. Bispecific T-cell engaging antibodies for cancer therapy. Cancer Research 2009, 69, 4941–4944. [Google Scholar] [CrossRef] [PubMed]
- Tran B, Horvath L, Dorff T, et al. Results from a phase I study of AMG 160, a half-life extended (HLE), PSMA- targeted, bispecific T-cell engager (BiTE®) immune therapy for metastatic castration-resistant prostate cancer (mCRPC). Annals of Oncology. [CrossRef]
- FDA approves first PSMA-targeted PET imaging drug for men with prostate cancer. Accessed June 6, 2021. https://www.fda.gov/news-events/press- announcements/fda-approves-first-psma-targeted- pet-imaging-drug-men-prostate-cancer.
- Morris MJ, Rowe SP, Gorin MA, et al. Diagnostic performance of 18 F-DCFPyL-PET/CT in men with biochemically recurrent prostate cancer: results from the CONDOR phase III, multicenter study. Clinical Cancer Research, 23 February 2021. [CrossRef]
- Pienta KJ, Gorin MA, Rowe SP, et al. A phase 2/3 prospective multicenter study of the diagnostic accuracy of prostate specific membrane antigen PET/CT with 18 F-DCFPyL in prostate cancer patients (OSPREY). Journal of Urology 2021, 206, 52–61. [Google Scholar] [CrossRef]
- Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. New England Journal of Medicine 2013, 369, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Novartis announces positive result of phase III study with radioligand therapy 177Lu-PSMA-617 in patients with advanced prostate cancer | Novartis. Accessed June 6, 2021. https://www.novartis.com/news/media-releases/novartis-announces-positive-result- phase-iii-study-radioligand-therapy-177lu-psma-617- patients-advanced-prostate-cancer.
- Sartor AO, Morris MJ, Krause BJ, et al. VISION: an international, prospective, open-label, multicenter, randomized phase 3 study of 177 Lu-PSMA-617 in the treatment of patients with progressive PSMA-positive metastatic castration-resistant prostate cancer (mCRPC). Journal of Clinical Oncology 2019, 37(15_ suppl):TPS5099. [CrossRef]
- Asim M, Tarish F, Zecchini HI, et al. Synthetic lethality between androgen receptor signalling and the PARP pathway in prostate cancer. Nature Communications 2017, 8, 1–10. [Google Scholar] [CrossRef]
- Virtanen V, Paunu K, Ahlskog JK, et al. PARP inhibitors in prostate cancer—The preclinical rationale and current clinical development. Genes 2019, 10, 565. [Google Scholar] [CrossRef]
- Bossi A, Dearnaley D, McKenzie M, et al. ATLAS: a phase 3 trial evaluating the efficacy of apalutamide (ARN-509) in patients with high-risk localized or locally advanced prostate cancer receiving primary radiation therapy. Annals of Oncology 2016, 27:vi263. [CrossRef]
- Dalela D, Löppenberg B, Sood A, et al. Contemporary role of the Decipher® test in prostate cancer management: current practice and future perspectives. Reviews in Urology 2016, 18, 1–9. [Google Scholar]
- Kneebone A, Fraser-Browne C, Duchesne GM, et al. Adjuvant radiotherapy versus early salvage radiotherapy following radical prostatectomy ( TROG 08.03/ANZUP RAVES): a randomised, controlled, phase 3, non-inferiority trial. The Lancet Oncology 2020, 21, 1331–1340. [Google Scholar] [CrossRef]
- Sargos P, Chabaud S, Latorzeff I, et al. Adjuvant radiotherapy versus early salvage radiotherapy plus short-term androgen deprivation therapy in men with localised prostate cancer after radical prostatectomy (GETUG-AFU 17): a randomised, phase 3 trial. The Lancet Oncology 2020, 21, 1341–1352. [Google Scholar] [CrossRef]
- Parker CC, Clarke NW, Cook AD, et al. Timing of radiotherapy after radical prostatectomy (RADICALS- RT): a randomised, controlled phase 3 trial. The Lancet 2020, 396, 1413–1421. [Google Scholar] [CrossRef]
- Den RB, Yousefi K, Trabulsi EJ, et al. Genomic classifier identifies men with adverse pathology after radical prostatectomy who benefit from adjuvant radiation therapy. Journal of Clinical Oncology 2015, 33, 944–951. [Google Scholar] [CrossRef]
- Zhao SG, Chang SL, Spratt DE, et al. Development and validation of a 24-gene predictor of response to postoperative radiotherapy in prostate cancer: a matched, retrospective analysis. The Lancet Oncology 2016, 17, 1612–1620. [Google Scholar] [CrossRef] [PubMed]
- Vale CL, Fisher D, Kneebone A, et al. Adjuvant or early salvage radiotherapy for the treatment of localised and locally advanced prostate cancer: a prospectively planned systematic review and meta-analysis of aggregate data. The Lancet 2020, 396, 1422–1431. [Google Scholar] [CrossRef]
- Loeb S, Giri VN. Clinical implications of germline testing in newly diagnosed prostate cancer. European Urology Oncology 2021, 4, 1–9. [Google Scholar] [CrossRef]
- Giri VN, Knudsen KE, Kelly WK, et al. Implementation of germline testing for prostate cancer: Philadelphia Prostate Cancer Consensus Conference 2019. Journal of Clinical Oncology 2020, 38, 2798–2811. [Google Scholar] [CrossRef]
- Abida W, Patnaik A, Campbell D, et al. Rucaparib in men with metastatic castration-resistant prostate cancer harboring a BRCA1 or BRCA2 gene alteration. Journal of Clinical Oncology 2020, 38, 3763–3772. [Google Scholar] [CrossRef]
- de Bono J, Mateo J, Fizazi K, et al. Olaparib for metastatic castration-resistant prostate cancer. New England Journal of Medicine 2020, 382, 2091–2102. [Google Scholar] [CrossRef] [PubMed]
- Antonarakis ES, Gomella LG, Petrylak DP. When and how to use PARP inhibitors in prostate cancer: a systematic review of the literature with an update on on-going trials. European Urology Oncology 2020, 3, 594–611. [Google Scholar] [CrossRef] [PubMed]
- Carter HB, Helfand B, Mamawala M, et al. Germline mutations in ATM and BRCA1/2 are associated with grade reclassification in men on active surveillance for prostate cancer. European Urology 2019, 75, 743–749. [Google Scholar] [CrossRef]
- Ribal MJ, Cornford P, Briganti A, et al. European Association of Urology guidelines office rapid reaction group: an organisation-wide collaborative effort to adapt the European Association of Urology guidelines recommendations to the coronavirus disease 2019 era. European Urology 2020, 78, 21–28. [Google Scholar] [CrossRef]
- Hanna TP, King WD, Thibodeau S, et al. Mortality due to cancer treatment delay: systematic review and meta- analysis. BMJ (Clinical Research Ed) 2020, 371:m4087. [CrossRef]
- Glasbey JC, Nepogodiev D, Simoes JFF, et al. Elective cancer surgery in COVID-19-free surgical pathways during the SARS-CoV-2 pandemic: an international, multicenter, comparative cohort study. Journal of Clinical Oncology 2021, 39, 66–78. [Google Scholar] [CrossRef]
- COVIDSurg Collaborative, GlobalSurg Collaborative. Timing of surgery following SARS-CoV-2 infection: an international prospective cohort study. Anaesthesia 2021, 76, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Lee LYW, Cazier JB, Angelis V, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. The Lancet 2020, 395, 1919–1926. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 and Cancer Consortium Registry. Accessed June 6, 2021. https://clinicaltrials.gov/ct2/show/NCT04354701.
- ASCO Survey on COVID-19 in Oncology (ASCO) Registry. Accessed June 6, 2021. https://clinicaltrials.gov/ct2/show/NCT04659135?term=NCT04659135&draw= 2&rank=1.
- ESMO-CoCARE Registry. Accessed June 6, 2021. ht tps://w w w. esmo. org/covid-19 - and- c ancer/registries-studies-and-surveys/esmo-cocare-registry.
- COVID-19 vaccination in cancer patients: ESMO statements. Accessed June 6, 2021. https://www.esmo. org/covid-19-and-cancer/covid-19-vaccination.
- ASCO. COVID-19 vaccines & patients with cancer. Accessed June 6, 2021. https://www.asco.org/asco- coronavirus-resourcescovid-19-vaccines-patients- cancer.
- Vaccination Advisory Committee: Recommendations of the NCCN COVID-19. Accessed June 6, 2021. https://www.nccn.org/covid-19.
- COVIDSurg Collaborative, GlobalSurg Collaborative. SARS-CoV-2 vaccination modelling for safe surgery to save lives: data from an international prospective cohort study. British Journal of Surgery. Published online 2021:1-8. [CrossRef]
- Rodriguez Socarrás M, Loeb S, Teoh JYC, et al. Telemedicine and smart working: recommendations of the European Association of Urology. European Urology 2020, 78, 812–819. [Google Scholar] [CrossRef]
- Rodler S, Apfelbeck M, Schulz GB, et al. Telehealth in uro-oncology beyond the pandemic: toll or lifesaver? European Urology Focus 2020, 6, 1097–1103. [Google Scholar] [CrossRef]
- Wallis CJD, Catto JWF, Finelli A, et al. The impact of the COVID-19 pandemic on genitourinary cancer care: re-envisioning the future. European Urology 2020, 78, 731–742. [Google Scholar] [CrossRef]
- Hlubocky FJ, Symington BE, McFarland DC, et al. Impact of the COVID-19 pandemic on oncologist burnout, emotional well-being, and moral distress: considerations for the cancer organization’s response for readiness, mitigation, and resilience. JCO Oncology Practice. Published online February 8, 2021:1-11. [CrossRef]
- Maringe C, Spicer J, Morris M, et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. The Lancet Oncology 2020, 21, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Oderda M, Roupret M, Marra G, et al. The impact of COVID-19 outbreak on uro-oncological practice across Europe: which burden of activity are we facing ahead? European Urology 2020, 78, 124–126. [Google Scholar] [CrossRef]
- Lughezzani G, Saita A, Lazzeri M, et al. Comparison of the diagnostic accuracy of micro-ultrasound and magnetic resonance imaging/ultrasound fusion targeted biopsies for the diagnosis of clinically significant prostate cancer. European Urology Oncology 2019, 2, 329–332. [Google Scholar] [CrossRef]
- Ghai S, Eure G, Fradet V, et al. Assessing cancer risk on novel 29 MHz micro-ultrasound images of the prostate: creation of the micro-ultrasound protocol for prostate risk identification. Journal of Urology 2016, 196, 562–569. [Google Scholar] [CrossRef]
- Abouassaly R, Klein EA, El-Shefai A, et al. Impact of using 29 MHz high-resolution micro-ultrasound in real- time targeting of transrectal prostate biopsies: initial experience. World Journal of Urology 2020, 38, 1201–1206. [Google Scholar] [CrossRef]
- Lughezzani G, Maffei D, Saita A, et al. Diagnostic accuracy of microultrasound in patients with a suspicion of prostate cancer at magnetic resonance imaging: a single-institutional prospective study. European Urology Focus 2020, S2405-4569, 30272-30278. [CrossRef]
- Klotz L, Lughezzani G, Maffei D, et al. Comparison of micro-ultrasound and multiparametric magnetic resonance imaging for prostate cancer: a multicenter, prospective analysis. Canadian Urological Association Journal 2020, 15, E11–E16. [Google Scholar] [CrossRef]
- Cornud F, Lefevre A, Flam T, et al. MRI-directed high- frequency (29MhZ) TRUS-guided biopsies: initial results of a single-center study. European Radiology 2020, 30, 4838–4846. [Google Scholar] [CrossRef] [PubMed]
- Claros OR, Tourinho-Barbosa RR, Fregeville A, et al. Comparison of initial experience with transrectal magnetic resonance imaging cognitive guided micro- ultrasound biopsies versus established transperineal robotic ultrasound magnetic resonance imaging fusion biopsies for prostate cancer. The Journal of Urology 2020, 203, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Socarrás ME, Gomez Rivas J, Cuadros Rivera V, et al. Prostate mapping for cancer diagnosis: the Madrid protocol. Transperineal prostate biopsies using multiparametric magnetic resonance imaging fusion and micro-ultrasound guided biopsies. The Journal of Urology 2020, 204, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Wiemer L, Hollenbach M, Heckmann R, et al. Evolution of targeted prostate biopsy by adding microultrasound to the magnetic resonance imaging pathway. European Urology Focus 2020, S2405-4569(20)30188-7. [CrossRef]
- Regis F, Casale P, Persico F, et al. Use of 29-MHz micro-ultrasound for local staging of prostate cancer in patients scheduled for radical prostatectomy: a feasibility study. European Urology Open Science 2020, 19:20-23. [CrossRef]
- Stabile A, Orczyk C, Hosking-Jervis F, et al. Medium- term oncological outcomes in a large cohort of men treated with either focal or hemi-ablation using high- intensity focused ultrasonography for primary localized prostate cancer. BJU International 2019, 124, 431–440. [Google Scholar] [CrossRef]
- Donaldson IA, Alonzi R, Barratt D, et al. Focal therapy: patients, interventions, and outcomes - a report from a consensus meeting. European Urology 2015, 67, 771–777. [Google Scholar] [CrossRef]
- Rastinehad AR, Anastos H, Wajswol E, et al. Gold nanoshell-localized photothermal ablation of prostate tumors in a clinical pilot device study. Proceedings of the National Academy of Sciences of the United States of America 2019, 116, 18590–18596. [Google Scholar] [CrossRef]
- Maruf M, George A, Canfield S, et al. Phase II clinical trial: short-term oncologic outcomes of nanoparticle- directed focal photothermal laser ablation. Journal of Urology 2020, 203(Supplement 4):e373-e374. [CrossRef]
- Turkbey B, Pinto PA, Mani H, et al. Prostate cancer: value of multiparametric MR imaging at 3 T for detection - histopathologic correlation. Radiology 2010, 255, 89–99. [Google Scholar] [CrossRef]
- Valerio M, Cerantola Y, Eggener SE, et al. New and established technology in focal ablation of the prostate: a systematic review. European Urology 2017, 71, 17–34. [Google Scholar] [CrossRef]
- Mottet N, van den Bergh RCN, Briers E, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer—2020 update. Part 1: screening, diagnosis, and local treatment with curative intent. European Urology 2021, 79, 243–262. [Google Scholar] [CrossRef] [PubMed]
- Kishan AU, Park SJ, King CR, et al. Dosimetric benefits of hemigland stereotactic body radiotherapy for prostate cancer: implications for focal therapy. British Journal of Radiology 2015, 88, 20150658. [Google Scholar] [CrossRef] [PubMed]
- Prada PJ, Cardenal J, García Blanco A, et al. Focal high-dose-rate brachytherapy for localized prostate cancer: toxicity and preliminary biochemical results. Strahlentherapie und Onkologie 2020, 196, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Brand DH, Tree AC, Ostler P, et al. Intensity-modulated fractionated radiotherapy versus stereotactic body radiotherapy for prostate cancer (PACE-B): acute toxicity findings from an international, randomised, open-label, phase 3, non-inferiority trial. The Lancet Oncology 2019, 20 (11):1531-1543. [CrossRef]
- Tocco BR, Kishan AU, Ma TM, et al. MR-guided radiotherapy for prostate cancer. Frontiers in Oncology 2020, 10:2763. [CrossRef]
- Kerkmeijer LGW, Groen VH, Pos FJ, et al. Focal boost to the intraprostatic tumor in external beam radiotherapy for patients with localized prostate cancer: results from the FLAME randomized phase III trial. Journal of Clinical Oncology 2021, 39, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Draulans C, van der Heide UA, Haustermans K, et al. Primary endpoint analysis of the multicentre phase II hypo-FLAME trial for intermediate and high risk prostate cancer. Radiotherapy and Oncology 2020, 147:92-98. [CrossRef]
- Connor MJ, Shah TT, Smigielska K, et al. Additional treatments to the local tumour for metastatic prostate cancer-assessment of novel treatment algorithms (IP2-ATLANTA): protocol for a multicentre, phase II randomised controlled trial. BMJ Open 2021, 11, 42953. [Google Scholar] [CrossRef]
- Fendler WP, Weber M, Iravani A, et al. Prostate- specific membrane antigen ligand positron emission tomography in men with nonmetastatic castration- resistant prostate cancer. Clinical Cancer Research 2019, 25, 7448–7454. [Google Scholar] [CrossRef] [PubMed]
- Howard LE, Moreira DM, de Hoedt A, et al. Thresholds for PSA doubling time in men with non-metastatic castration-resistant prostate cancer. BJU International 2017, 120(5B):E80-E86. [CrossRef]
- Fizazi K, Shore N, Tammela TL, et al. Darolutamide in nonmetastatic, castration-resistant prostate cancer. New England Journal of Medicine 2019, 380, 1235–1246. [Google Scholar] [CrossRef]
- Hussain M, Fizazi K, Saad F, et al. Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. New England Journal of Medicine 2018, 378, 2465–2474. [Google Scholar] [CrossRef]
- Smith MR, Saad F, Chowdhury S, et al. Apalutamide treatment and metastasis-free survival in prostate cancer. New England Journal of Medicine 2018, 378, 1408–1418. [Google Scholar] [CrossRef]
- Beauval J-B, Loriot Y, Hennequin C, et al. Loco- regional treatment for castration-resistant prostate cancer: is there any rationale? A critical review from the AFU-GETUG. Critical Reviews in Oncology/Hematology 2018, 122:144-149. [CrossRef]
- Parker C, Castro E, Fizazi K, et al. Prostate cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Annals of Oncology 2020, 31, 1119–1134. [Google Scholar] [CrossRef]
- Wyatt AW, Azad AA, Volik S v. , et al. Genomic alterations in cell-free DNA and enzalutamide resistance in castration-resistant prostate cancer. JAMA Oncology 2016, 2, 1598–1606. [Google Scholar] [CrossRef] [PubMed]
- Lallous N, Volik S v. , Awrey S, et al. Functional analysis of androgen receptor mutations that confer anti-androgen resistance identified in circulating cell-free DNA from prostate cancer patients. Genome Biology 2016, 17, 10. [Google Scholar] [CrossRef]
- Annala M, Struss WJ, Warner EW, et al. Treatment outcomes and tumor loss of heterozygosity in germline DNA repair–deficient prostate cancer. European Urology 2017, 72, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Hussain M, Mateo J, Fizazi K, et al. Survival with olaparib in metastatic castration-resistant prostate cancer. New England Journal of Medicine 2020, 383, 2345–2357. [Google Scholar] [CrossRef] [PubMed]
- de Wit R, de Bono J, Sternberg CN, et al. Cabazitaxel versus abiraterone or enzalutamide in metastatic prostate cancer. New England Journal of Medicine 2019, 381, 2506–2518. [CrossRef]
- Hofman MS, Emmett L, Sandhu S, et al. [177Lu] Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. The Lancet 2021, 397, 797–804. [CrossRef]
- Digital trends 2021. Accessed June 7, 2021. https://www. hootsuite.com/pages/digital-trends-2021.
- Statista. Where health app usage is most common. Accessed June 7, 2021. https://www.statista.com/chart/23161/health-app-usage-country-comparison/.
- Pereira-Azevedo N, Osório L, Fraga A, et al. Rotterdam Prostate Cancer Risk Calculator: development and usability testing of the mobile phone app. JMIR Cancer 2017, 3, e1. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Grid. Virtual tumour board. Accessed June 7, 2021. https://tmc.gov.in/ncg/index.php/activities-ncg/vrtual-tumor-board.
- Gudaru K, Blanco LT, Castellani D, et al. Connecting the urological community: the #UroSoMe experience. Journal of Endoluminal Endourology 2019, 2, e21–e29. [Google Scholar] [CrossRef]
- Bhatt NR, Czarniecki SW, Borgmann H, et al. A systematic review of the use of social media for dissemination of clinical practice guidelines. European Urology Focus 2020, S2405 - 4569, 30292-30293. [CrossRef]
- Loeb S, Roupret M, van Oort I, et al. Novel use of Twitter to disseminate and evaluate adherence to clinical guidelines by the European Association of Urology. BJU International 2017, 119, 820–822. [Google Scholar] [CrossRef]
- Dubin JM, Wyant WA, Balaji NC, et al. Telemedicine usage among urologists during the COVID-19 pandemic: cross-sectional study. Journal of Medical Internet Research 2020, 22, e21875. [Google Scholar] [CrossRef]
- Rivas JG, Socarras MR, Patruno G, et al. Perceived role of social media in urologic knowledge acquisition among young urologists: a European survey. European Urology Focus 2018, 4, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Dubin JM, Greer AB, Patel P, et al. Global survey of the roles and attitudes toward social media platforms amongst urology trainees. Urology 2021, 147:64-67. [CrossRef]
- Junwen S, Rongjiang W. The efficacy of the WeChat app combined with pelvic floor muscle exercise for the urinary incontinence after radical prostatectomy. BioMed Research International 2020, 2020:6947839. [CrossRef]
- Belarmino A, Walsh R, Alshak M, et al. Feasibility of a mobile health application to monitor recovery and patient-reported outcomes after robot-assisted radical prostatectomy. European Urology Oncology 2019, 2, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Gebbia V, Piazza D, Valerio MR, et al. Patients with cancer and COVID-19: a WhatsApp messenger-based survey of patients’ queries, needs, fears, and actions taken. JCO Global Oncology 2020, (6). [CrossRef]
- Loeb S, Mihalcea R, Perez-Rosas V, et al. Leveraging social media as a thermometer to gauge patient and caregiver concerns: COVID-19 and prostate cancer. European Urology Open Science. [CrossRef]
- Alsyouf M, Stokes P, Hur D, et al. ‘Fake news’ in urology: evaluating the accuracy of articles shared on social media in genitourinary malignancies. BJU International 2019, 124, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Loeb S, Reines K, Abu-Salha Y, et al. Quality of bladder cancer information on YouTube. European Urology 2021, 79, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Loeb S, Sengupta S, Butaney M, et al. Dissemination of misinformative and biased information about prostate cancer on YouTube. European Urology 2019, 75, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Xu AJ, Taylor J, Gao T, et al. TikTok and prostate cancer: misinformation and quality of information using validated questionnaires. BJU International. Published online April 2, 2021. [CrossRef]
- Charnock D, Shepperd S, Needham G, et al. DISCERN: an instrument for judging the quality of written consumer health information on treatment choices. Journal of Epidemiology and Community Health 1999, 53, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Pantanowitz L, Quiroga-Garza GM, Bien L, et al. An artificial intelligence algorithm for prostate cancer diagnosis in whole slide images of core needle biopsies: a blinded clinical validation and deployment study. The Lancet Digital Health 2020, 2, e407–e416. [Google Scholar] [CrossRef] [PubMed]
- PIONEER - European network of excellence for big data in prostate cancer. Accessed June 8, 2021. https://prostate-pioneer.eu/.
- Hoffer-Hawlik MA, Moran AE, Burka D, et al. Leveraging telemedicine for chronic disease management in low- and middle-income countries during Covid-19. Global Heart 2020, 15, 63. [Google Scholar] [CrossRef]
- Veneziano D, Tafuri A, Rivas JG, et al. Is remote live urologic surgery a reality? Evidences from a systematic review of the literature. World Journal of Urology 2020, 38, 2367–2376. [Google Scholar] [CrossRef]
This is an open access article under the terms of a license that permits non-commercial use, provided the original work is properly cited. © 2021 The Authors. Société Internationale d'Urologie Journal, published by the Société Internationale d'Urologie, Canada.
