Use of Urology-Based Clinical Practice Guidelines in International Settings
Abstract
:Introduction
Methods
Study design and participants
Study variables
Statistical analysis
Ethics
Results
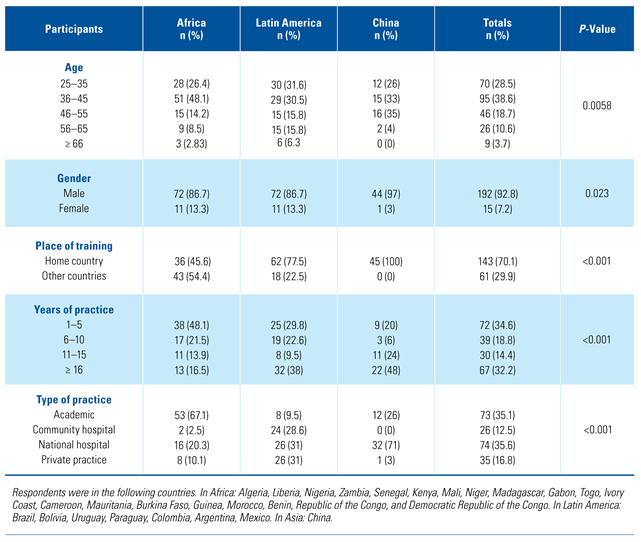
Demographics
Baseline factors contributing to clinical decision-making and access to CPGs
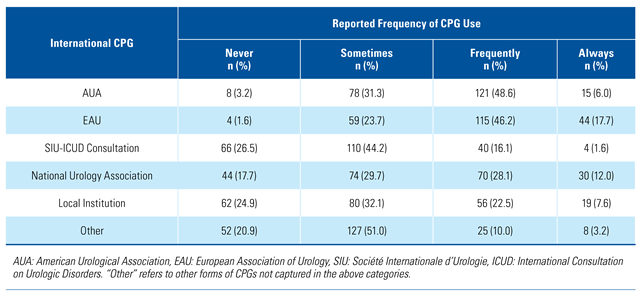
Use of urology CPGs in international settings
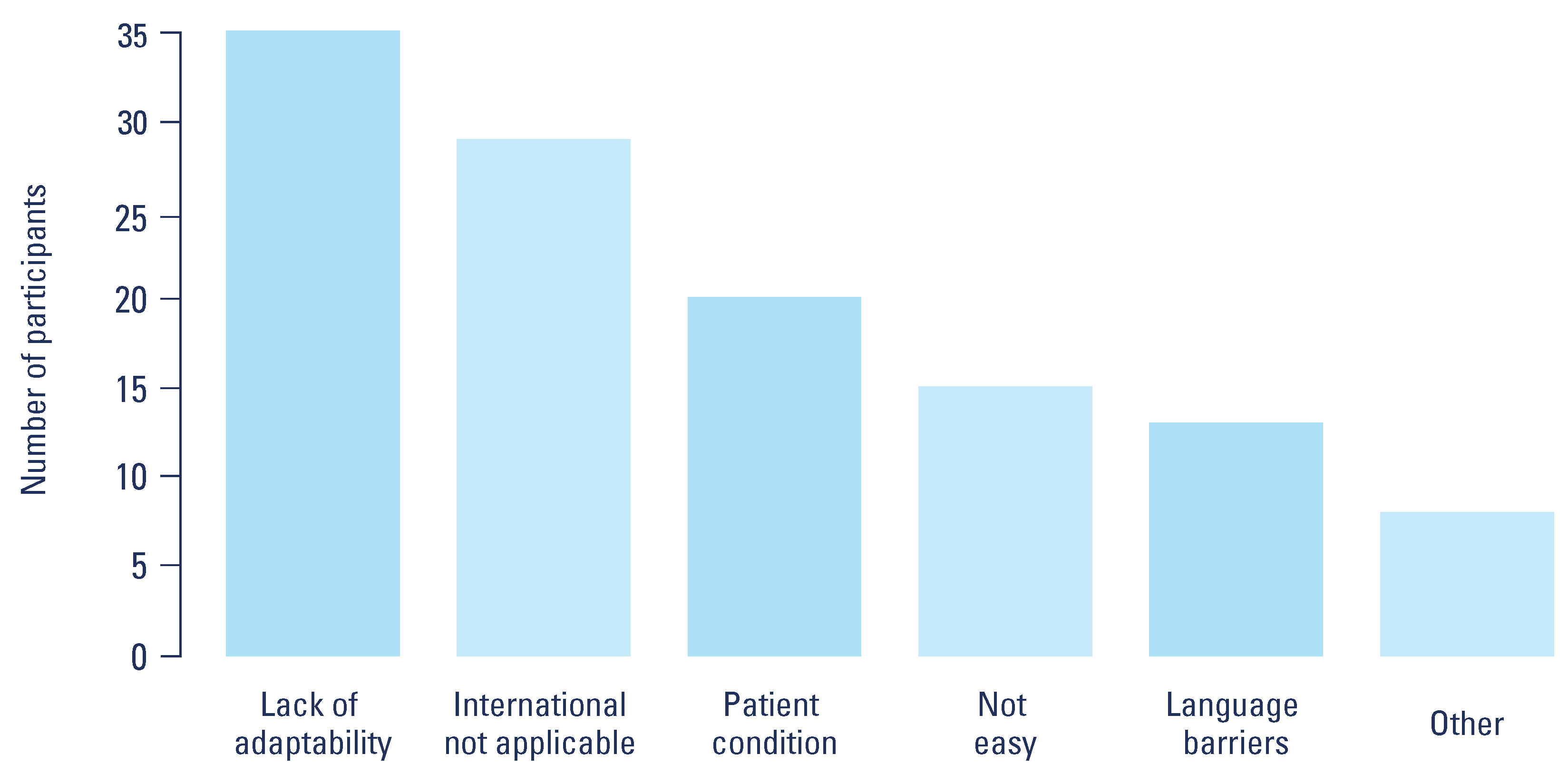
Reported barriers to CPG use
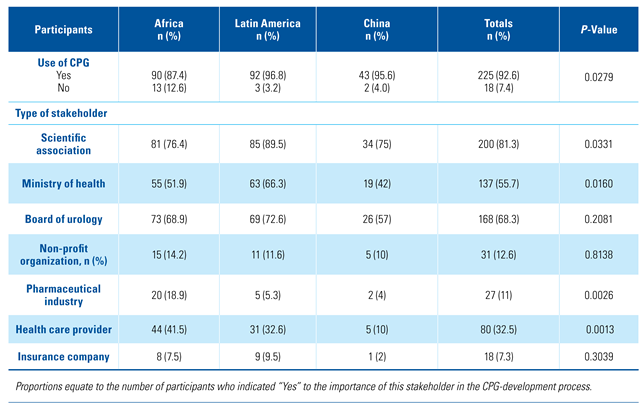
Stakeholder responsibility for the development of urology CPGs
Discussion
Conclusions
Conflicts of Interest
Abbreviations
| AUA | American Urological Association |
| CPG | Clinical practice guideline |
| EAU | European Association of Urology |
References
- Sackett, D.L.; Rosenberg, W.M.; Gray, J.A.; Haynes, R.B.; Richardson, W.S. Evidence based medicine: What it is and what it isn’t. BMJ 1996, 312, 71. [Google Scholar] [CrossRef] [PubMed]
- Kredo, T.; Bernhardsson, S.; Machingaidze, S.; Young, T.; Louw, Q.; Ochodo, E.; et al. Guide to clinical practice guidelines: The current state of play. Int. J. Qual. Health Care 2016, 28, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Bhaumik, S. Use of evidence for clinical practice guideline development. Trop. Parasitol. 2017, 7, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Developing a methodology for drawing up guidelines on best medical practices. (Recommendation (2001)13 and explanatory memorandum). Council of Europe. Z. Arztl. Fortbild Qualitätssich. 2002, 96 (Suppl. 3), 5–59. Available online: https://www.ncbi.nlm.nih.gov/pubmed/12964585 (accessed on 20 November 2020).
- Bahtsevani, C.; Udén, G.; Willman, A. Outcomes of evidence–based clinical practice guidelines: A systematic review. Int. J. Technol. Assess Health Care 2004, 20, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Lugtenberg, M.; Burgers, J.S.; Westert, G.P. Effects of evidence–based clinical practice guidelines on quality of care: A systematic review. Qual. Saf. Health Care 2009, 18, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Grimshaw, J.M.; Russell, I.T. Effect of clinical guidelines on medical practice: A systematic review of rigorous evaluations. Lancet 1993, 342, 1317–1322. [Google Scholar] [CrossRef] [PubMed]
- Breyer, B.N.; Fang, R.; Meeks, W.; Lightner, D.; Clemens, J.Q. Use of the American Urological Association clinical practice guidelines: Data from the AUA census. Urol. Pract. 2017, 4, 462–467 https://doi org/101016/jurpr201610003. [Google Scholar] [CrossRef] [PubMed]
- Cacciamani, G.; Artibani, W.; Briganti, A.; N’Dow, J. Adherence to the European Association of Urology Guidelines: A national survey among Italian urologists. Urol. Int. 2018, 100, 139–145. [Google Scholar] [CrossRef]
- Tomaškovic´, I.; Tomi, M.; Nikles, S.; Neretljak, I.; Milicic´, V. Croatian urologists’ clinical practice and compliance with guidelines in the management of non-neurogenic male lower urinary tract symptoms. Acta Clin. Croat. 2015, 54, 453–457. [Google Scholar]
- Strope, S.A.; Elliott, S.P.; Saigal, C.S.; Smith, A.; Wilt, T.J.; Wei, J.T.; et al. Urologist compliance with AUA best practice guidelines for benign prostatic hyperplasia in medicare population. Urology 2011, 78, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Van Rhijn, B.W.G.; Burger, M. Bladder cancer: Low adherence to guidelines in non-muscle-invasive disease. Nat. Rev. Urol. 2016, 13, 570–571. [Google Scholar] [CrossRef]
- Shinagare, A.B.; Silverman, S.G.; Gershanik, E.F.; Chang, S.L.; Khorasani, R. Evaluating hematuria: Impact of guideline adherence on urologic cancer diagnosis. Am. J. Med. 2014, 127, 625–632. [Google Scholar] [CrossRef]
- Ismaila, N.; Salako, O.; Mutiu, J.; Adebayo, O. Oncology guidelines usage in a low–and middle-income country. J. Glob. Oncol. 2018, 4, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Docherty, M.; Shaw, K.; Goulding, L.; Parke, H.; Eassom, E.; Ali, F.; et al. Evidence-based guideline implementation in low and middle income countries: Lessons for mental health care. Int. J. Ment. Health Syst. 2017, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Olayemi, E.; Asare, E.V.; Benneh-Akwasi Kuma, A.A. Guidelines in lower–middle income countries. Br. J. Haematol. 2017, 177, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research Electronic Data Capture (REDCap) –a metadata driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Woolf, S.; Schünemann, H.J.; Eccles, M.P.; Grimshaw, J.M.; Shekelle, P. Developing clinical practice guidelines: Types of evidence and outcomes; values and economics, synthesis, grading, and presentation and deriving recommendations. Implement Sci. 2012, 7, 1–12. [Google Scholar] [CrossRef]
- WHO. Guidelines in health care practice: Report on the WHO meeting, Schloss Velen, Borken, Germany, 26-28 January 1997. 1997;(45). https://apps.who.int/iris/handle/10665/107628.
- Cabrera, P.A.; Pardo, R. Review of evidence based clinical practice guidelines developed in Latin America and Caribbean during the last decade: An analysis of the methods for grading quality of evidence and topic prioritization. Glob. Health 2019, 15, 1–10. [Google Scholar] [CrossRef]
- Hesketh, T.; Zhu, W.X. Health in China. Traditional Chinese medicine: One country, two systems. BMJ 1997, 315, 115–117. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, C.; Shang, H.; Yang, K.; Norris, S.L. Clinical practice guidelines in China. BMJ 2018, 360. [Google Scholar] [CrossRef]
- Okwen, P.M.; Maweu, I.; Grimmer, K.; Margarita Dizon, J. Evaluation of all African clinical practice guidelines for hypertension: Quality and opportunities for improvement. J. Eval. Clin. Pract. 2019, 25, 565–574. [Google Scholar] [CrossRef]
- McCaul, M.; Ernstzen, D.; Temmingh, H.; Draper, B.; Galloway, M.; Kredo, T. Clinical practice guideline adaptation methods in resource–constrained settings: Four case studies from South Africa. BMJ Evid. Based Med. 2020, 25, 193–198, Epub ahead of print 10 July 2019: 1–6. Available online: https://www.ncbi.nlm.nih.gov/pubmed/31292208 (accessed on 20 November 2020). [CrossRef] [PubMed]
- Dahm, P.; Poolman, R.W.; Bhandari, M.; Fesperman, S.F.; Baum, J.; Kosiak, B.; et al. Perceptions and competence in evidence-based medicine: A survey of the American Urological Association membership. J. Urol. 2009, 181, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Fraser, H.S.F.; McGrath, S.J.D. Information technology and telemedicine in sub-Saharan Africa. BMJ 2000, 321, 465–466. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.; Arunachalam, S.; Kirsop, B. Open access: A giant leap towards bridging health inequities. Bull. World Health Organ. 2009, 87, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.; Haustein, S.; Mongeon, P.; Shu, F.; Ridde, V.; Larivière, V. Knowledge sharing in global health research –the impact, uptake and cost of open access to scholarly literature. Health Res. Policy Syst. 2017, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Shaghaghi, A.; Bhopal, R.S.; Sheikh, A. Approaches to recruiting “hard–to-reach” populations into re-search: A review of the literature. Health Promot. Perspect. 2011, 1, 86–94. [Google Scholar] [CrossRef]
- Valerio, M.A.; Rodriguez, N.; Winkler, P.; Lopez, J.; Dennison, M.; Liang, Y.; et al. Comparing two sampling methods to engage hard-to-reach communities in research priority setting. BMC Med. Res. Methodol. 2016, 16, 1–11. [Google Scholar] [CrossRef]
 |
 |
 |
This is an open access article under the terms of a license that permits non-commercial use, provided the original work is properly cited. © 2021 The Authors. Société Internationale d'Urologie Journal, published by the Société Internationale d'Urologie, Canada.
Share and Cite
Patino, G.; Ndoye, M.; Thomas, H.S.; Cohen, A.J.; Mmonu, N.A.; Chu, C.E.; Breyer, B.N. Use of Urology-Based Clinical Practice Guidelines in International Settings. Soc. Int. Urol. J. 2021, 2, 10-17. https://doi.org/10.48083/QVXE4949
Patino G, Ndoye M, Thomas HS, Cohen AJ, Mmonu NA, Chu CE, Breyer BN. Use of Urology-Based Clinical Practice Guidelines in International Settings. Société Internationale d’Urologie Journal. 2021; 2(1):10-17. https://doi.org/10.48083/QVXE4949
Chicago/Turabian StylePatino, German, Medina Ndoye, Hannah S. Thomas, Andrew J. Cohen, Nnenaya A. Mmonu, Carissa E. Chu, and Benjamin N. Breyer. 2021. "Use of Urology-Based Clinical Practice Guidelines in International Settings" Société Internationale d’Urologie Journal 2, no. 1: 10-17. https://doi.org/10.48083/QVXE4949
APA StylePatino, G., Ndoye, M., Thomas, H. S., Cohen, A. J., Mmonu, N. A., Chu, C. E., & Breyer, B. N. (2021). Use of Urology-Based Clinical Practice Guidelines in International Settings. Société Internationale d’Urologie Journal, 2(1), 10-17. https://doi.org/10.48083/QVXE4949




