Prognostic Role of Serum Adrenomedullin in Patients with Ventilator Associated Pneumonia
Abstract
:Highlights
- Ventilator associated pneumonia (VAP) is a common type of sepsis in mechanically ventilated patients that can progress to septic shock and increased mortality.
- Adrenomedullin (ADM) was found to be elevated in septic patients, so we explored the possible prognostic role of adrenomedullin in VAP patients.
- A total of 140 mechanically ventilated patients with proven VAP after medical ICU admission were consecutively enrolled into this prospective observational study.
- Serum ADM on enrollment could predict the unfavorable outcome with a sensitivity of 96.25%. After 5 days, it showed a sensitivity of 57.5% and a specificity of 100%.
- We found that serum adrenomedullin when measured at days 0 and 5 of VAP diagnosis may serve as an early predictor of unfavorable outcome (prolonged mechanical ventilation, septic shock, and mortality).
Abstract
1. Introduction
2. Patients and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- American Thoracic Society. Infectious Diseases Society of America: Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am. J. Respir. Crit. Care Med. 2005, 171, 388–416. [Google Scholar] [CrossRef] [PubMed]
- Kalanuria, A.A.; Ziai, W.; Mirski, M. Ventilator-associated pneumonia in the ICU. Crit. Care 2014, 18, 208. [Google Scholar] [CrossRef] [PubMed]
- Rea-Neto, A.; Youssef, N.C.; Tuche, F. Diagnosis of ventilator-associated pneumonia: A systematic review of the literature. Crit. Care 2008, 12, R56. [Google Scholar] [CrossRef]
- Afshari, A.; Pagani, L.; Harbarth, S. Year in review 2011: Critical Care-infection. Crit. Care Med. 2012, 16, 242. [Google Scholar] [CrossRef]
- Aydogdu, M.; Gursel, G. Predictive factors for septic shock in patients with ventilator-associated pneumonia. South Med. J. 2008, 101, 1222–1226. [Google Scholar] [CrossRef] [PubMed]
- Klompas, M. Clinician’s Corner: Does this patient have ventilator-associated pneumonia? JAMA 2007, 297, 1583–1593. [Google Scholar] [CrossRef] [PubMed]
- Geven, C.; Kox, M.; Pickkers, P. Adrenomedullin and Adrenomedullin-Targeted Therapy as Treatment Strategies Relevant for Sepsis. Front. Immunol. 2018, 9, 292. [Google Scholar] [CrossRef]
- Ueda, S.; Nishio, K.; Minamino, N.; Kubo, A.; Akai, Y.; Kangawa, K.; Matsuo, H.; Fujimura, Y.; Yoshioka, A.; Masui, K.; et al. Increased plasma levels of adrenomedullin in patients with systemic inflammatory response syndrome. Am. J. Respir. Crit. Care Med. 1999, 160, 132–136. [Google Scholar] [CrossRef]
- Chen, Y.-X.; Li, C.-S. The Predictive Value of Adrenomedullin for Development of Severe Sepsis and Septic Shock in Emergency Department. BioMed Res. Int. 2013, 2013, 960101. [Google Scholar] [CrossRef]
- Helmy, T.A.; Beshey, B.N. Prognostic role of adrenomedullin in sepsis. Int. J. Adv. Res. Biol. Sci. 2016, 3, 136–141. [Google Scholar]
- Gursel, G.; Demirtas, S. Value of APACHE II, SOFA and CPIS scores in predicting prognosis in patients with ventilator-associated pneumonia. Respiration 2006, 73, 503–508. [Google Scholar] [CrossRef]
- Madan, H.K.; Singh, R.; Karnik, N.D. Value of SOFA Scores in Predicting Prognosis in Patients with Ventilator Associated Pneumonia. Int. J. Anat. Radiol. Surg. 2016, 5, 6–11. [Google Scholar]
- Di Somma, S.; Magrini, L.; Travaglino, F.; Lalle, I.; Fiotti, N.; Cervellin, G.; Avanzi, G.C.; Lupia, E.; Maisel, A.; Hein, F.; et al. Opinion paper on innovative approach of biomarkers for infectious diseases and sepsis management in the emergency department. Clin. Chem. Lab. Med. 2013, 51, 1167–1175. [Google Scholar] [CrossRef]
- Kalil, A.C.; Metersky, M.L.; Klompas, M.; Muscedere, J.; Sweeney, D.A.; Palmer, L.B.; Napolitano, L.M.; O’Grady, N.P.; Bartlett, J.G.; Carratalà, J.; et al. Management of Adults with Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 2016, 63, e61–e111, Erratum in Clin. Infect. Dis. 2017, 64, 1298; Erratum in Clin. Infect. Dis. 2017, 65, 1435; Erratum in Clin. Infect. Dis. 2017, 65, 2161. [Google Scholar] [CrossRef]
- Dellinger, R.P.; Levy, M.M.; Rhodes, A.; Annane, D.; Gerlach, H.; Opal, S.M.; Sevransky, J.E.; Sprung, C.L.; Douglas, I.S.; Jaeschke, R.; et al. Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup: Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit. Care Med. 2013, 41, 580–637. [Google Scholar] [CrossRef]
- Wu, D.; Wu, C.; Zhang, S.; Zhong, Y. Risk Factors of Ventilator-Associated Pneumonia in Critically Ill Patients. Front. Pharmacol. 2019, 10, 482. [Google Scholar] [CrossRef]
- McEnery, T.; Martin-Loeches, I. Predicting ventilator-associated pneumonia. Ann. Transl. Med. 2020, 8, 670. [Google Scholar] [CrossRef]
- Khaleeq, G.; Garcha, P.; Hirani, A.; Patrick, H. Clinical pulmonary infection score (CPIS): Relationship to mortality in patients with ventilator associated pneumonia (VAP). Chest 2006, 130, 218S. [Google Scholar] [CrossRef]
- Zhou, X.-Y.; Ben, S.-Q.; Chen, H.-L.; Ni, S.-S. A comparison of APACHE II and CPIS scores for the prediction of 30-day mortality in patients with ventilator-associated pneumonia. Int. J. Infect. Dis. 2015, 30, 144–147. [Google Scholar] [CrossRef]
- Boeck, L.; Eggimann, P.; Smyrnios, N.; Pargger, H.; Thakkar, N.; Siegemund, M.; Morgenthaler, N.G.; Rakic, J.; Tamm, M.; Stolz, D. The Sequential Organ Failure Assessment score and copeptin for predicting survival in ventilator-associated pneumonia. J. Crit. Care 2012, 27, 523.e1–523.e9. [Google Scholar] [CrossRef]
- Núñez, S.A.; Roveda, G.; Zárate, M.S.; Emmerich, M.; Verón, M.T. Ventilator-associated pneumonia in patients on prolonged mechanical ventilation: Description, risk factors for mortality, and performance of the SOFA score. J. Bras. Pneumol. 2021, 47, e20200569. [Google Scholar] [CrossRef]
- Karakike, E.; Kyriazopoulou, E.; Tsangaris, I.; Routsi, C.; Vincent, J.L.; Giamarellos-Bourboulis, E.J. The early change of SOFA score as a prognostic marker of 28-day sepsis mortality: Analysis through a derivation and a validation cohort. Crit. Care 2019, 23, 387. [Google Scholar] [CrossRef]
- Póvoa, P.; Coelho, L.; Almeida, E.; Fernandes, A.; Mealha, R.; Moreira, P.; Sabino, H. C-reactive protein as a marker of ventilator-associated pneumonia resolution: A pilot study. Eur. Respir. J. 2005, 25, 804–812. [Google Scholar] [CrossRef]
- Ehlenz, K.; Koch, B.; Preuss, P.; Simon, B.; Koop, I.; Lang, R.E. High levels of circulating adrenomedullin in severe illness: Correlation with C-reactive protein and evidence against the adrenal medulla as site of origin. Exp. Clin. Endocrinol. Diabetes 1997, 105, 156–162. [Google Scholar] [CrossRef]
- Hillas, G.; Vassilakopoulos, T.; Plantza, P.; Rasidakis, A.; Bakakos, P. C-reactive protein and procalcitonin as predictors of survival and septic shock in ventilator-associated pneumonia. Eur. Respir. J. 2010, 35, 805–811. [Google Scholar] [CrossRef]
- Caironi, P.; Latini, R.; Struck, J.; Hartmann, O.; Bergmann, A.; Maggio, G.; Cavana, M.; Tognoni, G.; Pesenti, A.; Gattinoni, L.; et al. Circulating Biologically Active Adrenomedullin (bio-ADM) Predicts Hemodynamic Support Requirement and Mortality During Sepsis. Chest 2017, 152, 312–320. [Google Scholar] [CrossRef]
- Bou Chebl, R.; El Khuri, C.; Shami, A.; Rajha, E.; Faris, N.; Bachir, R.; Abou Dagher, G. Serum lactate is an independent predictor of hospital mortality in critically ill patients in the emergency department: A retrospective study. Scand. J. Trauma Resusc. Emerg. Med. 2017, 25, 69. [Google Scholar] [CrossRef]
- Lee, S.M.; An, W.S. New clinical criteria for septic shock: Serum lactate level as new emerging vital sign. J. Thorac. Dis. 2016, 8, 1388–1390. [Google Scholar] [CrossRef]
- Marino, R.; Struck, J.; Maisel, A.S.; Magrini, L.; Bergmann, A.; Di Somma, S. Plasma adrenomedullin is associated with short-term mortality and vasopressor requirement in patients admitted with sepsis. Crit. Care 2014, 18, R34. [Google Scholar] [CrossRef]
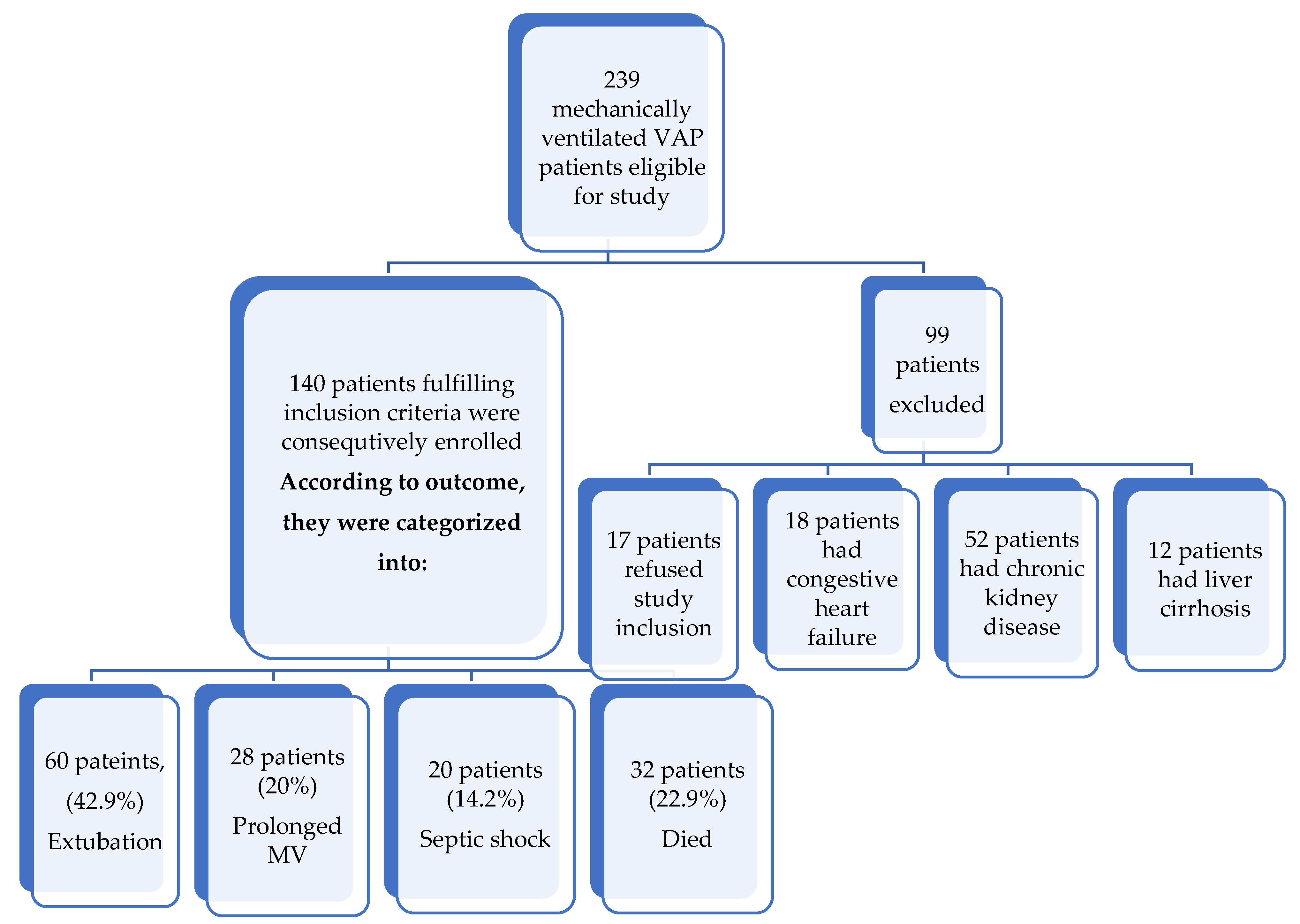
| Extubation (n = 60) | Prolonged MV (n = 28) | Septic Shock (n = 20) | Died (n = 32) | χ2 | p | |
|---|---|---|---|---|---|---|
| Age (years): Range Mean ± SD | 19–86 | 28–70 | 50–66 | 51–73 | (F) = | 0.061 |
| 51.3 ± 24.6 | 50.1 ± 14.9 | 58.0 ± 6.22 | 60.4 ± 7.8 | 2.515 | ||
| Sex: Male (No%) Female (No%) | 32 (53.3) | 16 (57.1) | 12 (60.0) | 22 (68.8) | 2.085 | 0.555 |
| 28 (46.7) | 12 (42.9) | 8 (40.0) | 10 (31.3) | |||
| Comorbidities: DM (%) HTN (%) IHD (%) COPD (%) | 44 (73.3) | 20 (71.4) | 15 (75.0) | 23 (71.86) | 2.101 | 0.989 |
| 38 (63.3) | 17 (60.7) | 12 (60.0) | 17 (53.13) | |||
| 27 (45.0) | 12 (42.9) | 8 (40.0) | 13 (40.63) | |||
| 12 (20.0) | 7 (25.0) | 3 (15.0) | 3 (9.38) | |||
| MV days before VAP Range Mean ± SD | 2–5 | 3–5 | 2–6 | 2–4 | (F) = 2.703 | 0.641 |
| 3.1 ± 0.98 | 2.58 ± 1.03 | 2.06 ± 1.9 | 3.06 ± 0.08 | |||
| Main diagnosis: Coma (%) Respiratory failure (%) Sepsis (%) Toxidromes (%) | 31 (51.67) | 12 (42.86) | 11 (55.0) | 18 (56.25) | 2.246 | 0.989 |
| 12 (20.0) | 7 (25.0) | 3 (15.0) | 5 (15.63) | |||
| 12 (20.0) | 5 (17.86) | 4 (20.0) | 6 (18.75) | |||
| 5 (8.3) | 4 (14.29) | 2 (10.0) | 3 (9.38) | |||
| Culture of Tracheal Aspirate at day 0: MRSA (%) Pseudomonas (%) Klebsiella (%) E-coli (%) Acinetobacter (%) Candida (%) Contaminated (%) | 4 (6.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4.015 | 0.999 |
| 0 (0.0) | 0 (0.0) | 4 (14.3) | 0 (0.0) | |||
| 20 (33.3) | 20 (71.4) | 12 (42.8) | 24 (75.0) | |||
| 4 (6.7) | 0 (0.0) | 0 (0.0) | 4 (12.5) | |||
| 32 (53.3) | 8 (28.6) | 12 (42.8) | 4 (12.5) | |||
| 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (12.5) | |||
| 4 (6.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Parameter: | Time Interval | Extubation (n = 60) | Prolonged MV (n = 28) | Septic Shock (n = 20) | Died (n = 32) | F/H | p |
|---|---|---|---|---|---|---|---|
| APACHE II (Mean ± SD) | Day 0 | 11.1 ± 4.6 | 12.9 ± 3.1 | 17.8 ± 5.4 | 17.1 ± 4.4 | 19.316 * | <0.001 * |
| pExtupation | 0.323 | <0.001 * | <0.001 * | ||||
| Sig. bet. Grps | p1 = 0.001 *, p2 = 0. 002 *, p3 = 0.950 | ||||||
| (Mean ± SD) pExtupation Sig. bet. Grps | Day 5 | 10.9 ±3.9 | 13.6 ± 2.9 | 21.3 ± 3.2 | 20.6 ± 2.9 | 20.932 * | <0.001 * |
| 0.291 | <0.001 * | <0.001 * | |||||
| p1 < 0.001 *, p2 = 0.001 *, p3 = 0.694 | |||||||
| CRP (mg/L) | |||||||
| (Mean ± SD) | Day 0 | 37.3 ± 33.7 | 45.7 ± 8.5 | 44.0 ± 44.4 | 44.6 ± 14.7 | 0.806 | 0.493 |
| (Mean ± SD) | Day 5 | 18.6 ± 7.54 | 28.3 ± 9.5 | 52.0 ± 19.2 | 34.4 ± 15.8 | 59.320 * | <0.001 * |
| pExtupation | <0.001 * | <0.001 * | <0.001 * | ||||
| Sig. bet. Grps | p1 < 0.001 *, p2 = 0.300, p3 = 0.006 * | ||||||
| Lactate (mmol/L) | |||||||
| (Mean ± SD) | Day 0 | 2.39 ± 0.38 | 2.75 ± 0.28 | 2.78 ± 0.42 | 2.97 ± 0.30 | 21.535 * | <0.001 * |
| pExtupation | <0.001 * | <0.001 * | <0.001 * | ||||
| Sig. bet. Grps | p1 = 0.994, p2 = 0.235, p3 = 0.235 | ||||||
| (Mean ± SD) | Day 5 | 2.08 ± 0.35 | 2.7 ± 0.54 | 3.64 ± 0.89 | 3.33 ± 0.66 | 49.363* | < 0.001* |
| pExtupation | <0.001 * | <0.001 * | <0.001 * | ||||
| Sig. bet. Grps | p1 < 0.001 *, p2 < 0.001 *, p3 = 0.855 | ||||||
| SOFA score | |||||||
| (Mean ± SD) | Day 0 | 6.07 ± 1.62 | 6.7 ± 2.16 | 7.0 ± 2.25 | 5.88 ± 1.29 | 6.368 | 0.095 |
| (Mean ± SD) | Day 5 | 3.60 ± 1.80 | 4.29 ± 1.86 | 6.60 ± 2.64 | 6.38 ± 2.03 | 41.485 * | <0.001 * |
| pExtupation | <0.001 * | <0.001 * | <0.001 * | ||||
| Sig. bet. Grps | p1 = 0.001 *, p2 = 0.001 *, p3 = 0.892 | ||||||
| Adrenomedullin (pg/mL) | |||||||
| (Mean ± SD) | Day 0 | 19.1 ± 8.42 | 34.1 ± 18.2 | 42.4 ± 26.7 | 49.8 ± 23.0 | 46.703* | <0.001 * |
| pExtupation | <0.001 * | <0.001 * | <0.001 * | ||||
| Sig. bet. Grps | p1 = 0.579, p2 = 0.027*, p3 = 0.149 | ||||||
| (Mean ± SD) | Day 5 | 7.82 ± 5.16 | 18.5 ± 9.66 | 52.5 ± 35.2 | 43.9 ± 29.1 | 74.411 * | <0.001 * |
| pExtupation | <0.001 * | <0.001 * | <0.001 * | ||||
| Sig. bet. Grps | p1 = 0.016 *, p2 = 0.010 *, p3 = 0.897 | ||||||
| Parameter: | Adrenomedullin (ADM) | |||
|---|---|---|---|---|
| Day 0 | Day 5 | |||
| rs | p | rs | p | |
| CRP | 0.085 $ | 0.320 | 0.702 $ | <0.001 * |
| SOFA score | 0.262 # | 0.049 * | 0.820 # | <0.001 * |
| APACHE II score | 0.437 # | <0.001 * | 0.814 # | <0.001 * |
| Lactate | 0.992 $ | <0.001 * | 0.990 $ | <0.001 * |
| Extubation (n = 60) | Prolonged M.V (n = 28) | Septic Shock (n = 20) | Died (n = 32) | F | p | |
|---|---|---|---|---|---|---|
| M.V (days) | ||||||
| (Mean ± SD) | 8.6 ± 1.09 | 14.4 ± 1.20 | 16.6 ± 1.90 | 14.38 ± 1.90 | 227.81 * | <0.001 * |
| pExtupation | <0.001 * | <0.001 * | <0.001 * | |||
| Sig. bet. Grps | p1 < 0.001 *, p2 = 0.999, p3 < 0.001 * | |||||
| ICU stay (days) | ||||||
| (Mean ± SD) | 11.13 ± 0.72 | 18.7 ± 1.5 | 20.8 ± 1.77 | 14.38 ± 1.90 | 337.6 * | <0.001 * |
| pExtupation | <0.001 * | <0.001 * | <0.001 * | |||
| Sig. bet. Grps | p1 < 0.001 *, p2 < 0.001 *, p3 < 0.001 * | |||||
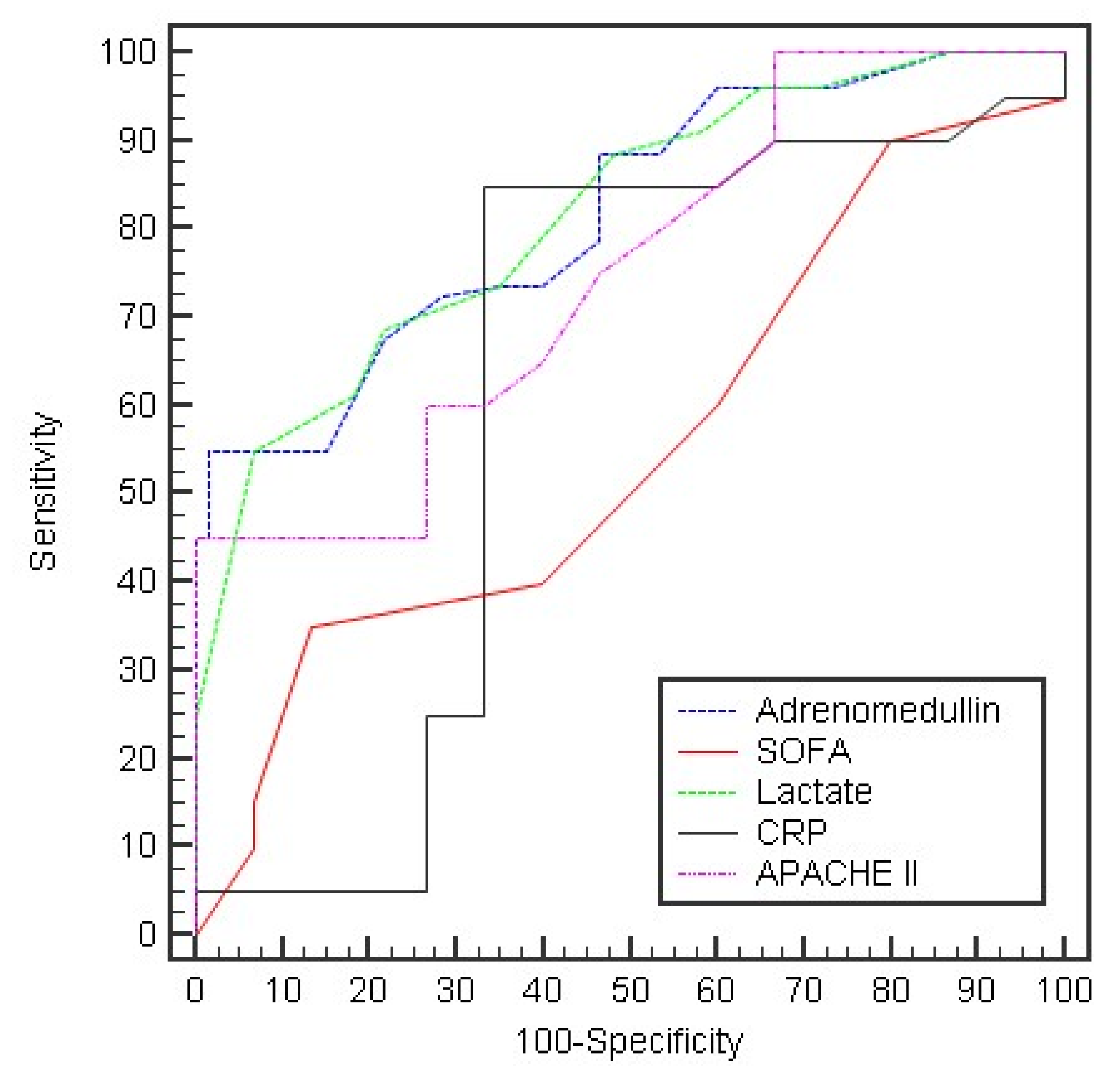
| Day 0: | AUC | p | 95% C.I | Cut off | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|---|---|
| Adrenomedullin | 0.818 * | <0.001 * | 0.751–0.885 | >16 | 96.25 | 40.0 | 68.1 | 88.9 |
| SOFA | 0.555 | 0.266 | 0.459–0.651 | ≤5 | 50.0 | 60.0 | 62.5 | 47.4 |
| Lactate | 0.817 * | <0.001 * | 0.749–0.885 | >2.9 | 55.0 | 93.33 | 91.7 | 60.9 |
| CRP | 0.552 | 0.296 | 0.450–0.654 | >22 | 60.0 | 66.67 | 70.6 | 55.6 |
| APACHE II | 0.747 * | <0.001 * | 0.667–0.826 | >12 | 65.0 | 60.0 | 68.4 | 56.3 |
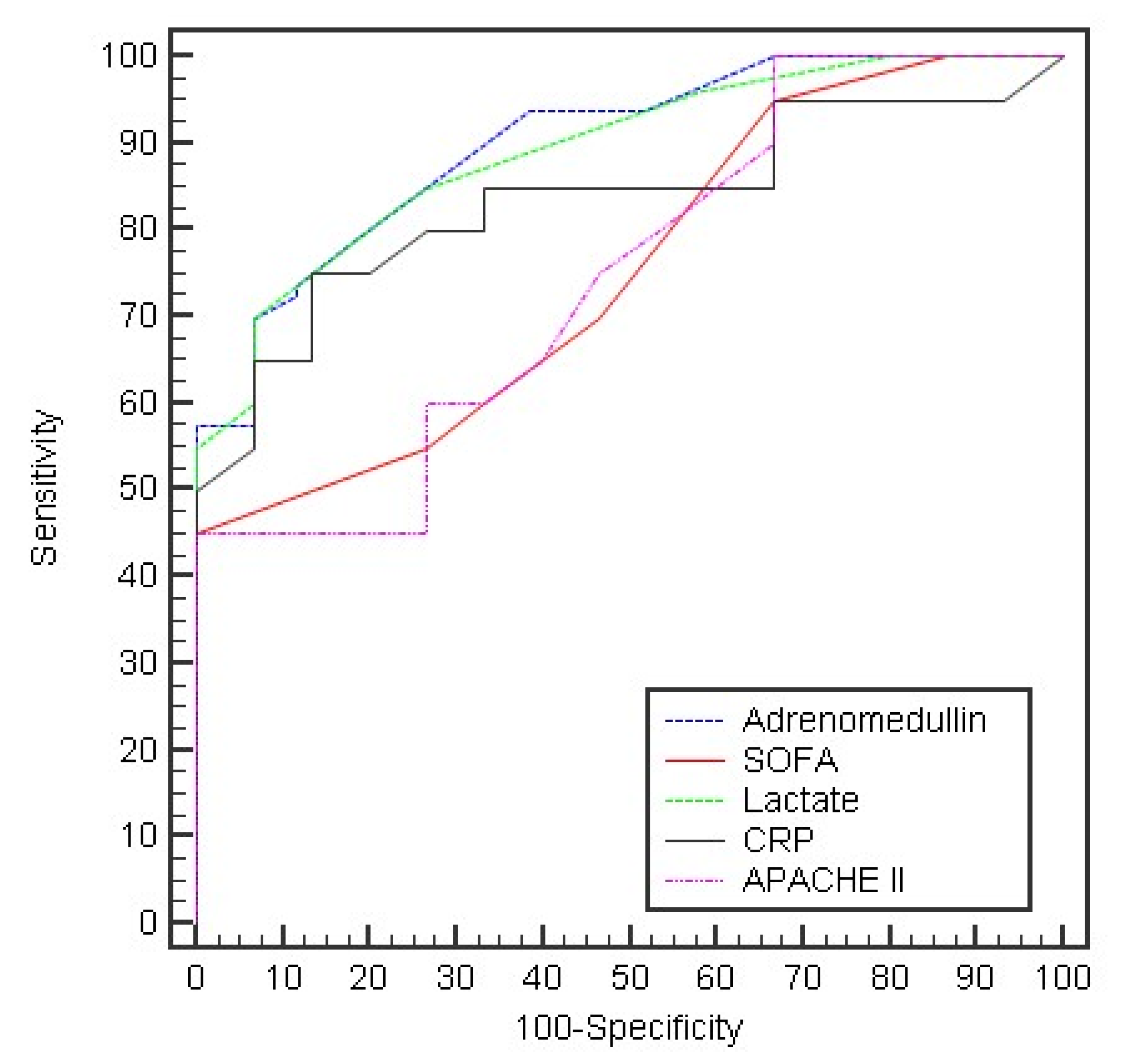
| Day 5: | ||||||||
| Adrenomedullin | 0.901 * | <0.001 * | 0.853–0.949 | >20 | 57.50 | 100.0 | 100.0 | 63.8 |
| SOFA Lactate CRP APACHE II | 0.752 * 0.893 * 0.835 * 0.747 * | <0.001 * <0.001 * <0.001 * <0.001 * | 0.673–0.830 0.842–0.943 | >5 >2.5 | 55.0 70.0 | 73.33 93.33 | 73.3 93.3 | 55.0 70.0 |
| 0.768–0.902 0.667–0.826 | >34 >12 | 55.0 65.0 | 93.33 60.0 | 91.7 68.4 | 60.9 56.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Helmy, T.A.; Tammam, H.H.; Leuis, M.E.; Beshey, B.N. Prognostic Role of Serum Adrenomedullin in Patients with Ventilator Associated Pneumonia. Adv. Respir. Med. 2022, 90, 349-359. https://doi.org/10.3390/arm90040044
Helmy TA, Tammam HH, Leuis ME, Beshey BN. Prognostic Role of Serum Adrenomedullin in Patients with Ventilator Associated Pneumonia. Advances in Respiratory Medicine. 2022; 90(4):349-359. https://doi.org/10.3390/arm90040044
Chicago/Turabian StyleHelmy, Tamer Abdallah, Haitham Hamdy Tammam, Michael Ebrahim Leuis, and Bassem Nashaat Beshey. 2022. "Prognostic Role of Serum Adrenomedullin in Patients with Ventilator Associated Pneumonia" Advances in Respiratory Medicine 90, no. 4: 349-359. https://doi.org/10.3390/arm90040044
APA StyleHelmy, T. A., Tammam, H. H., Leuis, M. E., & Beshey, B. N. (2022). Prognostic Role of Serum Adrenomedullin in Patients with Ventilator Associated Pneumonia. Advances in Respiratory Medicine, 90(4), 349-359. https://doi.org/10.3390/arm90040044






