A Unified Map of Airway Interactions: Secretome and Mechanotransduction Loops from Development to Disease
Highlights
- First closed-loop model mapping bidirectional secretome–mechanotransduction feedback in airways, where mechanical cues trigger cytokine release and vice versa through YAP/TAZ signalling.
- A novel cellular specialization framework defining epithelial cells as environmental activators, smooth muscle as mechanical actuators, and chondrocytes as calcium-dependent regulators in airway homeostasis.
- Enables targeted therapy of airway diseases by interrupting pathological feedback loops (e.g., YAP/TAZ-mediated feed-forward stiffness traps in asthma/COPD).
- Provides a system-based framework for airway tissue engineering by incorporating mechanotransduction feedback loops essential for functional airway constructs.
Abstract
1. Introduction: Gaps in Mapping Airway Interactomes
Anatomic Scope of the Review
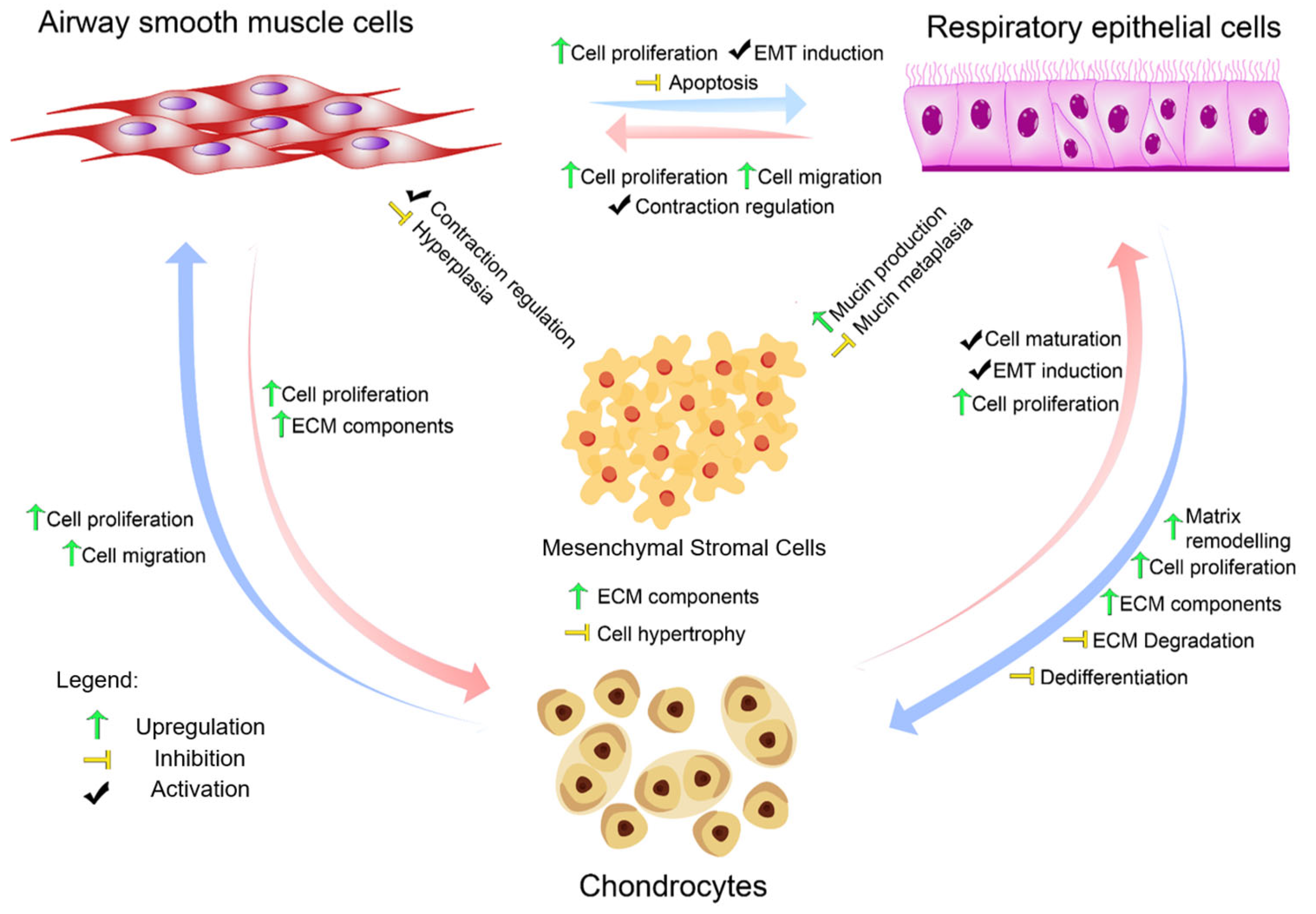
2. Airway Cell Specialisation in a Feedback Loop Model
2.1. Dynamic Secretome: Mechanical Drivers and Reciprocal Rewiring
| Secretory Signal | Stimulant | Effect on Airway Cells | References |
|---|---|---|---|
| Secreting Cell: Mesenchymal Stromal Cell | |||
| Activin A | Differentiation regulation | [66,67] | |
| Angiopoietin-1 | Vascular stabilisation | [68] | |
| Angiopoietin-2 | Shear forces | Angiogenic remodelling | [69] |
| Bone morphogenic protein-2 and 4 | Cyclic tensile strain, PGE2 | [70,71,72] | |
| Connective tissue Growth Factor | Fibrotic remodelling | [46,73,74,75] | |
| Fibroblast Growth Factor-2 | Hypoxia, TNF-α | Proliferation effects | [76,77] |
| Hepatocyte Growth Factor | Hypoxia, TNF-α | Epithelial repair | [76,77] |
| Insulin Growth Factor-1 | Hypoxia, TNF-α | Proliferation effects | [76,77,78,79] |
| Interleukin-1 | Inflammatory signalling | [79] | |
| Interleukin-6, 19, 23 | Cyclic tensile strain, Dexamethasone | Inflammatory modulation | [80] |
| Interleukin-7 | Lymphocyte support | [81] | |
| Interleukin-8 | Neutrophil chemotaxis | [80] | |
| Interleukin-10, 19, 20 | Anti-inflammatory effects | ||
| Osteoprotegerin | [67] | ||
| Platelet-derived Growth Factor | Proliferation effects, Remodelling signal | [82] | |
| Transforming Growth Factor-β | Hypoxia, TNF-α | Airway cell contraction and ECM remodelling across cell types | [67,77,83] |
| Vascular endothelial Growth Factor | Hypoxia, TNF-α | Angiogenic/remodelling cues affecting multiple airway cells | [67,76,77,78,79,84] |
| Secreting cell: Chondrocyte | |||
| Adrenomedullin | Hypoxia | Vasodilation, anti-apoptotic | [85,86] |
| Angiopoietin-like 4 | Hypoxia | [87] | |
| Angiopoietin-like 7 | Mechanical stress | Angiogenic signalling | [46,73] |
| Connective tissue Growth Factor | TGF-β, mechanical stress | Induces EMT in airway epithelial cells; supports perichondrium formation via fibroblast generation | [46,73,74,75,88] |
| Chitinase 3-like 2 | Inflammatory cytokines | ERK activation | [46,73,89] |
| Epidermal Growth Factor | EGF | Triggers chondrocyte PGE2 release, which increases AEC proliferation | [90,91] |
| Fibroblast Growth Factor-2 | Interleukin-1β | Maintains epithelial integrity; supports barrier/homeostasis | [92] |
| Interleukin-6 | Fluid shear stress | Pro-inflammatory activation | [93] |
| Nitric Oxide | Interleukin-1 | Vasodilation | [94] |
| Osteomodulin | Regulates mineralization | [46,73,95] | |
| Prostaglandin E2 | Increases proliferation of AECs (via paracrine loop). | [90] | |
| Transforming Growth Factor-α | [91] | ||
| Vascular endothelial Growth Factor | Angiogenic/remodelling cues affecting multiple airway cells | [96] | |
| Secreting cell: Airway Smooth Muscle Cell | |||
| Adrenomedullin | IL-1β, TNF-α | Vasodilation, angiogenesis | [97,98] |
| Amphiregulin | TNF-α, IL-4 | In AECs: ↑VEGF, ↑PGE2, ↑COX-2, ↑CXCL8; modulates ASM contraction/proliferation | [99,100] |
| Connective tissue Growth Factor | Injury, TGF-β | Overexpression promotes AEC senescence (pathologic); may drive EMT-like changes | [101,102,103] |
| Fibroblast Growth Factor-2 | TNF-α, IL-1β M | Mitogenic across airway cells (general) | [98,104] |
| Fibroblast Growth Factor-9 | Hypoxia, mechanical stress | Mitogenic across airway cells (general) | [105,106] |
| Fibroblast Growth Factor-10 | Epithelial injury | Promotes epithelial repair | [103,107] |
| Interleukin-6 | TNF-α, epithelial co-culture | Inflammatory response activation | [107,108,109] |
| Interleukin-8 | Interleukin-1β, TNF-α, epithelial injury | Neutrophil chemotaxis | [99,107,110] |
| Nerve Growth Factor | Interleukin-1β, inflammatory mediators | Neuronal sensitization | [98,111] |
| Nitric Oxide | Inflammatory cytokines | Smooth muscle relaxation | [106,108] |
| Prostaglandin E2 | Mechanical stress, cytokines | Bronchodilation, anti-inflammatory | [99,106] |
| Transforming Growth Factor-α | EGF receptor activation | Epithelial proliferation | [91,100] |
| Transforming Growth Factor-β1 | Mechanical injury, hypoxia | Drives ECM remodelling; promotes pathological changes across cell types (context-dependent) | [104,112] |
| Vascular endothelial Growth Factor | Angiotensin-2, Endothelin-1, TGF-β1, Bradykinin, | IL-4, IL-5, IL-13, PGE2 Angiogenic/remodelling cues affecting multiple airway cells | [113,114] |
| TGF-β1 | Mechanical stress, injury | ECM remodelling; activates epithelial responses (context-dependent) | [102,115] |
| Stem cell factor | Neutrophil elastase, Increased matrix stiffness | Mast cell activation | [104,116] |
| Secreting cell: Airway Epithelial Cell | |||
| Adrenomedullin | [117,118] | ||
| Amphiregulin | Cigarette smoke | [119] | |
| Angiopoietin | [117] | ||
| Chitinase 3-like 1 | Viral dsRNA, chitin | Induces IL-8 secretion | [46,89,120,121,122] |
| Endothelin-1 | Compression | ASMC proliferation, contraction | [117,123,124] |
| Epidermal Growth Factor | Induces chondrocyte PGE2 release → increases AEC proliferation (paracrine loop) | [91,117] | |
| Insulin Growth Factor-1 | Mitogenic across airway cells (general) | [117,125] | |
| Interleukin-1β | Thrombin, Trypsin, TNF-α | Stimulates chondrocyte FGF-2 secretion to maintain epithelial integrity | [29] |
| Interleukin-4, 10, 13, 22 | Induces mucus hyperproduction; promotes ciliated differentiation | [126] | |
| Interleukin-6 | Thrombin, Trypsin | [29,127] | |
| Interleukin-8 | Thrombin, Trypsin, TNF-α | [29,127] | |
| Nitric Oxide | Relaxes ASM by decreasing Ca2+ oscillations | [128] | |
| Platelet-derived Growth Factor (PDGF) | Inflammatory cytokines | Smooth muscle proliferation | [129] |
| Interleukin-11 | [29] | ||
| Prostaglandin E2 | Thrombin, Trypsin | Relaxes ASM (paracrine effect) | [127] |
| Prostaglandin D2 | Allergen exposure, inflammation | Bronchoconstriction | [130] |
| Transforming Growth Factor-β1, β2 | Thrombin, Trypsin, Hypoxia, Amphiregulin | Promotes ECM remodelling; cross-talk with ASM and cartilage | [29,99,117,131] |
| Tumour necrosis factor α | [29] | ||
| Vascular endothelial Growth Factor | Thrombin, Trypsin | Angiogenic/remodelling cues affecting multiple airway cells | [99,127,131] |
2.2. Mechanotransduction and ECM Feedback: YAP/TAZ Networks Unveiled
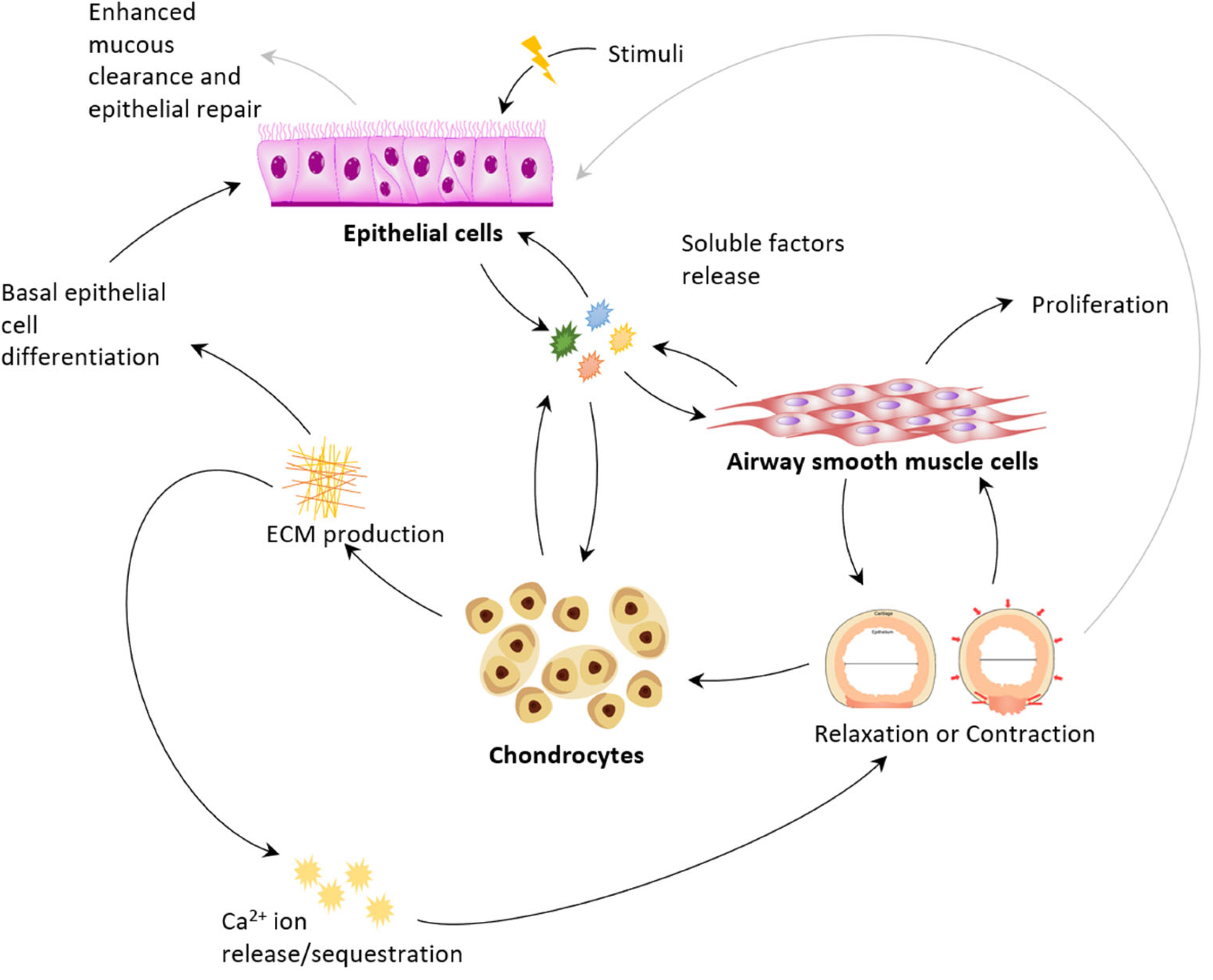
2.3. Secretome Mechanotransduction Feedback Signalling: Closed Loop Model and Control Points
3. Interactions of Airway Cells
3.1. Physiology: Epithelium as Activator Smooth Muscle as Actuator Cartilage as Regulator
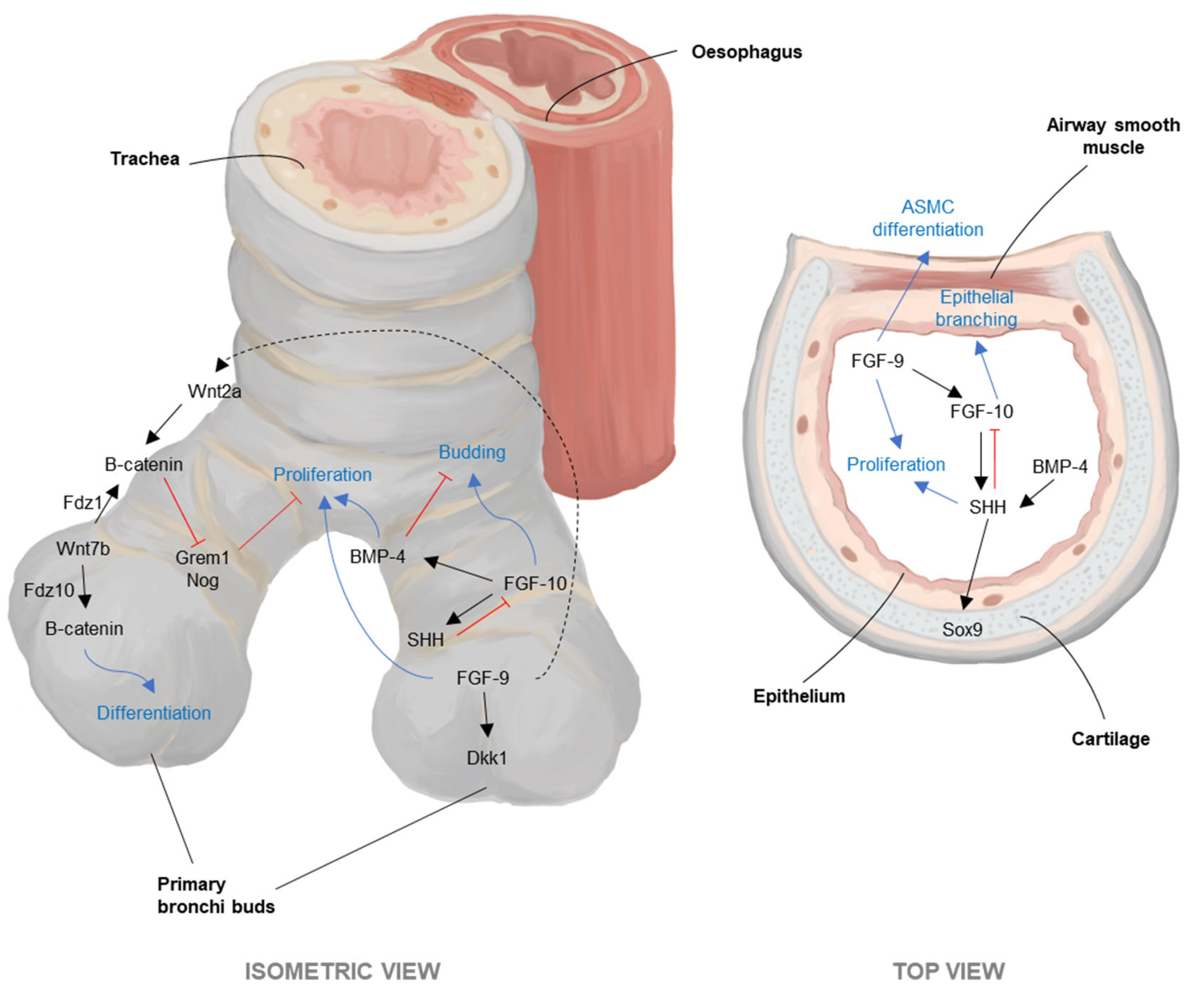
3.2. Development: ASM FGF10 and Peristalsis Pattern Cartilage and Epithelial Differentiation
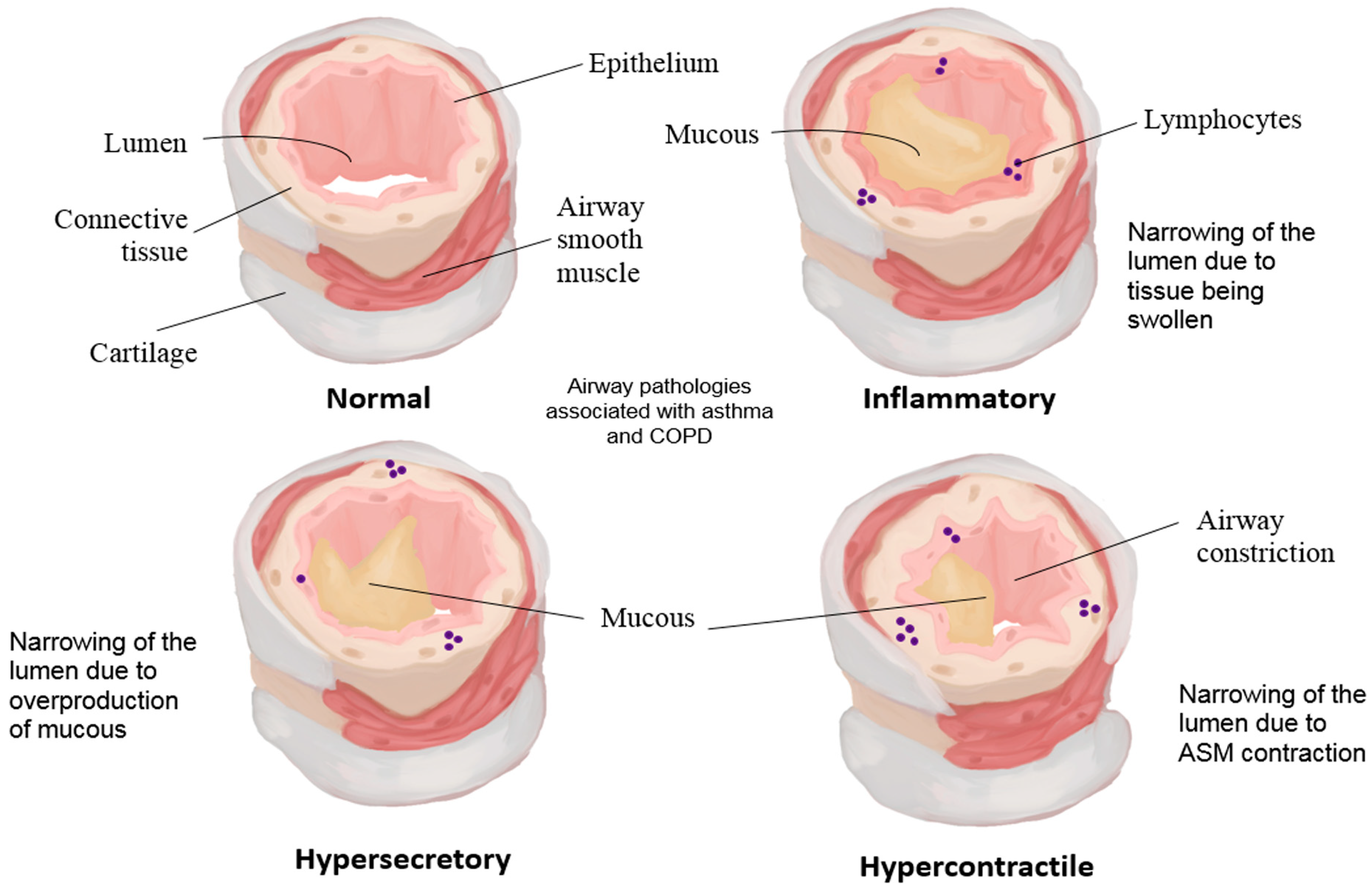
3.3. Disease: Developmental Programmes Misapplied in Asthma and COPD and the Feed-Forward Stiffness Trap
3.4. Mesenchymal Stromal Cells as Global Mediators
4. Implications for Tissue Engineering and Therapy Design
4.1. Composition and Failure Modes of Current Constructs
4.2. Paradigm Shift: From Cell-Centric to Systems-Level Engineering
4.3. Translational Considerations: Animal Model Selection and Scaling Effects
4.4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MSC | Mesenchymal Stromal Cell |
| AEC | Airway Epithelial Cell |
| ASMC | Airway Smooth Muscle Cell |
| ECM | Extracellular Matrix |
| YAP | Yes-associated Protein |
| TAZ | Transcriptional Coactivator with PDZ-binding Motif |
| TRPV4 | Transient Receptor Potential Vanilloid 4 |
| FGF-10 | Fibroblast Growth Factor 10 |
| COPD | Chronic Obstructive Pulmonary Disease |
| TGF-β | Transforming Growth Factor Beta |
| PGE2 | Prostaglandin E2 |
| NO | Nitric Oxide |
| IL-1β | Interleukin 1 Beta |
| VEGF | Vascular Endothelial Growth Factor |
| CTGF | Connective Tissue Growth Factor |
| EGF | Epidermal Growth Factor |
| IGF | Insulin Growth Factor |
| PDK1 | Three-Phosphoinositide Dependent Protein Kinase 1 |
| ROCK | Rho-associated, coiled-coil containing protein kinase |
| EMT | Epithelial–Mesenchymal Transition |
References
- Pierce, R.J.; Worsnop, C.J. Upper Airway function and dysfunction in respiration. Clin. Exp. Pharmacol. Physiol. 1999, 26, 1–10. [Google Scholar] [CrossRef]
- Joo, H.; Park, S.-Y.; Park, S.; Kim, S.-H.; Cho, Y.; Yoo, K.; Jung, K.; Rhee, C. Phenotype of Asthma-COPD Overlap in COPD and Severe Asthma Cohorts. J. Korean Med. Sci. 2022, 37, e236. [Google Scholar] [CrossRef]
- Kume, H.; Watanabe, N.; Suzuki, Y. Airway Disorders as Predictive Factors of Exacerbations in Asthma and COPD. In Airway Management in Emergency Medicine; Aslanidis, T., Bersot, C.D.A., Eds.; IntechOpen: London, UK, 2023. [Google Scholar]
- ten Hallers, E.J.O.; Rakhorst, G.; Marres, H.A.M.; Jansen, J.A.; van Kooten, T.G.; Schutte, H.K.; van Loon, J.P.; van der Houwen, E.B.; Verkerke, G.J. Animal models for tracheal research. Biomaterials 2004, 25, 1533–1543. [Google Scholar] [CrossRef] [PubMed]
- Vacanti, C.A.; Paige, K.T.; Kim, W.S.; Sakata, J.; Upton, J.; Vacanti, J.P. Experimental tracheal replacement using tissue-engineered cartilage. J. Pediatr. Surg. 1994, 29, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Wechselberger, G.; Russell, R.C.; Neumeister, M.W.; Schoeller, T.; Piza-Katzer, H.; Rainer, C. Successful transplantation of three tissue-engineered cell types using capsule induction technique and fibrin glue as a delivery vehicle. Plast. Reconstr. Surg. 2002, 110, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Weidenbecher, M.; Tucker, H.M.; Gilpin, D.A.; Dennis, J.E. Tissue-engineered trachea for airway reconstruction. Laryngoscope 2009, 119, 2118–2123. [Google Scholar] [CrossRef]
- Kojima, K.; Bonassar, L.J.; Roy, A.K.; Vacanti, C.A.; Cortiella, J. Autologous tissue-engineered trachea with sheep nasal chondrocytes. J. Thorac. Cardiovasc. Surg. 2002, 123, 1177–1184. [Google Scholar] [CrossRef]
- Olze, H.; Kaschke, O.; Müller, W.D. Investigations to improve the design of an alloplastic epithelialized tracheal replacement. HNO 1997, 45, 453–459. [Google Scholar] [CrossRef]
- Kim, J.; Suh, S.W.; Shin, J.Y.; Kim, J.H.; Choi, Y.S.; Kim, H. Replacement of a tracheal defect with a tissue-engineered prosthesis: Early results from animal experiments. J. Thorac. Cardiovasc. Surg. 2004, 128, 124–129. [Google Scholar] [CrossRef]
- Gonfiotti, A.; Jaus, M.O.; Barale, D.; Baiguera, S.; Comin, C.; Lavorini, F.; Fontana, G.; Sibila, O.; Rombolà, G.; Jungebluth, P.; et al. The first tissue-engineered airway transplantation: 5-year follow-up results. Lancet 2014, 383, 238–244, Retracted in Lancet 2023, 402, 1510.. [Google Scholar] [CrossRef]
- Vogel, G. Report Finds Trachea Surgeon Committed Misconduct. Available online: http://www.sciencemag.org/news/2015/05/report-finds-trachea-surgeon-committed-misconduct (accessed on 27 April 2017).
- Kojima, K.; Bonassar, L.J.; Roy, A.K.; Mizuno, H.; Cortiella, J.; Vacanti, C.A. A composite tissue-engineered trachea using sheep nasal chondrocyte and epithelial cells. FASEB J. 2003, 17, 823–828. [Google Scholar] [CrossRef]
- Gao, M.; Zhang, H.; Dong, W.; Bai, J.; Gao, B.; Xia, D.; Feng, B.; Chen, M.; He, X.; Yin, M.; et al. Tissue-engineered trachea from a 3D-printed scaffold enhances whole-segment tracheal repair. Sci. Rep. 2017, 7, 5246. [Google Scholar] [CrossRef]
- Hatachi, G.; Machino, R.; Tsuchiya, T.; Taura, Y.; Elgalad, A.; Taniguchi, D.; Takagi, K.; Matsumoto, K.; Gunge, K.; Matsuo, N.; et al. Scaffold-free trachea regeneration by tissue engineering with bio-3D printing†. Interact. Cardiovasc. Thorac. Surg. 2018, 26, 745–752. [Google Scholar] [CrossRef]
- Lin, C.; Yao, E.; Zhang, K.; Jiang, X.; Croll, S.; Thompson-Peer, K.; Chuang, P.-T. YAP is essential for mechanical force production and epithelial cell proliferation during lung branching morphogenesis. eLife 2017, 6, e21130. [Google Scholar] [CrossRef]
- Sanchez-Esteban, J.; Tsai, S.-W.; Sang, J.; Qin, J.; Torday, J.S.; Rubin, L.P. Effects of Mechanical Forces on Lung-Specific Gene Expression. Am. J. Med. Sci. 1998, 316, 200–204. [Google Scholar] [CrossRef]
- Yu, X.; Feng, L.; Han, Z.; Wu, B.; Wang, S.; Xiao, Y.; Li, F.; Zhang, L.; Cao, B.; Di, X.; et al. Crosstalk of dynamic functional modules in lung development of rhesus macaques. Mol. Biosyst. 2016, 12, 1342–1349. [Google Scholar] [CrossRef] [PubMed]
- Mieczkowski, B.; Seavey, B.F. Anatomy, Head and Neck, Trachea. In StatPearls; StatPearls Publishing: Tampa, FL, USA, 2023. [Google Scholar]
- Chawla, A. Imaging of Large and Small Airways: Basic to Advanced. In Thoracic Imaging; Springer: Singapore, 2019; pp. 31–64. [Google Scholar]
- Sin, D.D. What Single Cell RNA Sequencing Has Taught Us about Chronic Obstructive Pulmonary Disease. Tuberc. Respir. Dis. 2024, 87, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Li, J.; Che, L.; Yang, R.; Wu, Z.; Hu, G.; Zou, W.; Zhao, Z.; Zhou, Y.; Jiang, X.; et al. Single-cell transcriptomics reveals e-cigarette vapor-induced airway epithelial remodeling and injury. Respir. Res. 2024, 25, 353. [Google Scholar] [CrossRef] [PubMed]
- Renaut, S.; Saavedra Armero, V.; Boudreau, D.K.; Gaudreault, N.; Desmeules, P.; Thériault, S.; Mathieu, P.; Joubert, P.; Bossé, Y. Single-cell and single-nucleus RNA-sequencing from paired normal-adenocarcinoma lung samples provide both common and discordant biological insights. PLoS Genet. 2024, 20, e1011301. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, Y.; Guo, L.; Qian, J.; Ge, J.; Sinner, D.; Ding, H.; Califano, A.; Cardoso, W.V. Airway basal cells show regionally distinct potential to undergo metaplastic differentiation. eLife 2022, 11, e80083. [Google Scholar] [CrossRef]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef]
- Archer, C.W.; Francis-West, P. The chondrocyte. Int. J. Biochem. Cell Biol. 2003, 35, 401–404. [Google Scholar] [CrossRef]
- Rojas, M.; Xu, J.; Woods, C.R.; Mora, A.L.; Spears, W.; Roman, J.; Brigham, K.L. Bone marrow–derived mesenchymal stem cells in repair of the injured lung. Am. J. Respir. Cell Mol. Biol. 2005, 33, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Amrani, Y.; Panettieri, R.A. Airway smooth muscle: Contraction and beyond. Int. J. Biochem. Cell Biol. 2003, 35 (Suppl. S1), 272–276. [Google Scholar] [CrossRef] [PubMed]
- Mills, P.R.; Davies, R.J.; Devalia, J.L. Airway Epithelial Cells, Cytokines, and Pollutants. Am. J. Respir. Crit. Care Med. 1999, 160, S38–S43. [Google Scholar] [CrossRef] [PubMed]
- Jesenak, M.; Durdik, P.; Oppova, D.; Franova, S.; Diamant, Z.; Golebski, K.; Banovcin, P.; Vojtkova, J.; Novakova, E. Dysfunctional mucociliary clearance in asthma and airway remodeling—New insights into an old topic. Respir. Med. 2023, 218, 107372. [Google Scholar] [CrossRef] [PubMed]
- Roth, D.; Şahin, A.T.; Ling, F.; Tepho, N.; Senger, C.N.; Quiroz, E.J.; Calvert, B.A.; van der Does, A.M.; Güney, T.G.; Glasl, S.; et al. Structure and function relationships of mucociliary clearance in human and rat airways. Nat. Commun. 2025, 16, 2446. [Google Scholar] [CrossRef]
- Walentek, P. Signaling Control of Mucociliary Epithelia: Stem Cells, Cell Fates, and the Plasticity of Cell Identity in Development and Disease. Cells Tissues Organs 2022, 211, 736–753. [Google Scholar] [CrossRef]
- Guo, T.; He, C.; Venado, A.; Zhou, Y. Extracellular Matrix Stiffness in Lung Health and Disease. Compr. Physiol. 2022, 12, 3523–3558. [Google Scholar] [CrossRef]
- Horta, C.A.; Doan, K.; Yang, J. Mechanotransduction pathways in regulating epithelial-mesenchymal plasticity. Curr. Opin. Cell Biol. 2023, 85, 102245. [Google Scholar] [CrossRef]
- Feng, K.N.; Meng, P.; Zou, X.L.; Zhang, M.; Li, H.K.; Yang, H.L.; Li, H.T.; Zhang, T.T. IL-37 protects against airway remodeling by reversing bronchial epithelial–mesenchymal transition via IL-24 signaling pathway in chronic asthma. Respir. Res. 2022, 23, 244. [Google Scholar] [CrossRef]
- Novak, C.M.; Wheat, J.S.; Ghadiali, S.N.; Ballinger, M.N. Mechanomemory of pulmonary fibroblasts demonstrates reversibility of transcriptomics and contraction phenotypes. Biomaterials 2025, 314, 122830. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Ma, X.Y.; Liu, W.; Meridew, J.A.; Jones, D.L.; Haak, A.J.; Sicard, D.; Ligresti, G.; Tschumperlin, D.J. Nascent Lung Organoids Reveal Epithelium- and Bone Morphogenetic Protein–mediated Suppression of Fibroblast Activation. Am. J. Respir. Cell Mol. Biol. 2019, 61, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Miethe, S.; Kattler, K.; Colakoglu, B.; Walter, J.; Schneider-Daum, N.; Herr, C.; Garn, H.; Ritzmann, F.; Bals, R.; et al. Mutual Regulation of Transcriptomes between Murine Pneumocytes and Fibroblasts Mediates Alveolar Regeneration in Air-Liquid Interface Cultures. Am. J. Respir. Cell Mol. Biol. 2024, 70, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Song, Y.; Soto, J.; Hoffman, T.; Lin, X.; Zhang, A.; Chen, S.; Massad, R.N.; Han, X.; Qi, D.; et al. Viscoelastic extracellular matrix enhances epigenetic remodeling and cellular plasticity. Nat. Commun. 2025, 16, 4054. [Google Scholar] [CrossRef]
- Chang, Y.; Lee, J.W.N.; Holle, A.W. The mechanobiology of fibroblast activation in disease. APL Bioeng. 2025, 9, 021505. [Google Scholar] [CrossRef]
- Polacek, M.; Bruun, J.A.; Johansen, O.; Martinez, I. Differences in the secretome of cartilage explants and cultured chondrocytes unveiled by SILAC technology. J. Orthop. Res. 2010, 28, 1040–1049. [Google Scholar] [CrossRef]
- Polacek, M.; Bruun, J.-A.; Elvenes, J.; Figenschau, Y.; Martinez, I. The secretory profiles of cultured human articular chondrocytes and mesenchymal stem cells: Implications for autologous cell transplantation strategies. Cell Transplant. 2011, 20, 1381–1393. [Google Scholar] [CrossRef]
- Rosenthal, A.K.; Gohr, C.M.; Ninomiya, J.; Wakim, B.T. Proteomic analysis of articular cartilage vesicles from normal and osteoarthritic cartilage. Arthritis Rheum. 2011, 63, 401–411. [Google Scholar] [CrossRef]
- Cui, Y.; Yu, C.; Lu, Q.; Huang, X.; Lin, W.; Huang, T.; Cao, L.; Yang, Q. The Function of RhoA/ROCK Pathway and MYOCD in Airway Remodeling in Asthma. Int. Arch. Allergy Immunol. 2025, 186, 103–119. [Google Scholar] [CrossRef]
- Turcatel, G.; Millette, K.; Thornton, M.; Leguizamon, S.; Grubbs, B.; Shi, W.; Warburton, D. Cartilage rings contribute to the proper embryonic tracheal epithelial differentiation, metabolism, and expression of inflammatory genes. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 312, L196–L207. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, F.; Luo, R.; Cui, Y.; Zhang, Z.; Xu, M.; Zhao, Y.; He, Y.; Yang, W.; Li, N.; et al. YAP Alleviates Pulmonary Fibrosis Through Promoting Alveolar Regeneration via Modulating the Stemness of Alveolar Type 2 Cells. Stem Cells Dev. 2024, 33, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Rossios, C.; Pavlidis, S.; Gibeon, D.; Mumby, S.; Durham, A.; Ojo, O.; Horowitz, D.; Loza, M.; Baribaud, F.; Rao, N.; et al. Impaired innate immune gene profiling in airway smooth muscle cells from chronic cough patients. Biosci. Rep. 2017, 37, BSR20171090. [Google Scholar] [CrossRef] [PubMed]
- Hackett, N.R.; Shaykhiev, R.; Walters, M.S.; Wang, R.; Zwick, R.K.; Ferris, B.; Witover, B.; Salit, J.; Crystal, R.G. The Human Airway Epithelial Basal Cell Transcriptome. PLoS ONE 2011, 6, e18378. [Google Scholar] [CrossRef]
- Barrow, R.E.; Wang, C.Z.; Evans, M.J.; Herndon, D.N. Growth factors accelerate epithelial repair in sheep trachea. Lung 1993, 171, 335–344. [Google Scholar] [CrossRef]
- Retsch-Bogart, G.Z.; Stiles, A.D.; Moats-Staats, B.M.; Van Scott, M.R.; Boucher, R.C.; D’Ercole, A.J. Canine tracheal epithelial cells express the type 1 insulin-like growth factor receptor and proliferate in response to insulin-like growth factor I. Am. J. Respir. Cell Mol. Biol. 1990, 3, 227–234. [Google Scholar] [CrossRef]
- Lotz, M. Cytokines in Cartilage Injury and Repair. Clin. Orthop. Relat. Res. 2001, 391, S108–S115. [Google Scholar] [CrossRef]
- Pufe, T.; Harde, V.; Petersen, W.; Goldring, M.B.; Tillmann, B.; Mentlein, R. Vascular endothelial growth factor (VEGF) induces matrix metalloproteinase expression in immortalized chondrocytes. J. Pathol. 2004, 202, 367–374. [Google Scholar] [CrossRef]
- Aizawa, T.; Kon, T.; Einhorn, T.; Gerstenfeld, L. Induction of apoptosis in chondrocytes by tumor necrosis factor-alpha. J. Orthop. Res. 2001, 19, 785–796. [Google Scholar] [CrossRef]
- Ivkovic, S.; Yoon, B.S.; Popoff, S.N.; Safadi, F.F.; Libuda, D.E.; Stephenson, R.C.; Daluiski, A.; Lyons, K.M. Connective tissue growth factor coordinates chondrogenesis and angiogenesis during skeletal development. Development 2003, 130, 2779–2791. [Google Scholar] [CrossRef]
- Wang, Y.; Lou, S. Direct protective effect of interleukin-10 on articular chondrocytes in vitro. Chin. Med. J. 2001, 114, 723–725. [Google Scholar]
- Jikko, A.; Wakisaka, T.; Iwamoto, M.; Hiranuma, H.; Kato, Y.; Maeda, T.; Fujishita, M.; Fuchihata, H. Effects of interleukin-6 on proliferation and proteoglycan metabolism in articular chondrocyte cultures. Cell Biol. Int. 1998, 22, 615–621. [Google Scholar] [CrossRef]
- Govindaraju, V.; Michoud, M.-C.; Ferraro, P.; Arkinson, J.; Safka, K.; Valderrama-Carvajal, H.; Martin, J.G. The effects of interleukin-8 on airway smooth muscle contraction in cystic fibrosis. Respir. Res. 2008, 9, 76. [Google Scholar] [CrossRef] [PubMed]
- Kuperman, D.A.; Huang, X.; Koth, L.L.; Chang, G.H.; Dolganov, G.M.; Zhu, Z.; Elias, J.A.; Sheppard, D.; Erle, D.J. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat. Med. 2002, 8, 885. [Google Scholar] [CrossRef] [PubMed]
- Tschumperlin, D.J.; Drazen, J.M. Chronic effects of mechanical force on airways. Annu. Rev. Physiol. 2006, 68, 563–583. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Goswami, R.; Zhang, D.X.; Rahaman, S.O. TRPV4 regulates matrix stiffness and TGFβ1-induced epithelial-mesenchymal transition. J. Cell. Mol. Med. 2019, 23, 761–774. [Google Scholar] [CrossRef]
- Sharma, S.; Goswami, R.; Rahaman, S.O. The TRPV4-TAZ Mechanotransduction Signaling Axis in Matrix Stiffness- and TGFβ1-Induced Epithelial-Mesenchymal Transition. Cell. Mol. Bioeng. 2019, 12, 139–152. [Google Scholar] [CrossRef]
- Bandaru, P.; Cefaloni, G.; Vajhadin, F.; Lee, K.; Kim, H.-J.; Cho, H.-J.; Hartel, M.C.; Zhang, S.; Sun, W.; Goudie, M.J.; et al. Mechanical Cues Regulating Proangiogenic Potential of Human Mesenchymal Stem Cells through YAP-Mediated Mechanosensing. Small 2020, 16, 2001837. [Google Scholar] [CrossRef]
- Tan, Y.H.; Wang, K.C.W.; Chin, I.L.; Sanderson, R.W.; Li, J.; Kennedy, B.F.; Noble, P.B.; Choi, Y.S. Stiffness Mediated-Mechanosensation of Airway Smooth Muscle Cells on Linear Stiffness Gradient Hydrogels. Adv. Healthc. Mater. 2024, 13, 2304254. [Google Scholar] [CrossRef]
- Zhang, M.; Meng, N.; Wang, X.; Chen, W.; Zhang, Q. TRPV4 and PIEZO Channels Mediate the Mechanosensing of Chondrocytes to the Biomechanical Microenvironment. Membranes 2022, 12, 237. [Google Scholar] [CrossRef]
- Calloni, G.-W.; Stimamiglio, M.-A. Tuning mesenchymal stem cell secretome therapeutic potential through mechanotransduction. Biocell 2022, 46, 1375–1381. [Google Scholar] [CrossRef]
- Djouad, F.; Jackson, W.M.; Bobick, B.E.; Janjanin, S.; Song, Y.; Huang, G.T.; Tuan, R.S. Activin A expression regulates multipotency of mesenchymal progenitor cells. Stem Cell Res. Ther. 2010, 1, 11. [Google Scholar] [CrossRef]
- Burdon, T.J.; Paul, A.; Noiseux, N.; Prakash, S.; Shum-Tim, D. Bone Marrow Stem Cell Derived Paracrine Factors for Regenerative Medicine: Current Perspectives and Therapeutic Potential. Bone Marrow Res. 2011, 2011, 207326. [Google Scholar] [CrossRef]
- Fang, X.; Neyrinck, A.P.; Matthay, M.A.; Lee, J.W. Allogeneic human mesenchymal stem cells restore epithelial protein permeability in cultured human alveolar type II cells by secretion of angiopoietin-1. J. Biol. Chem. 2010, 285, 26211–26222. [Google Scholar] [CrossRef]
- Gardner, O.; Fahy, N.; Alini, M.; Stoddart, M. Differences in human mesenchymal stem cell secretomes during chondrogenic induction. Eur. Cells Mater. 2016, 30, 221–235. [Google Scholar] [CrossRef]
- Sumanasinghe, R.D.; Bernacki, S.H.; Loboa, E.G. Osteogenic differentiation of human mesenchymal stem cells in collagen matrices: Effect of uniaxial cyclic tensile strain on bone morphogenetic protein (BMP-2) mRNA expression. Tissue Eng. 2006, 12, 3459–3465. [Google Scholar] [CrossRef]
- Arikawa, T.; Omura, K.; Morita, I. Regulation of bone morphogenetic protein-2 expression by endogenous prostaglandin E2 in human mesenchymal stem cells. J. Cell. Physiol. 2004, 200, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Wislet-Gendebien, S.; Bruyere, F.; Hans, G.; Leprince, P.; Moonen, G.; Rogister, B. Nestin-positive mesenchymal stem cells favour the astroglial lineage in neural progenitors and stem cells by releasing active BMP4. BMC Neurosci. 2004, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Pan, Z.; Wan, H.; Li, Y.; Mao, G.; Zhao, J.; Zhang, F.; Pan, S. Mechanisms of Mechanical Stimulation in the Development of Respiratory System Diseases. Am. J. Physiol. Lung Cell Mol. Physiol. 2024, 327, L724–L739. [Google Scholar] [CrossRef] [PubMed]
- Omoto, S.; Nishida, K.; Yamaai, Y.; Shibahara, M.; Nishida, T.; Doi, T.; Asahara, H.; Nakanishi, T.; Inoue, H.; Takigawa, M. Expression and localization of connective tissue growth factor (CTGF/Hcs24/CCN2) in osteoarthritic cartilage. Osteoarthr. Cartil. 2004, 12, 771–778. [Google Scholar] [CrossRef]
- Fukunaga, T.; Yamashiro, T.; Oya, S.; Takeshita, N.; Takigawa, M.; Takano-Yamamoto, T. Connective tissue growth factor mRNA expression pattern in cartilages is associated with their type I collagen expression. Bone 2003, 33, 911–918. [Google Scholar] [CrossRef]
- Crisostomo, P.R.; Wang, Y.; Markel, T.A.; Wang, M.; Lahm, T.; Meldrum, D.R. Human mesenchymal stem cells stimulated by TNF-α, LPS, or hypoxia produce growth factors by an NFκB-but not JNK-dependent mechanism. Am. J. Physiol. Cell Physiol. 2008, 294, C675–C682. [Google Scholar] [CrossRef]
- Baraniak, P.R.; McDevitt, T.C. Stem cell paracrine actions and tissue regeneration. Regen. Med. 2010, 5, 121–143. [Google Scholar] [CrossRef]
- Chen, L.; Tredget, E.E.; Wu, P.Y.; Wu, Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE 2008, 3, e1886. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Li, T.S.; Suzuki, R.; Kobayashi, T.; Ito, H.; Ikeda, Y.; Matsuzaki, M.; Hamano, K. Cytokines produced by bone marrow cells can contribute to functional improvement of the infarcted heart by protecting cardiomyocytes from ischemic injury. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H886–H893. [Google Scholar] [CrossRef] [PubMed]
- Sumanasinghe, R.D.; Pfeiler, T.W.; Monteiro-Riviere, N.A.; Loboa, E.G. Expression of proinflammatory cytokines by human mesenchymal stem cells in response to cyclic tensile strain. J. Cell. Physiol. 2009, 219, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, Y.; Kanai, T.; Takahara, M.; Oshima, S.; Nakamura, T.; Okamoto, R.; Tsuchiya, K.; Watanabe, M. Bone marrow-mesenchymal stem cells are a major source of interleukin-7 and sustain colitis by forming the niche for colitogenic CD4 memory T cells. Gut 2013, 62, 1142–1152. [Google Scholar] [CrossRef]
- Windmolders, S.; De Boeck, A.; Koninckx, R.; Daniëls, A.; De Wever, O.; Bracke, M.; Hendrikx, M.; Hensen, K.; Rummens, J.-L. Mesenchymal stem cell secreted platelet derived growth factor exerts a pro-migratory effect on resident Cardiac Atrial appendage Stem Cells. J. Mol. Cell. Cardiol. 2014, 66, 177–188. [Google Scholar] [CrossRef]
- Spees, J.L.; Lee, R.H.; Gregory, C.A. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res. Ther. 2016, 7, 125. [Google Scholar] [CrossRef]
- Li, F.; Armstrong, G.B.; Tombran-Tink, J.; Niyibizi, C. Pigment epithelium derived factor upregulates expression of vascular endothelial growth factor by human mesenchymal stem cells: Possible role in PEDF regulated matrix mineralization. Biochem. Biophys. Res. Commun. 2016, 478, 1106–1110. [Google Scholar] [CrossRef]
- Chosa, E.; Hamada, H.; Kitamura, K.; Kuwasako, K.; Yanagita, T.; Eto, T.; Tajima, N. Expression of adrenomedullin and its receptor by chondrocyte phenotype cells. Biochem. Biophys. Res. Commun. 2003, 303, 379–386. [Google Scholar] [CrossRef]
- Velard, F.; Chatron-Colliet, A.; Côme, D.; Ah-Kioon, M.-D.; Lin, H.; Hafsia, N.; Cohen-Solal, M.; Ea, H.-K.; Lioté, F. Adrenomedullin and truncated peptide adrenomedullin(22-52) affect chondrocyte response to apoptotis in vitro: Downregulation of FAS protects chondrocyte from cell death. Sci. Rep. 2020, 10, 16740. [Google Scholar] [CrossRef]
- Murata, M.; Yudo, K.; Nakamura, H.; Chiba, J.; Okamoto, K.; Suematsu, N.; Nishioka, K.; Beppu, M.; Inoue, K.; Kato, T.; et al. Hypoxia upregulates the expression of angiopoietin-like-4 in human articular chondrocytes: Role of angiopoietin-like-4 in the expression of matrix metalloproteinases and cartilage degradation. J. Orthop. Res. 2009, 27, 50–57. [Google Scholar] [CrossRef]
- Huang, B.-L.; Brugger, S.M.; Lyons, K.M. Stage-specific control of connective tissue growth factor (CTGF/CCN2) expression in chondrocytes by Sox9 and β-catenin. J. Biol. Chem. 2010, 285, 27702–27712. [Google Scholar] [CrossRef]
- Hu, B.; Trinh, K.; Figueira, W.F.; Price, P.A. Isolation and sequence of a novel human chondrocyte protein related to mammalian members of the chitinase protein family. J. Biol. Chem. 1996, 271, 19415–19420. [Google Scholar] [CrossRef] [PubMed]
- Huh, Y.-H.; Kim, S.-H.; Kim, S.-J.; Chun, J.-S. Differentiation status-dependent regulation of cyclooxygenase-2 expression and prostaglandin E2 production by epidermal growth factor via mitogen-activated protein kinase in articular chondrocytes. J. Biol. Chem. 2003, 278, 9691–9697. [Google Scholar] [CrossRef] [PubMed]
- Ruocco, S.; Lallemand, A.; Tournier, J.M.; Gaillard, D. Expression and localization of epidermal growth factor, transforming growth factor-α, and localization of their common receptor in fetal human lung development. Pediatr. Res. 1996, 39, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Chien, S.-Y.; Huang, C.-Y.; Tsai, C.-H.; Wang, S.-W.; Lin, Y.-M.; Tang, C.-H. Interleukin-1β induces fibroblast growth factor 2 expression and subsequently promotes endothelial progenitor cell angiogenesis in chondrocytes. Clin. Sci. 2016, 130, 667–681. [Google Scholar] [CrossRef]
- Mohtai, M.; Gupta, M.K.; Donlon, B.; Ellison, B.; Cooke, J.; Gibbons, G.; Schurman, D.J.; Smith, R.L. Expression of interleukin-6 in osteoarthritic chondrocytes and effects of fluid-induced shear on this expression in normal human chondrocytes in vitro. J. Orthop. Res. 1996, 14, 67–73. [Google Scholar] [CrossRef]
- Stadler, J.; Stefanovic-Racic, M.; Billiar, T.R.; Curran, R.D.; Mcintyre, L.A.; Georgescu, H.I.; Simmons, R.L.; Evans, C.H. Articular chondrocytes synthesize nitric oxide in response to cytokines and lipopolysaccharide. J. Immunol. 1991, 147, 3915–3920. [Google Scholar] [CrossRef]
- Zappia, J.; Tong, Q.; Van der Cruyssen, R.; Cornelis, F.M.F.; Lambert, C.; Pinto Coelho, T.; Grisart, J.; Kague, E.; Lories, R.J.; Muller, M.; et al. Osteomodulin downregulation is associated with osteoarthritis development. Bone Res. 2023, 11, 49. [Google Scholar] [CrossRef] [PubMed]
- Lingaraj, K.; Poh, C.K.; Wang, W. Vascular endothelial growth factor (VEGF) is expressed during articular cartilage growth and re-expressed in osteoarthritis. Ann. Acad. Med. Singap. 2010, 39, 399. [Google Scholar] [CrossRef] [PubMed]
- Upton, P.D.; Wharton, J.; Davie, N.; Ghatei, M.A.; Smith, D.M.; Morrell, N.W. Differential adrenomedullin release and endothelin receptor expression in distinct subpopulations of human airway smooth-muscle cells. Am. J. Respir. Cell Mol. Biol. 2001, 25, 316–325. [Google Scholar] [CrossRef]
- Alagappan, V.K.T.; McKay, S.; Widyastuti, A.; Garrelds, I.M.; Bogers, A.J.J.C.; Hoogsteden, H.C.; Hirst, S.J.; Sharma, H.S. Proinflammatory cytokines upregulate mRNA expression and secretion of vascular endothelial growth factor in cultured human airway smooth muscle cells. Cell Biochem. Biophys. 2005, 43, 119–129. [Google Scholar] [CrossRef]
- Deacon, K.; Knox, A.J. Human airway smooth muscle cells secrete amphiregulin via bradykinin/COX-2/PGE(2), inducing COX-2, CXCL8, and VEGF expression in airway epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 309, L237–L249. [Google Scholar] [CrossRef]
- Shim, J.Y.; Park, S.W.; Kim, D.S.; Shim, J.W.; Jung, H.L.; Park, M.S. The Effect of Interleukin-4 and Amphiregulin on the Proliferation of Human Airway Smooth Muscle Cells and Cytokine Release. J. Korean Med. Sci. 2008, 23, 857–863. [Google Scholar] [CrossRef]
- Johnson, P.R.A.; Burgess, J.K.; Ge, Q.; Poniris, M.; Boustany, S.; Twigg, S.M.; Black, J.L. Connective Tissue Growth Factor Induces Extracellular Matrix in Asthmatic Airway Smooth Muscle. Am. J. Respir. Crit. Care Med. 2006, 173, 32–41. [Google Scholar] [CrossRef]
- Xie, S.; Sukkar, M.B.; Issa, R.; Oltmanns, U.; Nicholson, A.G.; Chung, K.F. Regulation of TGF-β1-induced connective tissue growth factor expression in airway smooth muscle cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005, 288, L68–L76. [Google Scholar] [CrossRef]
- Volckaert, T.; Dill, E.; Campbell, A.; Tiozzo, C.; Majka, S.; Bellusci, S.; De Langhe, S.P. Parabronchial smooth muscle constitutes an airway epithelial stem cell niche in the mouse lung after injury. J. Clin. Investig. 2011, 121, 4409–4419. [Google Scholar] [CrossRef]
- Oliveira, L.C.; Danilucci, T.M.; Chaves-Neto, A.H.; Campanelli, A.P.; Silva, T.C.; Oliveira, S.H. Tracheal Smooth Muscle Cells Stimulated by Stem Cell Factor-c-Kit Coordinate the Production of Transforming Growth Factor-beta1 and Fibroblast Growth Factor-2 Mediated by Chemokine (C-C Motif) Ligand 3. J. Interferon Cytokine Res. 2016, 36, 401–411. [Google Scholar] [CrossRef]
- Coffey, E.; Newman, D.R.; Sannes, P.L. Expression of Fibroblast Growth Factor 9 in Normal Human Lung and Idiopathic Pulmonary Fibrosis. J. Histochem. Cytochem. 2013, 61, 671–679. [Google Scholar] [CrossRef]
- Wen, F.-Q.; Liu, X.; Manda, W.; Terasaki, Y.; Kobayashi, T.; Abe, S.; Fang, Q.; Ertl, R.; Manouilova, L.; Rennard, S.I. TH2 Cytokine-enhanced and TGF-β-enhanced vascular endothelial growth factor production by cultured human airway smooth muscle cells is attenuated by IFN-γ and corticosteroids. J. Allergy Clin. Immunol. 2003, 111, 1307–1318. [Google Scholar] [CrossRef]
- O’Sullivan, M.J.; Jang, J.H.; Panariti, A.; Bedrat, A.; Ijpma, G.; Lemos, B.; Park, J.A.; Lauzon, A.M.; Martin, J.G. Airway Epithelial Cells Drive Airway Smooth Muscle Cell Phenotype Switching to the Proliferative and Pro-inflammatory Phenotype. Front. Physiol. 2021, 12, 687654. [Google Scholar] [CrossRef]
- Johnson, S.R.; Knox, A.J. Synthetic functions of airway smooth muscle in asthma. Trends Pharmacol. Sci. 1997, 18, 288–292. [Google Scholar] [CrossRef] [PubMed]
- McKay, S.; Hirst, S.J.; Haas, M.B.-d.; De Jongste, J.C.; Hoogsteden, H.C.; Saxena, P.R.; Sharma, H.S. Tumor necrosis factor-α enhances mRNA expression and secretion of interleukin-6 in cultured human airway smooth muscle cells. Am. J. Respir. Cell Mol. Biol. 2000, 23, 103–111. [Google Scholar] [CrossRef] [PubMed]
- John, M.; Au, B.-T.; Jose, P.J.; Lim, S.; Saunders, M.; Barnes, P.J.; Mitchell, J.A.; Belvisi, M.G.; Fan Chung, K. Expression and release of interleukin-8 by human airway smooth muscle cells: Inhibition by Th-2 cytokines and corticosteroids. Am. J. Respir. Cell Mol. Biol. 1998, 18, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Freund, V.; Pons, F.; Joly, V.; Mathieu, E.; Martinet, N.; Frossard, N. Upregulation of nerve growth factor expression by human airway smooth muscle cells in inflammatory conditions. Eur. Respir. J. 2002, 20, 458–463. [Google Scholar] [CrossRef]
- McKay, S.; De Jongste, J.C.; Saxena, P.R.; Sharma, H.S. Angiotensin II induces hypertrophy of human airway smooth muscle cells: Expression of transcription factors and transforming growth factor-β1. Am. J. Respir. Cell Mol. Biol. 1998, 18, 823–833. [Google Scholar] [CrossRef]
- Knox, A.J.; Corbett, L.; Stocks, J.; Holland, E.; Zhu, Y.M.; Pang, L. Human airway smooth muscle cells secrete vascular endothelial growth factor: Up-regulation by bradykinin via a protein kinase C and prostanoid-dependent mechanism. FASEB J. 2001, 15, 2480–2488. [Google Scholar] [CrossRef]
- Alagappan, V.K.T.; Willems-Widyastuti, A.; Seynhaeve, A.L.B.; Garrelds, I.M.; ten Hagen, T.L.M.; Saxena, P.R.; Sharma, H.S. Vasoactive peptides upregulate mRNA expression and secretion of vascular endothelial growth factor in human airway smooth muscle cells. Cell Biochem. Biophys. 2007, 47, 109–118. [Google Scholar] [CrossRef]
- Fehrenbach, H.; Wagner, C.; Wegmann, M. Airway remodeling in asthma: What really matters. Cell Tissue Res. 2017, 367, 551–569. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-Y.; Ho, S.-C.; Lin, H.-C.; Lin, S.-M.; Liu, C.-Y.; Huang, C.-D.; Wang, C.-H.; Chung, K.F.; Kuo, H.-P. Neutrophil-derived elastase induces TGF-β1 secretion in human airway smooth muscle via NF-κB pathway. Am. J. Respir. Cell Mol. Biol. 2006, 35, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Gerayeli, F.V.; Park, H.Y.; Milne, S.; Li, X.; Yang, C.X.; Tuong, J.; Eddy, R.L.; Vahedi, S.M.; Guinto, E.; Cheung, C.Y.; et al. Single-cell sequencing reveals cellular landscape alterations in the airway mucosa of patients with pulmonary long COVID. Eur. Respir. J. 2024, 64, 2301947. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.; Miller, M.; Unsworth, E.J.; Siegfried, J.M.; Cuttitta, F. Expression of adrenomedullin in normal human lung and in pulmonary tumors. Endocrinology 1995, 136, 4099–4105. [Google Scholar] [CrossRef]
- Maunders, H.; Patwardhan, S.; Phillips, J.; Clack, A.; Richter, A. Human bronchial epithelial cell transcriptome: Gene expression changes following acute exposure to whole cigarette smoke in vitro. Am. J. Physiol. Lung Cell Mol. Physiol. 2007, 292, L1248–L1256. [Google Scholar] [CrossRef]
- Recklies, A.D.; White, C.; Hua, L. The chitinase 3-like protein human cartilage glycoprotein 39 (HC-gp39) stimulates proliferation of human connective-tissue cells and activates both extracellular signal-regulated kinase-and protein kinase B-mediated signalling pathways. Biochem. J. 2002, 365, 119–126. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, M.N.; Kim, E.G.; Leem, J.S.; Baek, S.M.; Kim, M.J.; Kim, K.W.; Sohn, M.H. Chitinase 3-like 1 is involved in the induction of IL-8 expression by double-stranded RNA in airway epithelial cells. Biochem. Biophys. Res. Commun. 2022, 592, 106–112. [Google Scholar] [CrossRef]
- Hübner, K.; Karwelat, D.; Pietsch, E.; Beinborn, I.; Winterberg, S.; Bedenbender, K.; Benedikter, B.J.; Schmeck, B.; Vollmeister, E. NF-κB-mediated inhibition of microRNA-149-5p regulates Chitinase-3-like 1 expression in human airway epithelial cells. Cell. Signal. 2020, 67, 109498. [Google Scholar] [CrossRef]
- Fagan, K.A.; McMurtry, I.F.; Rodman, D.M. Role of endothelin-1 in lung disease. Respir. Res. 2001, 2, 90. [Google Scholar] [CrossRef]
- Lan, B.; Mitchel, J.A.; O’Sullivan, M.J.; Park, C.Y.; Kim, J.H.; Cole, W.C.; Butler, J.P.; Park, J.-A. Airway epithelial compression promotes airway smooth muscle proliferation and contraction. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 315, L645–L652. [Google Scholar] [CrossRef]
- Chetty, A.; Andersson, S.; Lassus, P.; Nielsen, H.C. Insulin-like growth factor-1 (IGF-1) and IGF-1 receptor (IGF-1R) expression in human lung in RDS and BPD. Pediatr. Pulmonol. 2004, 37, 128–136. [Google Scholar] [CrossRef]
- Simões, F.B.; Kmit, A.; Amaral, M.D. Cross-talk of inflammatory mediators and airway epithelium reveals the cystic fibrosis transmembrane conductance regulator as a major target. ERJ Open Res. 2021, 7, 00247–02021. [Google Scholar] [CrossRef] [PubMed]
- Asokananthan, N.; Graham, P.T.; Fink, J.; Knight, D.A.; Bakker, A.J.; McWilliam, A.S.; Thompson, P.J.; Stewart, G.A. Activation of Protease-Activated Receptor (PAR)-1, PAR-2, and PAR-4 Stimulates IL-6, IL-8, and Prostaglandin E2 Release from Human Respiratory Epithelial Cells. J. Immunol. 2002, 168, 3577–3585. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.H.; De Raeve, H.R.; Rice, T.W.; Stuehr, D.J.; Thunnissen, F.; Erzurum, S.C. Continuous nitric oxide synthesis by inducible nitric oxide synthase in normal human airway epithelium in vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 7809–7813. [Google Scholar] [CrossRef]
- Levine, S.J. Bronchial epithelial cell-cytokine interactions in airway inflammation. J. Investig. Med. 1995, 43, 241–249. [Google Scholar]
- Suto, W.; Sakai, H.; Chiba, Y. Sustained exposure to prostaglandin D(2) augments the contraction induced by acetylcholine via a DP(1) receptor-mediated activation of p38 in bronchial smooth muscle of naive mice. J. Smooth Muscle Res. 2019, 55, 1–13. [Google Scholar] [CrossRef]
- Boussat, S.; Eddahibi, S.; Coste, A.; Fataccioli, V.; Gouge, M.; Housset, B.; Adnot, S.; Maitre, B. Expression and regulation of vascular endothelial growth factor in human pulmonary epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 279, L371–L378. [Google Scholar] [CrossRef]
- Huether, S.E.; McCance, K.L. Pathophysiology: The biologic basis for disease in adults and children. Dimens. Crit. Care Nurs. 1994, 13, 315. [Google Scholar] [CrossRef]
- Gaona, I.P.; McCall, A.S.; Geis, N.M.; Colvard, A.C.; DiGiovanni, G.T.; Sherrill, T.P.; Singha, U.K.; Nichols, D.S.; Serezani, A.P.; David, H.E.; et al. Sustained Yap/Taz activation promotes aberrant alveolar epithelial cell differentiation and drives persistent fibrotic remodeling. bioRxiv 2025. [Google Scholar] [CrossRef]
- Mohri, Z.; Del Rio Hernandez, A.; Krams, R. The emerging role of YAP/TAZ in mechanotransduction. J. Thorac. Dis. 2017, 9, E507–E509. [Google Scholar] [CrossRef]
- John, A.E.; Wilson, M.R.; Habgood, A.; Porte, J.; Tatler, A.L.; Stavrou, A.; Miele, G.; Jolly, L.; Knox, A.J.; Takata, M.; et al. Loss of epithelial Gq and G11 signaling inhibits TGFβ production but promotes IL-33–mediated macrophage polarization and emphysema. Sci. Signal. 2016, 9, ra104. [Google Scholar] [CrossRef]
- Ni, K.; Che, B.; Gu, R.; Wang, C.; Pan, Y.; Li, J.; Liu, L.; Luo, M.; Deng, L. Single-Cell Hypertrophy Promotes Contractile Function of Cultured Human Airway Smooth Muscle Cells via Piezo1 and YAP Auto-Regulation. Cells 2024, 13, 1697. [Google Scholar] [CrossRef]
- Govorova, I.A.; Nikitochkina, S.Y.; Vorotelyak, E.A. Influence of intersignaling crosstalk on the intracellular localization of YAP/TAZ in lung cells. Cell Commun. Signal 2024, 22, 289. [Google Scholar] [CrossRef] [PubMed]
- Scheraga, R.G.; Southern, B.D.; Grove, L.M.; Olman, M.A. The Role of Transient Receptor Potential Vanilloid 4 in Pulmonary Inflammatory Diseases. Front. Immunol. 2017, 8, 503. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, J.; Chen, R.; Shi, F.; Xiong, Y. Airway epithelial cells promote in vitro airway smooth muscle cell proliferation by activating the Wnt/β-catenin pathway. Respir. Physiol. Neurobiol. 2025, 331, 104368. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, R.G.; Su, X.; Su, G.; Scotton, C.J.; Camerer, E.; Laurent, G.J.; Davis, G.E.; Chambers, R.C.; Matthay, M.A.; Sheppard, D. Ligation of protease-activated receptor 1 enhances α v β 6 integrin–dependent TGF-β activation and promotes acute lung injury. J. Clin. Investig. 2006, 116, 1606–1614. [Google Scholar] [CrossRef]
- Alsubait, D.; Rajani, H.F.; Shan, L.; Koussih, L.; Halayko, A.J.; Lamkhioued, B.; Gounni, A.S. Expression of Semaphorin3E/PlexinD1 in human airway smooth muscle cells of patients with COPD. Am. J. Physiol. Lung Cell Mol. Physiol. 2024, 327, L831–L838. [Google Scholar] [CrossRef]
- Goldsmith, A.M.; Bentley, J.K.; Zhou, L.; Jia, Y.; Bitar, K.N.; Fingar, D.C.; Hershenson, M.B. Transforming growth factor-beta induces airway smooth muscle hypertrophy. Am. J. Respir. Cell Mol. Biol. 2006, 34, 247–254. [Google Scholar] [CrossRef]
- Zaleskas, J.M.; Kinner, B.; Freyman, T.M.; Yannas, I.V.; Gibson, L.J.; Spector, M. Growth factor regulation of smooth muscle actin expression and contraction of human articular chondrocytes and meniscal cells in a collagen-GAG matrix. Exp. Cell Res. 2001, 270, 21–31. [Google Scholar] [CrossRef]
- McMillan, S.J.; Xanthou, G.; Lloyd, C.M. Manipulation of Allergen-Induced Airway Remodeling by Treatment with Anti-TGF-β Antibody: Effect on the Smad Signaling Pathway. J. Immunol. 2005, 174, 5774–5780. [Google Scholar] [CrossRef]
- Postma, D.S.; Timens, W. Remodeling in asthma and chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2006, 3, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Le, A.V.; Cho, J.Y.; Miller, M.; McElwain, S.; Golgotiu, K.; Broide, D.H. Inhibition of Allergen-Induced Airway Remodeling in Smad 3-Deficient Mice. J. Immunol. 2007, 178, 7310–7316. [Google Scholar] [CrossRef] [PubMed]
- Raeburn, D.; Rodger, I.W.; Hay, D.W.P.; Fedan, J.S. The dependence of airway smooth muscle on extracellular Ca2+ for contraction is influenced by the presence of cartilage. Life Sci. 1986, 38, 1499–1505. [Google Scholar] [CrossRef] [PubMed]
- Hay, D.W.; Farmer, S.G.; Raeburn, D.; Robinson, V.A.; Fleming, W.W.; Fedan, J.S. Airway epithelium modulates the reactivity of guinea-pig respiratory smooth muscle. Eur. J. Pharmacol. 1986, 129, 11–18. [Google Scholar] [CrossRef]
- Kılıç, A.; Ameli, A.; Park, J.-A.; Kho, A.T.; Tantisira, K.; Santolini, M.; Cheng, F.; Mitchel, J.A.; McGill, M.; O’Sullivan, M.J.; et al. Mechanical forces induce an asthma gene signature in healthy airway epithelial cells. Sci. Rep. 2020, 10, 966. [Google Scholar] [CrossRef]
- Mwase, C.; Phung, T.-K.N.; O’Sullivan, M.J.; Mitchel, J.A.; De Marzio, M.; Kılıç, A.; Weiss, S.T.; Fredberg, J.J.; Park, J.-A. Mechanical Compression of Human Airway Epithelial Cells Induces Release of Extracellular Vesicles Containing Tenascin C. Cells 2022, 11, 256. [Google Scholar] [CrossRef]
- Dunne, O.M.; Martin, S.L.; Sergeant, G.P.; McAuley, D.F.; O’Kane, C.M.; Button, B.; McGarvey, L.P.; Lundy, F.T. TRPV2 modulates mechanically Induced ATP Release from Human bronchial epithelial cells. Respir. Res. 2024, 25, 188. [Google Scholar] [CrossRef]
- Zhao, L.; Liang, Y.-T.; Tian, D.-B.; Zhang, R.-G.; Huang, J.; Zhu, Y.-X.; Zhou, W.-L.; Zhang, Y.-L. Regulation of smooth muscle contractility by the epithelium in rat tracheas: Role of prostaglandin E2 induced by the neurotransmitter acetylcholine. Ann. Transl. Med. 2021, 9, 313. [Google Scholar] [CrossRef]
- Jairaman, A.; Prakriya, M. Calcium Signaling in Airway Epithelial Cells: Current Understanding and Implications for Inflammatory Airway Disease. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 772–783. [Google Scholar] [CrossRef]
- Yao, Y.; Zheng, M.; Borkar, N.A.; Thompson, M.A.; Zhang, E.Y.; Koloko Ngassie, M.L.; Wang, S.; Pabelick, C.M.; Vogel, E.R.; Prakash, Y.S. Role of STIM1 in stretch-induced signaling in human airway smooth muscle. Am. J. Physiol. Lung Cell. Mol. Physiol. 2024, 327, L150–L159. [Google Scholar] [CrossRef]
- Luo, M.; Ni, K.; Gu, R.; Qin, Y.; Guo, J.; Che, B.; Pan, Y.; Li, J.; Liu, L.; Deng, L. Chemical Activation of Piezo1 Alters Biomechanical Behaviors toward Relaxation of Cultured Airway Smooth Muscle Cells. Biol. Pharm. Bull. 2023, 46, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Migulina, N.; Kelley, B.; Zhang, E.Y.; Pabelick, C.M.; Prakash, Y.S.; Vogel, E.R. Mechanosensitive Channels in Lung Health and Disease. Compr. Physiol. 2023, 13, 5157–5178. [Google Scholar] [CrossRef] [PubMed]
- Burgess, J.K.; Gosens, R. Mechanotransduction and the extracellular matrix: Key drivers of lung pathologies and drug responsiveness. Biochem. Pharmacol. 2024, 228, 116255. [Google Scholar] [CrossRef]
- McVicar, R.N.; Smith, E.; Melameka, M.; Bush, A.; Goetz, G.; Constantino, G.; Kumar, M.; Kwong, E.; Snyder, E.Y.; Leibel, S.L. iPSC-Derived Epithelial, Mesenchymal, Endothelial, and Immune Cell Co-Culture to Model Airway Barrier Integrity in Lung Health and Disease. J. Vis. Exp. 2024, 214, e67247. [Google Scholar] [CrossRef]
- Nishida, T.; Kubota, S.; Nakanishi, T.; Kuboki, T.; Yosimichi, G.; Kondo, S.; Takigawa, M. CTGF/Hcs24, a hypertrophic chondrocyte-specific gene product, stimulates proliferation and differentiation, but not hypertrophy of cultured articular chondrocytes. J. Cell Physiol. 2002, 192, 55–63. [Google Scholar] [CrossRef]
- Weksler, N.B.; Lunstrum, G.P.; Reid, E.S.; Horton, W.A. Differential effects of fibroblast growth factor (FGF) 9 and FGF2 on proliferation, differentiation and terminal differentiation of chondrocytic cells in vitro. Biochem. J. 1999, 342 Pt 3, 677–682. [Google Scholar] [CrossRef]
- Takeda, N.; Sumi, Y.; Préfontaine, D.; Abri, J.A.; Heialy, N.A.; Al-Ramli, W.; Michoud, M.C.; Martin, J.G.; Hamid, Q. Epithelium-derived chemokines induce airway smooth muscle cell migration. Clin. Exp. Allergy 2009, 39, 1018–1026. [Google Scholar] [CrossRef]
- Walker, F.; Kato, A.; Gonez, L.J.; Hibbs, M.L.; Pouliot, N.; Levitzki, A.; Burgess, A.W. Activation of the Ras/mitogen-activated protein kinase pathway by kinase-defective epidermal growth factor receptors results in cell survival but not proliferation. Mol. Cell. Biol. 1998, 18, 7192–7204. [Google Scholar] [CrossRef]
- Malavia, N.K.; Raub, C.B.; Mahon, S.B.; Brenner, M.; Jr, R.A.P.; George, S.C. Airway Epithelium Stimulates Smooth Muscle Proliferation. Am. J. Respir. Cell Mol. Biol. 2009, 41, 297–304. [Google Scholar] [CrossRef]
- Ramis, J.; Middlewick, R.; Pappalardo, F.; Cairns, J.T.; Stewart, I.D.; John, A.E.; Naveed, S.U.; Krishnan, R.; Miller, S.; Shaw, D.E.; et al. Lysyl oxidase like 2 is increased in asthma and contributes to asthmatic airway remodelling. Eur. Respir. J. 2022, 60, 2004361. [Google Scholar] [CrossRef]
- Sanchez-Adams, J.; Leddy, H.A.; McNulty, A.L.; O’Conor, C.J.; Guilak, F. The Mechanobiology of Articular Cartilage: Bearing the Burden of Osteoarthritis. Curr. Rheumatol. Rep. 2014, 16, 451. [Google Scholar] [CrossRef] [PubMed]
- Mauck, R.L.; Soltz, M.A.; Wang, C.C.B.; Wong, D.D.; Chao, P.-H.G.; Valhmu, W.B.; Hung, C.T.; Ateshian, G.A. Functional Tissue Engineering of Articular Cartilage Through Dynamic Loading of Chondrocyte-Seeded Agarose Gels. J. Biomech. Eng. 2000, 122, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Wang, Y.; Wang, K. Prostaglandin E(2) stimulates normal bronchial epithelial cell growth through induction of c-Jun and PDK1, a kinase implicated in oncogenesis. Respir. Res. 2015, 16, 149. [Google Scholar] [CrossRef] [PubMed]
- Guzy, R.D.; Stoilov, I.; Elton, T.J.; Mecham, R.P.; Ornitz, D.M. Fibroblast Growth Factor 2 Is Required for Epithelial Recovery, but Not for Pulmonary Fibrosis, in Response to Bleomycin. Am. J. Respir. Cell Mol. Biol. 2015, 52, 116–128. [Google Scholar] [CrossRef]
- Gupta, P.; Markham, A.; Morgan, R.M. Ca2+ ion sequestration by guinea-pig tracheal cartilage: Its influence on trachealis reactivity to KCl. Br. J. Pharmacol. 1991, 104, 123–127. [Google Scholar] [CrossRef]
- Ramchandani, R.; Shen, X.; Elmsley, C.L.; Ambrosius, W.T.; Gunst, S.J.; Tepper, R.S. Differences in airway structure in immature and mature rabbits. J. Appl. Physiol. 2000, 89, 1310–1316. [Google Scholar] [CrossRef]
- Alber, A.B.; Marquez, H.A.; Ma, L.; Kwong, G.; Thapa, B.R.; Villacorta-Martin, C.; Lindstrom-Vautrin, J.; Bawa, P.; Wang, F.; Luo, Y.; et al. Directed differentiation of mouse pluripotent stem cells into functional lung-specific mesenchyme. Nat. Commun. 2023, 14, 3488. [Google Scholar] [CrossRef]
- Leibel, S.L.; McVicar, R.N.; Winquist, A.M.; Niles, W.D.; Snyder, E.Y. Generation of Complete Multi−Cell Type Lung Organoids From Human Embryonic and Patient-Specific Induced Pluripotent Stem Cells for Infectious Disease Modeling and Therapeutics Validation. Curr. Protoc. Stem Cell Biol. 2020, 54, e118. [Google Scholar] [CrossRef]
- Kishimoto, K.; Furukawa, K.T.; Luz-Madrigal, A.; Yamaoka, A.; Matsuoka, C.; Habu, M.; Alev, C.; Zorn, A.M.; Morimoto, M. Bidirectional Wnt signaling between endoderm and mesoderm confers tracheal identity in mouse and human cells. Nat. Commun. 2020, 11, 4159. [Google Scholar] [CrossRef]
- Bottasso-Arias, N.; Leesman, L.; Burra, K.; Snowball, J.; Shah, R.; Mohanakrishnan, M.; Xu, Y.; Sinner, D. BMP4 and Wnt signaling interact to promote mouse tracheal mesenchyme morphogenesis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2022, 322, L224–L242. [Google Scholar] [CrossRef]
- Luo, Y.; Cao, K.; Chiu, J.; Chen, H.; Wang, H.-J.; Thornton, M.E.; Grubbs, B.H.; Kolb, M.; Parmacek, M.S.; Mishina, Y.; et al. Defective mesenchymal Bmpr1a-mediated BMP signaling causes congenital pulmonary cysts. eLife 2024, 12, RP91876. [Google Scholar] [CrossRef]
- Pansky, B. Development Of The Lower Respiratory System: Larynx And Trachea. In Review of Medical Embryology; McGraw-Hill: Columbus, OH, USA, 1982. [Google Scholar]
- Mailleux, A.A.; Kelly, R.; Veltmaat, J.M.; De Langhe, S.P.; Zaffran, S.; Thiery, J.P.; Bellusci, S. Fgf10 expression identifies parabronchial smooth muscle cell progenitors and is required for their entry into the smooth muscle cell lineage. Development 2005, 132, 2157–2166. [Google Scholar] [CrossRef]
- Pansky, B. Development Of The Lower Respiratory System: The Bronchi And Surrounding Structures. In Review of Medical Embryology; McGraw-Hill: Columbus, OH, USA, 1982; Volume 59. [Google Scholar]
- Bellusci, S.; Grindley, J.; Emoto, H.; Itoh, N.; Hogan, B.L. Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development 1997, 124, 4867–4878. [Google Scholar] [CrossRef] [PubMed]
- Jesudason, E.C.; Smith, N.P.; Connell, M.G.; Spiller, D.G.; White, M.R.; Fernig, D.G.; Losty, P.D. Peristalsis of airway smooth muscle is developmentally regulated and uncoupled from hypoplastic lung growth. Am. J. Physiol. Lung Cell Mol. Physiol. 2006, 291, L559–L565. [Google Scholar] [CrossRef] [PubMed]
- Parvez, O.; Voss, A.-M.; de Kok, M.; Roth-Kleiner, M.; Belik, J. Bronchial Muscle Peristaltic Activity in the Fetal Rat. Pediatr. Res. 2006, 59, 756. [Google Scholar] [CrossRef] [PubMed]
- Cairns, D.M.; Lee, P.G.; Uchimura, T.; Seufert, C.R.; Kwon, H.; Zeng, L. The role of muscle cells in regulating cartilage matrix production. J. Orthop. Res. 2010, 28, 529–536. [Google Scholar] [CrossRef]
- Shannon, J.M.; Nielsen, L.D.; Gebb, S.A.; Randell, S.H. Mesenchyme specifies epithelial differentiation in reciprocal recombinants of embryonic lung and trachea. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 1998, 212, 482–494. [Google Scholar] [CrossRef]
- Schittny, J.C.; Miserocchi, G.; Sparrow, M.P. Spontaneous Peristaltic Airway Contractions Propel Lung Liquid through the Bronchial Tree of Intact and Fetal Lung Explants. Am. J. Respir. Cell Mol. Biol. 2000, 23, 11–18. [Google Scholar] [CrossRef]
- Featherstone, N.C.; Jesudason, E.C.; Connell, M.G.; Fernig, D.G.; Wray, S.; Losty, P.D.; Burdyga, T.V. Spontaneous propagating calcium waves underpin airway peristalsis in embryonic rat lung. Am. J. Respir. Cell Mol. Biol. 2005, 33, 153–160. [Google Scholar] [CrossRef]
- Jesudason, E.C. Airway smooth muscle: An architect of the lung? Thorax 2009, 64, 541–545. [Google Scholar] [CrossRef]
- Perez-Zoghbi, J.F.; Bai, Y.; Sanderson, M.J. Nitric oxide induces airway smooth muscle cell relaxation by decreasing the frequency of agonist-induced Ca2+ oscillations. J. Gen. Physiol. 2010, 135, 247–259. [Google Scholar] [CrossRef]
- Sherman, T.S.; Chen, Z.; Yuhanna, I.S.; Lau, K.S.; Margraf, L.R.; Shaul, P.W. Nitric oxide synthase isoform expression in the developing lung epithelium. Am. J. Physiol. 1999, 276 Pt 1, L383–L390. [Google Scholar] [CrossRef]
- Halayko, A.J.; Salari, H.; MA, X.; Stephens, N.L. Markers of airway smooth muscle cell phenotype. Am. J. Physiol. Lung Cell. Mol. Physiol. 1996, 270, L1040–L1051. [Google Scholar] [CrossRef]
- Lan, R.S.; Knight, D.A.; Stewart, G.A.; Henry, P.J. Role of PGE2 in protease-activated receptor-1, −2 and −4 mediated relaxation in the mouse isolated trachea. Br. J. Pharmacol. 2001, 132, 93–100. [Google Scholar] [CrossRef]
- Barnett, K.; Jacoby, D.B.; Nadel, J.A.; Lazarus, S.C. The effects of epithelial cell supernatant on contractions of isolated canine tracheal smooth muscle. Am. Rev. Respir. Dis. 1988, 138, 780–783. [Google Scholar] [CrossRef] [PubMed]
- Panitch, H.B.; Wolfson, M.R.; Shaffer, T.H. Epithelial modulation of preterm airway smooth muscle contraction. J. Appl. Physiol. 1993, 74, 1437. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J.; Cuss, F.M.; Palmer, J.B. The effect of airway epithelium on smooth muscle contractility in bovine trachea. Br. J. Pharmacol. 1985, 86, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Sonnylal, S.; Xu, S.; Jones, H.; Tam, A.; Sreeram, V.R.; Ponticos, M.; Norman, J.; Agrawal, P.; Abraham, D.; de Crombrugghe, B. Connective tissue growth factor causes EMT-like cell fate changes in vivo and in vitro. J. Cell Sci. 2013, 126, 2164–2175. [Google Scholar] [CrossRef]
- Câmara, J.; Jarai, G. Epithelial-mesenchymal transition in primary human bronchial epithelial cells is Smad-dependent and enhanced by fibronectin and TNF-α. Fibrogenes. Tissue Repair. 2010, 3, 2. [Google Scholar] [CrossRef]
- Gospodarowicz, D.; Moran, J.S. Mitogenic effect of fibroblast growth factor on early passage cultures of human and murine fibroblasts. J. Cell Biol. 1975, 66, 451–457. [Google Scholar] [CrossRef]
- Aros, C.J.; Pantoja, C.J.; Gomperts, B.N. Wnt signaling in lung development, regeneration, and disease progression. Commun. Biol. 2021, 4, 601. [Google Scholar] [CrossRef] [PubMed]
- Eenjes, E.; Tibboel, D.; Wijnen, R.M.H.; Rottier, R.J. Lung epithelium development and airway regeneration. Front. Cell Dev. Biol. 2022, 10, 1022457. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Ciminieri, C.; Hu, Q.; Lehmann, M.; Königshoff, M.; Gosens, R. WNT Signalling in Lung Physiology and Pathology. Handb. Exp. Pharmacol. 2021, 269, 305–336. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef]
- Lin, L.; Yang, L.; Wang, N.; Chen, S.; Du, X.; Chen, R.; Zhang, H.; Kong, X. FGF10 protects against LPS-induced epithelial barrier injury and inflammation by inhibiting SIRT1-ferroptosis pathway in acute lung injury in mice. Int. Immunopharmacol. 2024, 127, 111426. [Google Scholar] [CrossRef]
- Peng, W.; Song, Y.; Zhu, G.; Zeng, Y.; Cai, H.; Lu, C.; Abuduxukuer, Z.; Song, X.; Gao, X.; Ye, L.; et al. FGF10 attenuates allergic airway inflammation in asthma by inhibiting PI3K/AKT/NF-κB pathway. Cell Signal 2024, 113, 110964. [Google Scholar] [CrossRef]
- Ma, Q.; Ma, Y.; Dai, X.; Ren, T.; Fu, Y.; Liu, W.; Han, Y.; Wu, Y.; Cheng, Y.; Zhang, T.; et al. Regeneration of functional alveoli by adult human SOX9(+) airway basal cell transplantation. Protein Cell 2018, 9, 267–282. [Google Scholar] [CrossRef]
- Sun, D.; Llora Batlle, O.; van den Ameele, J.; Thomas, J.C.; He, P.; Lim, K.; Tang, W.; Xu, C.; Meyer, K.B.; Teichmann, S.A.; et al. SOX9 maintains human foetal lung tip progenitor state by enhancing WNT and RTK signalling. Embo J. 2022, 41, e111338. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Huang, Y.; Chen, H.; Sui, P. Unlocking lung regeneration: Insights into progenitor cell dynamics and metabolic control. Cell Regen. 2024, 13, 31. [Google Scholar] [CrossRef]
- Osei, E.T.; Booth, S.; Hackett, T.-L. What Have In Vitro Co-Culture Models Taught Us about the Contribution of Epithelial-Mesenchymal Interactions to Airway Inflammation and Remodeling in Asthma? Cells 2020, 9, 1694. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, K.; Jaslove, J.M.; Tao, H.; Zhu, M.; Hopyan, S.; Nelson, C.M. Patterning the embryonic pulmonary mesenchyme. iScience 2022, 25, 103838. [Google Scholar] [CrossRef]
- Uwagboe, I.E.; Mumby, S.; Dunlop, I.E.; Adcock, I.M. Does mechanobiology drive respiratory disease? Biomechanical induction of mucus hypersecretion in human bronchial organoids using a photocontrolled biomaterial gel. bioRxiv 2025. [Google Scholar] [CrossRef]
- Al Yazeedi, S.; Guo, T.J.F.; Sohd, J.; Abokor, F.A.; Baher, J.Z.; Yee, L.; Cheung, C.; Sin, D.D.; Osei, E.T. Dynamic mechanical stimulation of alveolar epithelial-fibroblast models using the Flexcell tension system to study of lung disease mechanisms. Front. Med. 2025, 12, 1552803. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhou, Y.; Wang, R.; Lin, Y.; Lan, H.; Li, Y.; Wang, D.-Y.; Dong, J.; Li, K.; Yan, Y.; et al. YAP as a potential therapeutic target for myofibroblast formation in asthma. Respir. Res. 2025, 26, 51. [Google Scholar] [CrossRef]
- Pan, J.; Luk, C.; Kent, G.; Cutz, E.; Yeger, H. Pulmonary Neuroendocrine Cells, Airway Innervation, and Smooth Muscle Are Altered in Cftr Null Mice. Am. J. Respir. Cell Mol. Biol. 2006, 35, 320–326. [Google Scholar] [CrossRef]
- Bonvin, E.; Le Rouzic, P.; Bernaudin, J.F.; Cottart, C.H.; Vandebrouck, C.; Crie, A.; Leal, T.; Clement, A.; Bonora, M. Congenital tracheal malformation in cystic fibrosis transmembrane conductance regulator-deficient mice. J. Physiol. 2008, 586, 3231–3243. [Google Scholar] [CrossRef]
- Meyerholz, D.K.; Stoltz, D.A.; Namati, E.; Ramachandran, S.; Pezzulo, A.A.; Smith, A.R.; Rector, M.V.; Suter, M.J.; Kao, S.; McLennan, G.; et al. Loss of cystic fibrosis transmembrane conductance regulator function produces abnormalities in tracheal development in neonatal pigs and young children. Am. J. Respir. Crit. Care Med. 2010, 182, 1251–1261. [Google Scholar] [CrossRef]
- Haraguchi, M.; Shimura, S.; Shirato, K. Morphometric Analysis of Bronchial Cartilage in Chronic Obstructive Pulmonary Disease and Bronchial Asthma. Am. J. Respir. Crit. Care Med. 1999, 159, 1005–1013. [Google Scholar] [CrossRef]
- Carroll, N.; Elliot, J.; Morton, A.; James, A. The Structure of Large and Small Airways in Nonfatal and Fatal Asthma. Am. Rev. Respir. Dis. 1993, 147, 405–410. [Google Scholar] [CrossRef]
- Regamey, N.; Ochs, M.; Hilliard, T.N.; Mühlfeld, C.; Cornish, N.; Fleming, L.; Saglani, S.; Alton, E.W.F.W.; Bush, A.; Jeffery, P.K.; et al. Increased Airway Smooth Muscle Mass in Children with Asthma, Cystic Fibrosis, and Non-Cystic Fibrosis Bronchiectasis. Am. J. Respir. Crit. Care Med. 2008, 177, 837–843. [Google Scholar] [CrossRef]
- Ma, X.; Cheng, Z.; Kong, H.; Wang, Y.; Unruh, H.; Stephens, N.L.; Laviolette, M. Changes in biophysical and biochemical properties of single bronchial smooth muscle cells from asthmatic subjects. Am. J. Physiol. Lung Cell Mol. Physiol. 2002, 283, L1181–L1189. [Google Scholar] [CrossRef]
- Matsumoto, H.; Moir, L.M.; Oliver, B.G.; Burgess, J.K.; Roth, M.; Black, J.L.; McParland, B.E. Comparison of gel contraction mediated by airway smooth muscle cells from patients with and without asthma. Thorax 2007, 62, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Naylor, B. The Shedding of the Mucosa of the Bronchial Tree in Asthma. Thorax 1962, 17, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, D.L.; Merkhofer, R.M.; Ober, C.; Kujoth, G.C.; Niu, M.; Keller, N.P.; Gern, J.E.; Brockman-Schneider, R.A.; Evans, M.D.; Jackson, D.J.; et al. Club Cell TRPV4 Serves as a Damage Sensor Driving Lung Allergic Inflammation. Cell Host Microbe 2020, 27, 614–628.e616. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.A.; Li, T.-F.; Kim, K.-O.; Drissi, H.; Zuscik, M.J.; Zhang, X.; O’Keefe, R.J. Prostaglandin E2 inhibits BMP signaling and delays chondrocyte maturation. J. Orthop. Res. 2009, 27, 785–792. [Google Scholar] [CrossRef]
- Kinner, B.; Spector, M. Smooth muscle actin expression by human articular chondrocytes and their contraction of a collagen—Glycosaminoglycan matrix in vitro. J. Orthop. Res. 2001, 19, 233–241. [Google Scholar] [CrossRef]
- Marlovits, S.; Hombauer, M.; Truppe, M.; Vecsei, V.; Schlegel, W. Changes in the ratio of type-I and type-II collagen expression during monolayer culture of human chondrocytes. Bone Jt. J. 2004, 86, 286–295. [Google Scholar] [CrossRef]
- Parreno, J.; Raju, S.; Wu, P.-H.; Kandel, R.A. MRTF-A signaling regulates the acquisition of the contractile phenotype in dedifferentiated chondrocytes. Matrix Biol. 2017, 62, 3–14. [Google Scholar] [CrossRef]
- Zhong, B.; Du, J.; Liu, F.; Sun, S. The Role of Yes-Associated Protein in Inflammatory Diseases and Cancer. MedComm 2025, 6, e70128. [Google Scholar] [CrossRef]
- Kim, K.W.; Jee, H.M.; Park, Y.H.; Choi, B.S.; Sohn, M.H.; Kim, K.E. Relationship between amphiregulin and airway inflammation in children with asthma and eosinophilic bronchitis. Chest 2009, 136, 805–810. [Google Scholar] [CrossRef]
- Benayoun, L.; Druilhe, A.; Dombret, M.-C.; Aubier, M.; Pretolani, M. Airway Structural Alterations Selectively Associated with Severe Asthma. Am. J. Respir. Crit. Care Med. 2003, 167, 1360–1368. [Google Scholar] [CrossRef]
- Jang, J.-H.; Chand, H.S.; Bruse, S.; Doyle-Eisele, M.; Royer, C.; McDonald, J.; Qualls, C.; Klingelhutz, A.J.; Lin, Y.; Mallampalli, R.; et al. Connective Tissue Growth Factor Promotes Pulmonary Epithelial Cell Senescence and Is Associated with COPD Severity. J. Chronic Obstr. Pulm. Dis. 2017, 14, 228–237. [Google Scholar] [CrossRef]
- Chung, K.F. The Role of Airway Smooth Muscle in the Pathogenesis of Airway Wall Remodeling in Chronic Obstructive Pulmonary Disease. Proc. Am. Thorac. Soc. 2005, 2, 347–354. [Google Scholar] [CrossRef] [PubMed]
- López-Posadas, R.; Bagley, D.C.; Pardo-Pastor, C.; Ortiz-Zapater, E. The epithelium takes the stage in asthma and inflammatory bowel diseases. Front. Cell Dev. Biol. 2024, 12, 1258859. [Google Scholar] [CrossRef] [PubMed]
- Hicks-Berthet, J.; Ning, B.; Federico, A.; Tilston-Lunel, A.; Matschulat, A.; Ai, X.; Lenburg, M.E.; Beane, J.; Monti, S.; Varelas, X. Yap/Taz inhibit goblet cell fate to maintain lung epithelial homeostasis. Cell Rep. 2021, 36, 109347. [Google Scholar] [CrossRef] [PubMed]
- Chamorro-Herrero, I.; Zambrano, A. Modeling of Respiratory Diseases Evolving with Fibrosis from Organoids Derived from Human Pluripotent Stem Cells. Int. J. Mol. Sci. 2023, 24, 4413. [Google Scholar] [CrossRef]
- Lama, V.N.; Smith, L.; Badri, L.; Flint, A.; Andrei, A.-C.; Murray, S.; Wang, Z.; Liao, H.; Toews, G.B.; Krebsbach, P.H.; et al. Evidence for tissue-resident mesenchymal stem cells in human adult lung from studies of transplanted allografts. J. Clin. Investig. 2007, 117, 989–996. [Google Scholar] [CrossRef]
- Akram, K.M.; Patel, N.; Spiteri, M.A.; Forsyth, N.R. Lung Regeneration: Endogenous and Exogenous Stem Cell Mediated Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 128. [Google Scholar] [CrossRef]
- Summer, R.; Fitzsimmons, K.; Dwyer, D.; Murphy, J.; Fine, A. Isolation of an adult mouse lung mesenchymal progenitor cell population. Am. J. Respir. Cell Mol. Biol. 2007, 37, 152–159. [Google Scholar] [CrossRef]
- Martin, J.; Helm, K.; Ruegg, P.; Varella-Garcia, M.; Burnham, E.; Majka, S. Adult lung side population cells have mesenchymal stem cell potential. Cytotherapy 2008, 10, 140–151. [Google Scholar] [CrossRef]
- Jarvinen, L.; Badri, L.; Wettlaufer, S.; Ohtsuka, T.; Standiford, T.J.; Toews, G.B.; Pinsky, D.J.; Peters-Golden, M.; Lama, V.N. Lung Resident Mesenchymal Stem Cells Isolated From Human Lung Allografts Inhibit T Cell Proliferation via a Soluble Mediator. J. Immunol. 2008, 181, 4389–4396. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Zhou, J.; Rong, L.; Seeley, E.J.; Pan, J.; Zhu, X.; Liu, J.; Wang, Q.; Tang, X.; Qu, J.; et al. Fibroblast Growth Factor-10 (FGF-10) Mobilizes Lung-resident Mesenchymal Stem Cells and Protects Against Acute Lung Injury. Sci. Rep. 2016, 6, 21642. [Google Scholar] [CrossRef] [PubMed]
- Le Visage, C.; Dunham, B.; Flint, P.; Leong, K.W. Coculture of mesenchymal stem cells and respiratory epithelial cells to engineer a human composite respiratory mucosa. Tissue Eng. 2004, 10, 1426–1435. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Yeung, S.C.; Liang, Y.; Liang, X.; Ding, Y.; Ip, M.S.M.; Tse, H.-F.; Mak, J.C.W.; Lian, Q. Mitochondrial Transfer of Induced Pluripotent Stem Cell–Derived Mesenchymal Stem Cells to Airway Epithelial Cells Attenuates Cigarette Smoke–Induced Damage. Am. J. Respir. Cell Mol. Biol. 2014, 51, 455–465. [Google Scholar] [CrossRef]
- Serikov, V.B.; Popov, B.; Mikhailov, V.M.; Gupta, N.; Matthay, M.A. Evidence of Temporary Airway Epithelial Repopulation and Rare Clonal Formation by BM-derived Cells Following Naphthalene Injury in Mice. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2007, 290, 1033–1045. [Google Scholar] [CrossRef]
- Urbanek, K.; De Angelis, A.; Spaziano, G.; Piegari, E.; Matteis, M.; Cappetta, D.; Esposito, G.; Russo, R.; Tartaglione, G.; De Palma, R.; et al. Intratracheal Administration of Mesenchymal Stem Cells Modulates Tachykinin System, Suppresses Airway Remodeling and Reduces Airway Hyperresponsiveness in an Animal Model. PLoS ONE 2016, 11, e0158746. [Google Scholar] [CrossRef]
- Taillé, C.; Almolki, A.; Benhamed, M.; Zedda, C.; Mégret, J.; Berger, P.; Lesèche, G.; Fadel, E.; Yamaguchi, T.; Marthan, R. Heme oxygenase inhibits human airway smooth muscle proliferation via a bilirubin-dependent modulation of ERK1/2 phosphorylation. J. Biol. Chem. 2003, 278, 27160–27168. [Google Scholar] [CrossRef]
- Marinas-Pardo, L.; Mirones, I.; Amor-Carro, O.; Fraga-Iriso, R.; Lema-Costa, B.; Cubillo, I.; Rodriguez Milla, M.A.; Garcia-Castro, J.; Ramos-Barbon, D. Mesenchymal stem cells regulate airway contractile tissue remodeling in murine experimental asthma. Allergy 2014, 69, 730–740. [Google Scholar] [CrossRef]
- Cooke, M.; Allon, A.; Cheng, T.; Kuo, A.; Kim, H.; Vail, T.; Marcucio, R.; Schneider, R.; Lotz, J.; Alliston, T. Structured three-dimensional co-culture of mesenchymal stem cells with chondrocytes promotes chondrogenic differentiation without hypertrophy. Osteoarthr. Cartil. 2011, 19, 1210–1218. [Google Scholar] [CrossRef]
- Bian, L.; Zhai, D.Y.; Mauck, R.L.; Burdick, J.A. Coculture of Human Mesenchymal Stem Cells and Articular Chondrocytes Reduces Hypertrophy and Enhances Functional Properties of Engineered Cartilage. Tissue Eng. Part A 2011, 17, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Kim, H.-J.; Im, G.-I. PTHrP promotes chondrogenesis and suppresses hypertrophy from both bone marrow-derived and adipose tissue-derived MSCs. Biochem. Biophys. Res. Commun. 2008, 373, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Dickhut, A.; Rickert, M.; Richter, W. Human articular chondrocytes secrete parathyroid hormone–related protein and inhibit hypertrophy of mesenchymal stem cells in coculture during chondrogenesis. Arthritis Rheum. 2010, 62, 2696–2706. [Google Scholar] [CrossRef] [PubMed]
- Meretoja, V.V.; Dahlin, R.L.; Kasper, F.K.; Mikos, A.G. Enhanced Chondrogenesis in Co-Cultures with Articular Chondrocytes and Mesenchymal Stem Cells. Biomaterials 2012, 33, 6362–6369. [Google Scholar] [CrossRef] [PubMed]
- Qing, C.; Wei-ding, C.; Wei-min, F. Co-culture of chondrocytes and bone marrow mesenchymal stem cells in vitro enhances the expression of cartilaginous extracellular matrix components. Braz. J. Med. Biol. Res. 2011, 44, 303–310. [Google Scholar] [CrossRef]
- Tsuchiya, K.; Chen, G.; Ushida, T.; Matsuno, T.; Tateishi, T. The effect of coculture of chondrocytes with mesenchymal stem cells on their cartilaginous phenotype in vitro. Mater. Sci. Eng. C 2004, 24, 391–396. [Google Scholar] [CrossRef]
- Marin, A.E. Repopulation of De-Epithelialized Tracheal Grafts; University of Toronto: Toronto, ON, Canada, 2019. [Google Scholar]
- Zhou, Q.; Saijo, Y. Chapter 6—Induced pluripotent stem cells for trachea engineering. In iPSCs in Tissue Engineering; Birbrair, A., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 143–165. [Google Scholar]
- West, A.R.; Osagie, J.; Syeda, S.; Guimond, M.; Parrrenas, L.; Haroon, A.; Imaseun, P.; Turner-Brannen, E. Development of a 3D Bioprinted Airway Smooth Muscle Model for Manipulating Structure and Measuring Contraction. In C109. Even Better Than the Real Thing: Advanced Models of Lung Disease; American Thoracic Society: New York, NY, USA, 2023; Volume 207, p. A6174. [Google Scholar]
- Kwong, G.; Marquez, H.A.; Yang, C.; Wong, J.Y.; Kotton, D.N. Generation of a Purified iPSC-Derived Smooth Muscle-like Population for Cell Sheet Engineering. Stem Cell Rep. 2019, 13, 499–514. [Google Scholar] [CrossRef]
- Varma, R.; Marin-Araujo, A.E.; Rostami, S.; Waddell, T.K.; Karoubi, G.; Haykal, S. Short-Term Preclinical Application of Functional Human Induced Pluripotent Stem Cell-Derived Airway Epithelial Patches. Adv. Heal. Mater. 2021, 10, e2100957. [Google Scholar] [CrossRef]
- Noel, F.E.E.; Karamaoun, C.; Dempsey, J.A.; Mauroy, B. How mammals adapt their breath to body activity—And how this depends on body size. Peer Commun. Math. Comput. Biol. 2020, 1, 100005. [Google Scholar]
- Basil, M.C.; Morrisey, E.E. Lung regeneration: A tale of mice and men. Semin. Cell Dev. Biol. 2020, 100, 88–100. [Google Scholar] [CrossRef]
- Noël, F.; Karamaoun, C.; Demsey, J.; Mauroy, B. The origin of the allometric scaling of lung ventilation in mammals. Peer Commun. J. 2022, 2, e2. [Google Scholar] [CrossRef]
- Li, Y.; Xu, G.K. Editorial: Mechanobiology at multiple scales. Front. Bioeng. Biotechnol. 2023, 11, 1226198. [Google Scholar] [CrossRef]
- Nayak, P.S.; Wang, Y.; Najrana, T.; Priolo, L.M.; Rios, M.; Shaw, S.K.; Sanchez-Esteban, J. Mechanotransduction via TRPV4 regulates inflammation and differentiation in fetal mouse distal lung epithelial cells. Respir. Res. 2015, 16, 60. [Google Scholar] [CrossRef]
- Castro, M.G.B.; Varble, N.A.; Yung, R.C.; Wood, B.J.; Karanian, J.W.; Pritchard, W.F. In Vivo Characterization of the Swine Airway Morphometry and Motion Based on Computed Tomographic Imaging During Respiration. J. Biomech. Eng. 2020, 142, 121009. [Google Scholar] [CrossRef] [PubMed]
- Mariano, C.A.; Sattari, S.; Maghsoudi-Ganjeh, M.; Tartibi, M.; Lo, D.D.; Eskandari, M. Novel Mechanical Strain Characterization of Ventilated ex vivo Porcine and Murine Lung using Digital Image Correlation. Front. Physiol. 2020, 11, 600492. [Google Scholar] [CrossRef] [PubMed]
- Parzianello Egúsquiza, M.G.; Otsuki, D.A.; Costa Auler Junior, J.O. Ex Vivo Porcine Experimental Model for Studying and Teaching Lung Mechanics. J. Vis. Exp. 2024, 206, e64850. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Van Hassel, J.; Pinezich, M.R.; Diane, M.; Hudock, M.R.; Kaslow, S.R.; Gavaudan, O.P.; Fung, K.; Kain, M.L.; Lopez, H., 2nd; et al. Recovery of extracorporeal lungs using cross-circulation with injured recipient swine. J. Thorac. Cardiovasc. Surg. 2024, 167, e106–e130. [Google Scholar] [CrossRef]
- Mia, M.M.; Selvan, A.; Nilanthi, U.; Singh, M.K. YAP/TAZ activation in fibroblasts coordinates fibrotic remodeling, fibroinflammation, and epithelial dysfunction in pulmonary fibrosis. bioRxiv 2025. [Google Scholar] [CrossRef]
- Novak, C.; Ballinger, M.N.; Ghadiali, S. Mechanobiology of Pulmonary Diseases: A Review of Engineering Tools to Understand Lung Mechanotransduction. J. Biomech. Eng. 2021, 143, 110801. [Google Scholar] [CrossRef]
- Madissoon, E.; Oliver, A.; Kleshchevnikov, V.; Wilbrey-Clark, A.; Polnski, K.; Mamanova, L.; Bolt, L.; Pett, P.; Dabrowska, M.; Tuck, L.; et al. The multi-omics spatial lung atlas reveales new cell states and their functions in airway mesenchyme. ERJ Open Res. 2022, 8, 260. [Google Scholar]
- Megas, S.; Wilbrey-Clark, A.; Maartens, A.; Teichmann, S.A.; Meyer, K.B. Spatial Transcriptomics of the Respiratory System. Annu. Rev. Physiol. 2025, 87, 447–470. [Google Scholar] [CrossRef]
- Ma, S.; Wang, W.; Zhou, J.; Liao, S.; Hai, C.; Hou, Y.; Zhou, Z.; Wang, Z.; Su, Y.; Zhu, Y.; et al. Lamination-based organoid spatially resolved transcriptomics technique for primary lung and liver organoid characterization. Proc. Natl. Acad. Sci. USA 2024, 121, e2408939121. [Google Scholar] [CrossRef]
| Scaffold Type | Typical Cellularization | Key Limitations | References |
|---|---|---|---|
| Synthetic polymer scaffolds (PCL, PLA) | Epithelial cells, MSCs, chondrocytes | Variable epithelial coverage; mechanical mismatch causing stenosis | [252,253] |
| Decellularized tracheal grafts | Epithelial cells + MSCs/ chondrocytes | Higher stenosis rates with single-cell seeding; contamination risk | [252,253] |
| 3D-bioprinted hydrogels | Airway epithelial progenitors, MSC-derived SMCs | Mechanical collapse under contractile load; immature SMC phenotype | [254,255] |
| Composite biomaterials (silk-collagen) | hiPSC-derived epithelial patches | Short-term integration only; long-term durability unproven | [256] |
| Cell Selection and Differentiation | ||
|---|---|---|
| Aspect | Traditional Strategy | Mechanotransduction-Informed Strategy |
| Cell Sources | Primary cells, basic iPSC differentiation | iPSCs engineered for enhanced mechanotransduction (TRPV4, YAP/TAZ) |
| Differentiation Goals | Achieve cell type identity | Achieve functional mechanotransduction networks |
| Quality Metrics | Cell viability, marker expression | Mechanotransduction pathway activity, cellular specialisation roles |
| Scaffold Design Philosophy | ||
| Aspect | Traditional Strategy | Mechanotransduction-Informed Strategy |
| Material Selection | Biocompatible polymers, decellularized matrices | Biomimetic stiffness gradients (2–50 kPa) |
| Mechanical Properties | Uniform stiffness matching native tissue | Spatially varied stiffness to guide YAP/TAZ activation |
| Design Rationale | Provide structural support | Create mechanotransduction- responsive environments |
| Failure Prevention | Mechanical reinforcement | Dynamic adaptation through feedback loops |
| Culture and Conditioning Approaches | ||
| Aspect | Traditional Strategy | Mechanotransduction-Informed Strategy |
| Bioreactor Design | Static or simple dynamic culture | Multi-compartment mechanotransduction platforms |
| Mechanical Stimulation | Generic cyclic loading | Pathway-specific conditioning (YAP/TAZ, TRPV4, TGF-β) |
| Monitoring Parameters | Cell growth, basic function | Real-time mechanotransduction pathway activity |
| Maturation Goals | Tissue-like structure | Functional homeostatic mechanisms |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Polish Respiratory Society. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tugade, C.; Ramis, J. A Unified Map of Airway Interactions: Secretome and Mechanotransduction Loops from Development to Disease. Adv. Respir. Med. 2025, 93, 51. https://doi.org/10.3390/arm93060051
Tugade C, Ramis J. A Unified Map of Airway Interactions: Secretome and Mechanotransduction Loops from Development to Disease. Advances in Respiratory Medicine. 2025; 93(6):51. https://doi.org/10.3390/arm93060051
Chicago/Turabian StyleTugade, Crizaldy, and Jopeth Ramis. 2025. "A Unified Map of Airway Interactions: Secretome and Mechanotransduction Loops from Development to Disease" Advances in Respiratory Medicine 93, no. 6: 51. https://doi.org/10.3390/arm93060051
APA StyleTugade, C., & Ramis, J. (2025). A Unified Map of Airway Interactions: Secretome and Mechanotransduction Loops from Development to Disease. Advances in Respiratory Medicine, 93(6), 51. https://doi.org/10.3390/arm93060051






