The Clinical Applications of Tissue Biomarkers in Prostate Cancer
Abstract
:Introduction
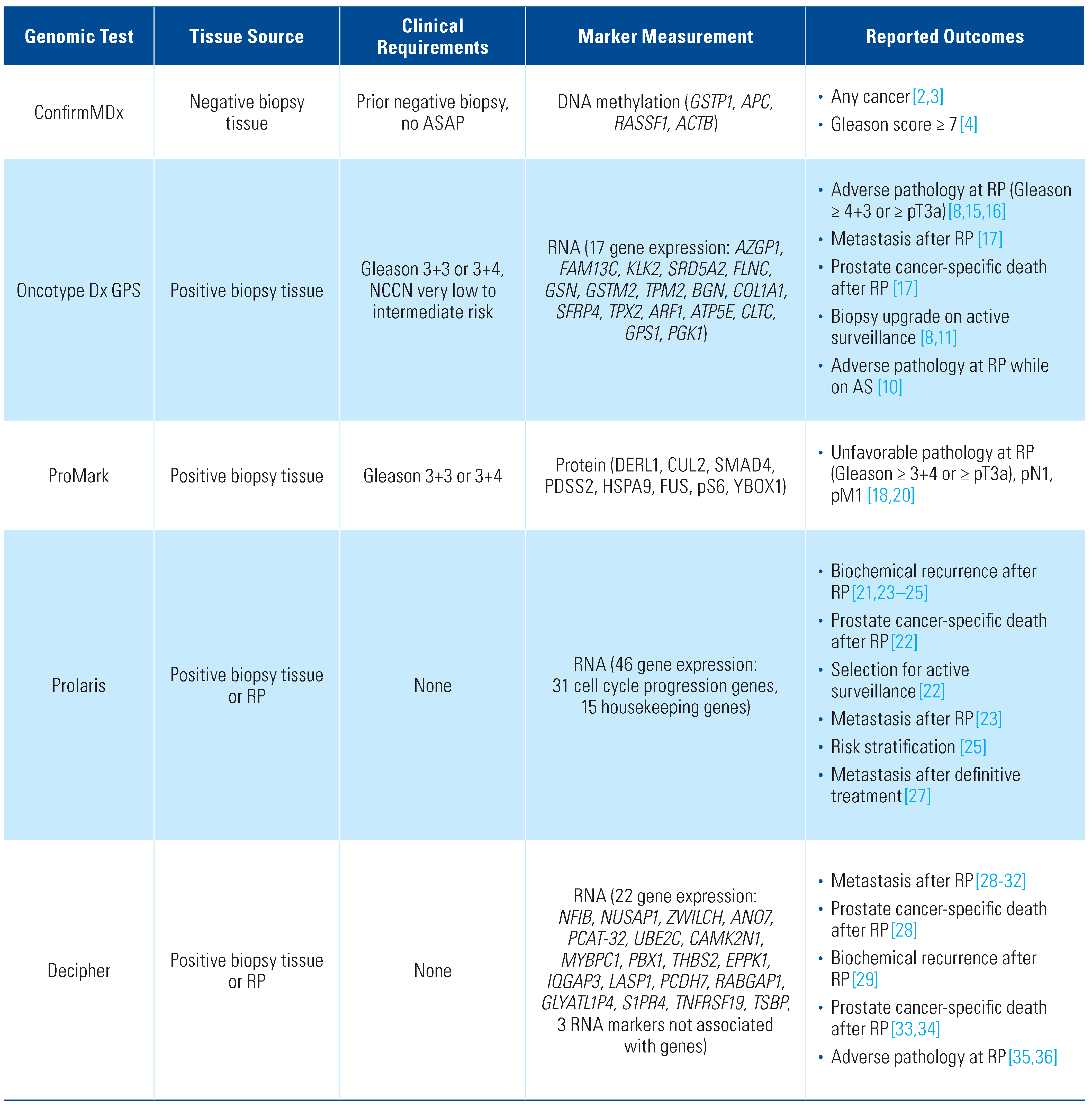
ConfirmMDX
Description of assay
Prediction of cancer after negative biopsy
Oncotype Dx Genomic Prostate Score (GPS)
Description of assay
Prediction of disease progression in active surveillance
Prediction of adverse pathology at surgery
Prediction of post-operative outcomes
ProMark
Description of assay
Prediction of adverse pathology following surgery
Prolaris
Description of assay
Identification of men suitable for active surveillance
Prediction of post-operative outcomes
Use in risk prediction
Decipher
Description of assay
Prediction of post-operative outcomes
Use in risk stratification
Emerging tissue biomarkers
Future Challenges and Directions
Conflicts of Interest
Abbreviations
| AP | adverse pathology |
| AS | active surveillance |
| BCR | biochemical recurrence |
| CAPRA | Cancer of the Prostate Risk Assessment |
| CCP | cell cycle progression |
| CCR | cell cycle risk |
| DRE | digital rectal examination |
| GPS | genomic prostate score |
| NCCN | National Comprehensive Cancer Network |
| NPV | negative predictive value |
| PCa | prostate cancer |
| PCR | polymerase chain reaction |
| RP | radical prostatectomy |
References
- Van Neste, L.; Bigley, J.; Toll, A.; et al. A tissue biopsy-based epigenetic multiplex PCR assay for prostate cancer detection. BMC Urol. 2012, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Stewart, G.D.; Van Neste, L.; Delvenne, P.; et al. Clinical utility of an epigenetic assay to detect occult prostate cancer in histopathologically negative biopsies: Results of the MATLOC study. J. Urol. 2013, 189, 1110–1116. [Google Scholar] [CrossRef] [PubMed]
- Partin, A.W.; Van Neste, L.; Klein, E.A.; et al. Clinical validation of an epigenetic assay to predict negative histopathological results in repeat prostate biopsies. J. Urol. 2014, 192, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, R.L., Jr.; Van Neste, L.; Moses, K.A.; et al. Evaluation of an epigenetic assay for predicting repeat prostate biopsy outcome in African American men. Urology 2019, 128, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, D.; Goddard, A.D.; Natraj, N.; et al. Analytical validation of the Oncotype DX prostate cancer assay—A clinical RT-PCR assay optimized for prostate needle biopsies. BMC Genom. 2013, 14, 690. [Google Scholar] [CrossRef]
- Mohler, J.L.; Antonarakis, E.S.; Armstrong, A.J.; et al. Prostate Cancer, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2019, 17, 479–505. [Google Scholar] [CrossRef]
- Eggener, S.E.; Rumble, R.B.; Armstrong, A.J.; et al. Molecular biomarkers in localized prostate cancer: ASCO guideline. J. Clin. Oncol. 2020, 38, 1474–1494. [Google Scholar] [CrossRef] [PubMed]
- Kornberg, Z.; Cowan, J.E.; Westphalen, A.C.; et al. Genomic Prostate Score, PI-RADS version 2 and progression in men with prostate cancer on active surveillance. J. Urol. 2019, 201, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Kornberg, Z.; Cooperberg, M.R.; Cowan, J.E.; et al. A 17-gene genomic prostate score as a predictor of adverse pathology for men on active surveillance. J. Urol. 2019, 202, 702–709. [Google Scholar] [CrossRef]
- Lin, D.W.; Zheng, Y.; McKenney, J.K.; et al. 17-Gene Genomic prostate score test results in the Canary Prostate Active Surveillance Study (PASS) Cohort. J. Clin. Oncol. 2020, 38, 1549–1557. [Google Scholar] [CrossRef]
- Cedars, B.E.; Washington, S.L., 3rd; Cowan, J.E.; et al. Stability of a 17-gene genomic prostate score in serial testing of men on active surveillance of early stage prostate cancer. J. Urol. 2020, 202, 696–701. [Google Scholar] [CrossRef]
- Lonergan, P.E.; Washington, S.L., 3rd; Cowan, J.E.; et al. Risk factors for biopsy reclassification over time in men on active surveillance for early stage prostate cancer. J. Urol. 2020, 204, 1216–1221. [Google Scholar] [CrossRef] [PubMed]
- Nyame, Y.A.; Grimberg, D.C.; Greene, D.J.; et al. Genomic scores are independent of disease volume in men with favorable risk prostate cancer: Implications for choosing men for active surveillance. J. Urol. 2018, 199, 438–444. [Google Scholar] [CrossRef]
- Gaffney, C.; Golan, R.; Cantu, M.D.; et al. The clinical utility of the genomic prostate score in men with very low to intermediate risk prostate cancer. J. Urol. 2019, 202, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.A.; Cooperberg, M.R.; Magi-Galluzzi, C.; et al. A 17-gene assay to predict prostate cancer aggressiveness in the context of Gleason grade heterogeneity, tumor multifocality, and biopsy undersampling. Eur. Urol. 2014, 66, 550–560. [Google Scholar] [CrossRef]
- Eggener, S.; Karsh, L.I.; Richardson, T.; et al. A 17-gene panel for prediction of adverse prostate cancer pathologic features: Prospective clinical validation and utility. Urology 2019, 126, 76–82. [Google Scholar] [CrossRef]
- Van Den Eeden, S.K.; Lu, R.; Zhang, N.; et al. A biopsy-based 17-gene genomic prostate score as a predictor of metastases and prostate cancer death in surgically treated men with clinically localized disease. Eur. Urol. 2018, 73, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Shipitsin, M.; Small, C.; Giladi, E.; et al. Automated quantitative multiplex immunofluorescence in situ imaging identifies phospho-S6 and phospho-PRAS40 as predictive protein biomarkers for prostate cancer lethality. Proteome Sci. 2014, 12, 40. [Google Scholar] [CrossRef] [PubMed]
- Shipitsin, M.; Small, C.; Choudhury, S.; et al. Identification of proteomic biomarkers predicting prostate cancer aggressiveness and lethality despite biopsy-sampling error. Br. J. Cancer. 2014, 111, 1201–1212. [Google Scholar] [CrossRef]
- Blume-Jensen, P.; Berman, D.M.; Rimm, D.L.; et al. Development and clinical validation of an in situ biopsy-based multimarker assay for risk stratification in prostate cancer. Clin. Cancer Res. 2015, 21, 2591–2600. [Google Scholar] [CrossRef]
- Cuzick, J.; Swanson, G.P.; Fisher, G.; et al. Prognostic value of an RNA expression signature derived from cell cycle proliferation genes in patients with prostate cancer: A retrospective study. Lancet Oncol. 2011, 12, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.W.; Crawford, E.D.; Keane, T.; et al. Identification of men with low-risk biopsy-confirmed prostate cancer as candidates for active surveillance. Urol. Oncol. 2018, 36, 310.e7–310.e13. [Google Scholar] [CrossRef] [PubMed]
- Bishoff, J.T.; et al. Prognostic utility of the cell cycle progression score generated from biopsy in men treated with prostatectomy. J. Urol. 2014, 192, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Tosoian, J.J.; Chappidi, M.R.; Bishoff, J.T.; et al. Prognostic utility of biopsy-derived cell cycle progression score in patients with National Comprehensive Cancer Network low-risk prostate cancer undergoing radical prostatectomy: Implications for treatment guidance. BJU Int. 2017, 120, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Cooperberg, M.R.; Simko, J.P.; Cowan, J.E.; et al. Validation of a cell-cycle progression gene panel to improve risk stratification in a contemporary prostatectomy cohort. J. Clin. Oncol. 2013, 31, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Kagawa-Singer, M.; Valdez Dadia, A.; Yu, M.C.; et al. Cancer, culture, and health disparities: Time to chart a new course? CA Cancer J. Clin. 2010, 60, 12–39. [Google Scholar] [CrossRef] [PubMed]
- Canter, D.J.; Reid, J.; Latsis, M.; et al. Comparison of the prognostic utility of the cell cycle progression score for predicting clinical outcomes in African American and Non-African American men with localized prostate cancer. Eur. Urol. 2019, 75, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Erho, N.; Crisan, A.; Vergara, I.A.; et al. Discovery and validation of a prostate cancer genomic classifier that predicts early metastasis following radical prostatectomy. PLoS ONE 2013, 8, e66855. [Google Scholar] [CrossRef]
- Ross, A.E.; Feng, F.Y.; Ghadessi, M.; et al. A genomic classifier predicting metastatic disease progression in men with biochemical recurrence after prostatectomy. Prostate Cancer Prostatic Dis. 2014, 17, 64–69. [Google Scholar] [CrossRef]
- Ross, A.E.; Johnson, M.H.; Yousefi, K.; et al. Tissue-based genomics augments post-prostatectomy risk stratification in a natural history cohort of intermediate- and high-risk men. Eur. Urol. 2016, 69, 157–165. [Google Scholar] [CrossRef]
- Klein, E.A.; Yousefi, K.; Haddad, Z.; et al. A genomic classifier improves prediction of metastatic disease within 5 years after surgery in node-negative high-risk prostate cancer patients managed by radical prostatectomy without adjuvant therapy. Eur. Urol. 2015, 67, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Spratt, D.E.; Dai, D.L.Y.; Den, R.B.; et al. Performance of a prostate cancer genomic classifier in predicting metastasis in men with prostate-specific antigen persistence postprostatectomy. Eur. Urol. 2018, 74, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Cooperberg, M.R.; Davicioni, E.; Anamaria Crisan, A.; et al. Combined value of validated clinical and genomic risk stratification tools for predicting prostate cancer mortality in a high-risk prostatectomy cohort. Eur. Urol. 2015, 67, 326–333. [Google Scholar] [CrossRef]
- Karnes, R.J.; Choeurng, V.; Ross, A.E.; et al. Validation of a genomic risk classifier to predict prostate cancer-specific mortality in men with adverse pathologic features. Eur. Urol. 2018, 73, 168–175. [Google Scholar] [CrossRef]
- Kim, H.L.; Li, P.; Huang, H.-C.; et al. Validation of the Decipher test for predicting adverse pathology in candidates for prostate cancer active surveillance. Prostate Cancer Prostatic Dis. 2019, 22, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Herlemann, A.; Huang, H.-C.; Alam, R.; et al. Decipher identifies men with otherwise clinically favorable-intermediate risk disease who may not be good candidates for active surveillance. Prostate Cancer Prostatic Dis. 2020, 23, 136–143. [Google Scholar] [CrossRef]
- Zhao, S.G.; Chang, S.L.; Spratt, D.E.; et al. Development and validation of a 24-gene predictor of response to postoperative radiotherapy in prostate cancer: A matched, retrospective analysis. Lancet Oncol 2016, 17, 1612–1620. [Google Scholar] [CrossRef]
This is an open access article under the terms of a license that permits non-commercial use, provided the original work is properly cited. © 2020 The Authors. Société Internationale d'Urologie Journal, published by the Société Internationale d'Urologie, Canada.
Share and Cite
Lonergan, P.E.; Washington, S.L., III; Meng, M.V.; Eapen, R. The Clinical Applications of Tissue Biomarkers in Prostate Cancer. Soc. Int. Urol. J. 2020, 1, 23-29. https://doi.org/10.48083/TABR2149
Lonergan PE, Washington SL III, Meng MV, Eapen R. The Clinical Applications of Tissue Biomarkers in Prostate Cancer. Société Internationale d’Urologie Journal. 2020; 1(1):23-29. https://doi.org/10.48083/TABR2149
Chicago/Turabian StyleLonergan, Peter E., Samuel L. Washington, III, Maxwell V. Meng, and Renu Eapen. 2020. "The Clinical Applications of Tissue Biomarkers in Prostate Cancer" Société Internationale d’Urologie Journal 1, no. 1: 23-29. https://doi.org/10.48083/TABR2149
APA StyleLonergan, P. E., Washington, S. L., III, Meng, M. V., & Eapen, R. (2020). The Clinical Applications of Tissue Biomarkers in Prostate Cancer. Société Internationale d’Urologie Journal, 1(1), 23-29. https://doi.org/10.48083/TABR2149




