Role of Cr Element in Highly Dense Passivation of Fe-Based Amorphous Alloy
Abstract
:1. Introduction
2. Experimental
2.1. Sample Preparation
2.2. Structural Analysis
2.3. Electrochemical Tests
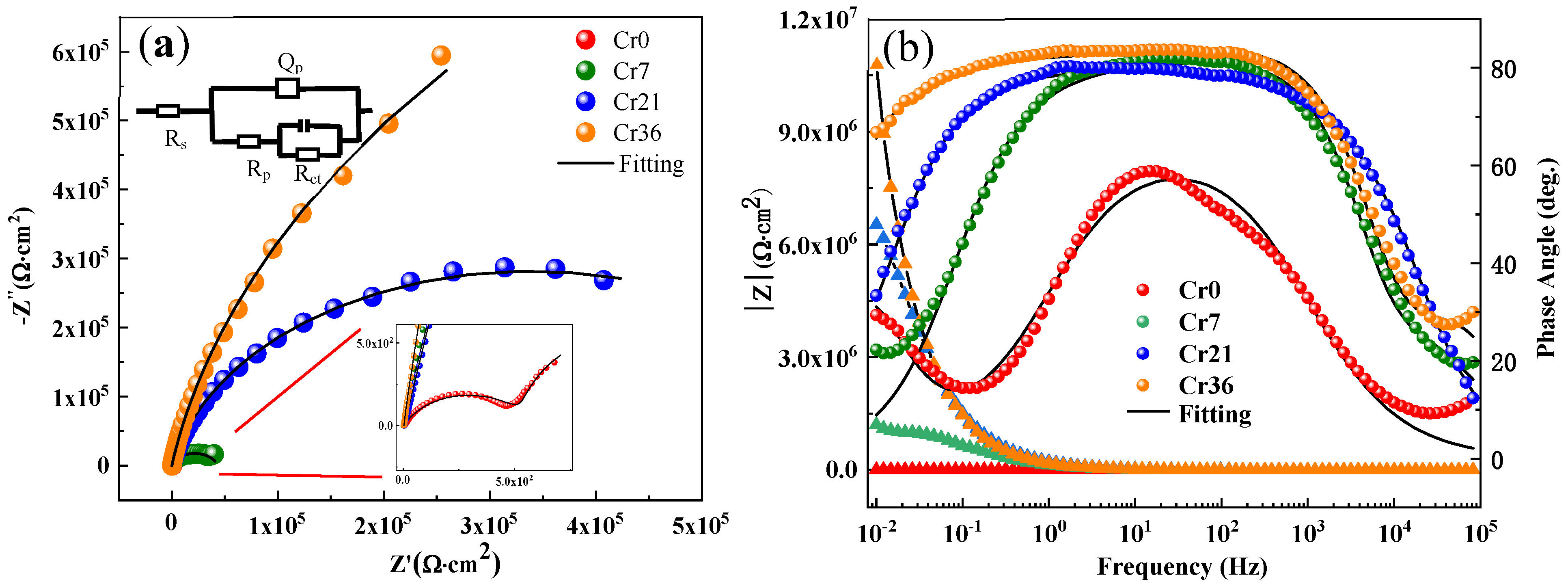
3. Results and Discussion
Microstructure
4. Conclusions
- (1)
- With increasing Cr content cCr (x), the stability and glass formability (GFA) of Fe-based alloys firstly increased until x = 21 and then decreased drastically as x = 36. Simultaneously, the CrFeB phase and clusters in the samples tended to form with strong orientations in the ingots and as-spun ribbons, which deteriorated the GFA of the alloy, as its abundance was high enough.
- (2)
- With increasing cCr, the corrosion rate Rim, corrosion current icorr, and deduced corrosion rate CR of Fe-based ribbons increased monotonically, as well as their hardness, whereas their pitting resistance increased until x = 7 and decreased as x = 21, indicating that the most stable passive film formed at a proper cCr, which was confirmed by XPS data. This work can give a new clue to design and prepare highly corrosion-resistant Fe-based alloys.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, C.-Y.; Lin, T.-J.; Sheu, H.-H.; Lee, H.-B. A study on corrosion and corrosion-wear behavior of Fe-based amorphous alloy coating prepared by high velocity oxygen fuel method. J. Mater. Res. Technol. 2021, 15, 4880–4895. [Google Scholar] [CrossRef]
- Farmer, J.; Choi, J.-S.; Saw, C.; Haslam, J.; Day, D.; Hailey, P.; Lian, T.; Rebak, R.; Perepezko, J.; Payer, J.; et al. Iron-Based Amorphous Metals: High-Performance Corrosion-Resistant Material Development. Metall. Mater. Trans. A 2009, 40, 1289–1305. [Google Scholar] [CrossRef]
- Ibrahim, M.Z.; Sarhan, A.A.D.; Kuo, T.Y.; Yusof, F.; Hamdi, M.; Lee, T.M. Developing a new laser cladded FeCrMoCB metallic glass layer on nickel-free stainless-steel as a potential superior wear-resistant coating for joint replacement implants. Surf. Coat. Technol. 2020, 392, 125755. [Google Scholar] [CrossRef]
- Souza, C.A.C.; Ribeiro, D.V.; Kiminami, C.S. Corrosion resistance of Fe-Cr-based amorphous alloys: An overview. J. Non-Cryst. Solids 2016, 442, 56–66. [Google Scholar] [CrossRef]
- Montemor, M.F.; Simões, A.m.p.; Ferreira, M.G.S.; Belo, M.D.C. The role of Mo in the chemical composition and semiconductive behaviour of oxide films formed on stainless steels. Corros. Sci. 1999, 41, 17–34. [Google Scholar] [CrossRef]
- Chen, Q.; Yan, Z.; Guo, L.; Zhang, H.; Zhang, L.; Kim, K.; Li, X.; Wang, W. Enhancing the acid orange dye degradation efficiency of Mg-based glassy alloys with introducing porous structure and zinc oxide. J. Alloys Compd. 2020, 831, 154817. [Google Scholar] [CrossRef]
- Stokłosa, Z.; Rasek, J.; Kwapuliński, P.; Badura, G.; Haneczok, G.; Pająk, L.; Lelątko, J.; Kolano-Burian, A. Magnetic, electrical and plastic properties of Fe76Nb2Si13B9, Fe75Ag1Nb2Si13B9 and Fe75Cu1Nb2Si13B9 amorphous alloys. J. Alloys Compd. 2011, 509, 9050–9054. [Google Scholar] [CrossRef]
- Li, L.Y.; Sun, C.H.; Ruan, Y.; Wei, B. Duplex corrosion mechanisms correlated to α/γ phase selection in containerlessly processed Fe-Cr-Ni-Mo alloy. Corros. Sci. 2023, 221, 111311. [Google Scholar] [CrossRef]
- Kobayashi, A.; Yano, S.; Kimura, H.; Inoue, A. Mechanical property of Fe-base metallic glass coating formed by gas tunnel type plasma spraying. Surf. Coat. Technol. 2008, 202, 2513–2518. [Google Scholar] [CrossRef]
- Yoon, S.; Kim, J.; Bae, G.; Kim, B.; Lee, C. Formation of coating and tribological behavior of kinetic sprayed Fe-based bulk metallic glass. J. Alloys Compd. 2011, 509, 347–353. [Google Scholar] [CrossRef]
- Zenebe, D.; Yi, S.; Kim, S.S. Sliding friction and wear behavior of Fe-based bulk metallic glass in 3.5% NaCl solution. J. Mater. Sci. 2011, 47, 1446–1451. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, L.; Chan, K.C.; Chen, Q.; Tang, C.Y. Wear behavior of HVOF-sprayed Fe-based amorphous coatings. Intermetallics 2012, 29, 80–85. [Google Scholar] [CrossRef]
- Lekakh, S.N.; Buchely, M.; Li, M.; Godlewski, L. Effect of Cr and Ni concentrations on resilience of cast Nb-alloyed heat resistant austenitic steels at extreme high temperatures. Mater. Sci. Eng. A 2023, 873, 145027. [Google Scholar] [CrossRef]
- Xiang, S.; Fan, Z.; Chen, T.; Lian, X.; Guo, Y. Microstructure evolution and creep behavior of nitrogen-bearing austenitic Fe–Cr–Ni heat-resistant alloys with various carbon contents. J. Mater. Res. Technol. 2023, 23, 316–330. [Google Scholar] [CrossRef]
- Long, Z.L.; Chang, C.T.; Ding, Y.H.; Shao, Y.; Zhang, P.; Shen, B.L.; Inoue, A. Corrosion behavior of Fe-based ferromagnetic (Fe, Ni)–B–Si–Nb bulk glassy alloys in aqueous electrolytes. J. Non-Cryst. Solids 2008, 354, 4609–4613. [Google Scholar] [CrossRef]
- Hashimoto, K. What we have learned from studies on chemical properties of amorphous alloys? Appl. Surf. Sci. 2011, 257, 8141–8150. [Google Scholar] [CrossRef]
- Tsai, P.H.; Xiao, A.C.; Li, J.B.; Jang, J.S.C.; Chu, J.P.; Huang, J.C. Prominent Fe-based bulk amorphous steel alloy with large supercooled liquid region and superior corrosion resistance. J. Alloys Compd. 2014, 586, 94–98. [Google Scholar] [CrossRef]
- Zohdi, H.; Shahverdi, H.R.; Hadavi, S.M.M. Effect of Nb addition on corrosion behavior of Fe-based metallic glasses in Ringer’s solution for biomedical applications. Electrochem. Commun. 2011, 13, 840–843. [Google Scholar] [CrossRef]
- Ni, H.S.; Liu, X.H.; Chang, X.C.; Hou, W.L.; Liu, W.; Wang, J.Q. High performance amorphous steel coating prepared by HVOF thermal spraying. J. Alloys Compd. 2009, 467, 163–167. [Google Scholar] [CrossRef]
- Otsubo, F.; Kishitake, K. Corrosion Resistance of Fe-16%Cr-30%Mo-(C,B,P) Amorphous Coatings Sprayed by HVOF and APS Processes. Mater. Trans. 2005, 46, 80–83. [Google Scholar] [CrossRef]
- Garfias-Mesias, L.F.; Sykes, J.M.; Tuck, C.D.S. The effect of phase compositions on the pitting corrosion of 25 Cr duplex stainless steel in chloride solutions. Corros. Sci. 1996, 38, 1319–1330. [Google Scholar] [CrossRef]
- Cleland, J.H. What does the pitting resistance equivalent really tell us? Eng. Fail. Anal. 1996, 3, 65–69. [Google Scholar] [CrossRef]
- Liljas, M.; Johansson, P.; Liu, H.-P.; Olsson, C.-O.A. Development of a Lean Duplex Stainless Steel. Steel Res. Int. 2008, 79, 466–473. [Google Scholar] [CrossRef]
- Tan, M.-W.; Akiyama, E.; Kawashima, A.; Asami, K.; Hashimoto, K. The effect of air exposure on the corrosion behavior of amorphous Fe-8Cr-Mo-13P-7C alloys in 1 M HCl. Corros. Sci. 1995, 37, 1289–1301. [Google Scholar] [CrossRef]
- Jung, K.; Ahn, S.; Kim, Y.; Oh, S.; Ryu, W.-H.; Kwon, H. Alloy design employing high Cr concentrations for Mo-free stainless steels with enhanced corrosion resistance. Corros. Sci. 2018, 140, 61–72. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Q.; Xie, L.; Zhang, G.; Mu, B.; Chang, C.; Li, H.; Ma, X. Development of novel Fe-based bulk metallic glasses with excellent wear and corrosion resistance by adjusting the Cr and Mo contents. Intermetallics 2023, 153, 107801. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, H.; Wang, L.; Li, M.; Si, R.; Sun, D. Research on the structure-activity relationship of Fe-Cr alloy in marine environment based on synchrotron radiation: Effect of Cr content. Surf. Interfaces 2021, 26, 101370. [Google Scholar] [CrossRef]
- Hua, Y.; Mohammed, S.; Barker, R.; Neville, A. Comparisons of corrosion behaviour for X65 and low Cr steels in high pressure CO2-saturated brine. J. Mater. Sci. Technol. 2020, 41, 21–32. [Google Scholar] [CrossRef]
- Yan, J.; Liu, T.; Wang, M.; Sun, J.; Dong, S.; Zhao, L.; Wang, Q. Constitutional supercooling and corresponding microstructure transition triggered by high magnetic field gradient during directional solidification of Al-Fe eutectic alloy. Mater. Charact. 2022, 188, 111920. [Google Scholar] [CrossRef]
- Qi, Z.G.; Chen, Q.; Feng, Y.; Liu, H.Z.; Wang, Z.X.; Song, Z.Q.; Yan, Z.C.; Guo, L.Y.; Zhang, X.H.; Wang, W.M. Light activated methylene blue degradation by magnetic Fe80P C20-(x = 0, 4, 7 and 13) glassy ribbons. J. Alloys Compd. 2023, 953, 170135. [Google Scholar] [CrossRef]
- Meng, L.L.; Li, X.Y.; Pang, J.; Wang, L.; An, B.; Yin, L.J.; Song, K.K.; Wang, W.M. Casting Atmosphere Effects on the Precipitates, Magnetism, and Corrosion Resistance of Fe78Si9B13 Glassy Alloys. Metall. Mater. Trans. A 2013, 44, 5122–5133. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, B.; Fu, Y.; Hu, X.; Cao, X.; Pan, Z.; Wei, Y.; Luo, H.; Li, X. Effect of cold deformation on corrosion behavior of selective laser melted 316L stainless steel bipolar plates in a simulated environment for proton exchange membrane fuel cells. Corros. Sci. 2022, 201, 110257. [Google Scholar] [CrossRef]
- Lu, K.; Sui, M.L.; Wang, J.T. Eutectic crystallization products and their orientation relationship in amorphous TM-M alloys. J. Mater. Sci. Lett. 1990, 630–632. [Google Scholar] [CrossRef]
- Lu, Z.P.; Liu, C.T. A new glass-forming ability criterion for bulk metallic glasses. Acta Mater. 2002, 50, 3501–3512. [Google Scholar] [CrossRef]
- Han, B.; Chen, Y.; Tao, W.; Lei, Z.; Li, H.; Guo, S.; Li, P. Nano-indentation investigation on the local softening of equiaxed zone in 2060-T8/2099-T83 aluminum-lithium alloys T-joints welded by double-sided laser beam welding. J. Alloys Compd. 2018, 756, 145–162. [Google Scholar] [CrossRef]
- Hatada, R.; Flege, S.; Ashraf, M.N.; Timmermann, A.; Schmid, C.; Ensinger, W. The Influence of Preparation Conditions on the Structural Properties and Hardness of Diamond-Like Carbon Films, Prepared by Plasma Source Ion Implantation. Coatings 2020, 10, 360. [Google Scholar] [CrossRef]
- Jiang, B.; Doi, K.; Tsuchiya, K.; Kawano, Y.; Kori, A.; Ikushima, K. Micromechanical properties of steel corrosion products in concrete studied by nano-indentation technique. Corros. Sci. 2020, 163, 108304. [Google Scholar] [CrossRef]
- Amin, M.A.; Abd El Rehim, S.S.; Abdel-Fatah, H.T.M. Electrochemical frequency modulation and inductively coupled plasma atomic emission spectroscopy methods for monitoring corrosion rates and inhibition of low alloy steel corrosion in HCl solutions and a test for validity of the Tafel extrapolation method. Corros. Sci. 2009, 51, 882–894. [Google Scholar] [CrossRef]
- Amin, M.A.; Khaled, K.F.; Fadl-Allah, S.A. Testing validity of the Tafel extrapolation method for monitoring corrosion of cold rolled steel in HCl solutions – Experimental and theoretical studies. Corros. Sci. 2010, 52, 140–151. [Google Scholar] [CrossRef]
- Zhang, S.D.; Zhang, W.L.; Wang, S.G.; Gu, X.J.; Wang, J.Q. Characterisation of three-dimensional porosity in an Fe-based amorphous coating and its correlation with corrosion behaviour. Corros. Sci. 2015, 93, 211–221. [Google Scholar] [CrossRef]
- Chong, K.; Gao, Y.; Zhang, Z.; Zou, Y.; Liang, X. Thermal stability and corrosion behavior of a novel Zr22.5Ti22.5Hf22.5Ni22.5Ta10 high-entropy amorphous alloy. Corros. Sci. 2023, 213, 110979. [Google Scholar] [CrossRef]
- Berger, J.E.; Jorge, A.M.; Koga, G.Y.; Roche, V.; Kiminami, C.S.; Bolfarini, C.; Botta, W.J. Influence of chromium concentration and partial crystallization on the corrosion resistance of FeCrNiB amorphous alloys. Mater. Charact. 2021, 179, 111369. [Google Scholar] [CrossRef]
- Botta, W.J.; Berger, J.E.; Kiminami, C.S.; Roche, V.; Nogueira, R.P.; Bolfarini, C. Corrosion resistance of Fe-based amorphous alloys. J. Alloys Compd. 2014, 586, S105–S110. [Google Scholar] [CrossRef]
- Coimbrão, D.D.; Zepon, G.; Koga, G.Y.; Godoy Pérez, D.A.; Paes de Almeida, F.H.; Roche, V.; Lepretre, J.C.; Jorge, A.M.; Kiminami, C.S.; Bolfarini, C.; et al. Corrosion properties of amorphous, partially, and fully crystallized Fe68Cr8Mo4Nb4B16 alloy. J. Alloys Compd. 2020, 826, 154123. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, S.; Xu, X.; Miao, J.; Zhang, W.; Wang, T.; Chen, C.; Wei, R.; Li, F. Effect of the substitution of Si for B on thermal stability, magnetic properties and corrosion resistance in novel Fe-rich amorphous soft magnetic alloy. Intermetallics 2021, 138, 107306. [Google Scholar] [CrossRef]
- Han, Y.; Chang, C.T.; Zhu, S.L.; Inoue, A.; Louzguine-Luzgin, D.V.; Shalaan, E.; Al-Marzouki, F. Fe-based soft magnetic amorphous alloys with high saturation magnetization above 1.5 T and high corrosion resistance. Intermetallics 2014, 54, 169–175. [Google Scholar] [CrossRef]
- Han, Y.; Kong, F.L.; Han, F.F.; Inoue, A.; Zhu, S.L.; Shalaan, E.; Al-Marzouki, F. New Fe-based soft magnetic amorphous alloys with high saturation magnetization and good corrosion resistance for dust core application. Intermetallics 2016, 76, 18–25. [Google Scholar] [CrossRef]
- Koga, G.Y.; Nogueira, R.P.; Roche, V.; Yavari, A.R.; Melle, A.K.; Gallego, J.; Bolfarini, C.; Kiminami, C.S.; Botta, W.J. Corrosion properties of Fe–Cr–Nb–B amorphous alloys and coatings. Surf. Coat. Technol. 2014, 254, 238–243. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Sun, Y.; He, A.; Dong, Y.; Wang, Y. Effect of annealing temperature on magnetic properties and corrosion resistance of Fe75.8Si12B8Nb2.6Cu0.6P1 alloy. J. Mater. Res. Technol. 2021, 15, 3880–3894. [Google Scholar] [CrossRef]
- Meghwal, A.; Pinches, S.; King, H.J.; Schulz, C.; Stanford, N.; Hall, C.; Berndt, C.C.; Ang, A.S.M. Fe-based amorphous coating for high-temperature wear, marine and low pH environments. Materialia 2022, 25, 101549. [Google Scholar] [CrossRef]
- Sunbul, S.E.; Akyol, S.; Onal, S.; Ozturk, S.; Sozeri, H.; Icin, K. Effect of Co, Cu, and Mo alloying metals on electrochemical and magnetic properties of Fe-B alloy. J. Alloys Compd. 2023, 947, 169652. [Google Scholar] [CrossRef]
- Vasić, M.M.; Simatović, I.S.; Radović, L.; Minić, D.M. Influence of microstructure of composite amorphous/nanocrystalline Fe72Ni8Si10B10 alloy on the corrosion behavior in various environments. Corros. Sci. 2022, 204, 110403. [Google Scholar] [CrossRef]
- Wu, L.; Zhou, Z.; Zhang, K.; Zhang, X.; Wang, G. Electrochemical and passive film evaluation on the corrosion resistance variation of Fe-based amorphous coating affected by high temperature. J. Non-Cryst. Solids 2022, 597, 121892. [Google Scholar] [CrossRef]
- Wang, P.; Qi, W.; Yang, K.; Qiao, Y.; Wang, X.; Zheng, T.; Bai, C.; Liu, Z.; Zhang, X. Systematic investigation of the oxidation behavior of Fe-Cr-Al cladding alloys in high-temperature steam. Corros. Sci. 2022, 207, 110595. [Google Scholar] [CrossRef]
- Qin, C.; Hu, Q.; Li, Y.; Wang, Z.; Zhao, W.; Louzguine-Luzgin, D.V.; Inoue, A. Novel bioactive Fe-based metallic glasses with excellent apatite-forming ability. Mater. Sci. Eng. C 2016, 69, 513–521. [Google Scholar] [CrossRef]
- Wu, G.; Tan, X.; Li, G.; Hu, C. Effect of preparation method on the physical and catalytic property of nanocrystalline Fe2O3. J. Alloys Compd. 2010, 504, 371–376. [Google Scholar] [CrossRef]
- Tian, W.-P.; Yang, H.-W.; Zhang, S.-D. Synergistic Effect of Mo, W, Mn and Cr on the Passivation Behavior of a Fe-Based Amorphous Alloy Coating. Acta Metall. Sin. (Engl. Lett.) 2017, 31, 308–320. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, S.L.; Zheng, Y.G.; Ke, W.; Sun, W.H.; Wang, J.Q. Electrochemical behaviour of Fe-based metallic glasses in acidic and neutral solutions. Corros. Sci. 2012, 63, 159–173. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, S.D.; Sun, W.H.; Gao, Y.; Wang, J.Q. Enhanced corrosion resistance in Fe-based amorphous coatings through eliminating Cr-depleted zones. Corros. Sci. 2018, 136, 161–173. [Google Scholar] [CrossRef]
- Xu, D.D.; Zhou, B.L.; Wang, Q.Q.; Zhou, J.; Yang, W.M.; Yuan, C.C.; Xue, L.; Fan, X.D.; Ma, L.Q.; Shen, B.L. Effects of Cr addition on thermal stability, soft magnetic properties and corrosion resistance of FeSiB amorphous alloys. Corros. Sci. 2018, 138, 20–27. [Google Scholar] [CrossRef]
- Zhai, H.; Yuan, H.; Li, W.; Zhang, X.; Li, X.; Cai, A. Corrosion resistance mechanisms of detonation sprayed Fe-based amorphous coating on AZ31B magnesium alloy. J. Non-Cryst. Solids 2022, 576, 121276. [Google Scholar] [CrossRef]
- Čekerevac, M.; Simičić, M.; Bujanović, L.N.; Popović, N. The influence of silicate and sulphate anions on the anodic corrosion and the transpassivity of iron and silicon-rich steel in concentrated KOH solution. Corros. Sci. 2012, 64, 204–212. [Google Scholar] [CrossRef]
- Luo, H.; Su, H.; Dong, C.; Li, X. Passivation and electrochemical behavior of 316L stainless steel in chlorinated simulated concrete pore solution. Appl. Surf. Sci. 2017, 400, 38–48. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, B.; Ming, J.; Yan, Q.; Wang, M.; Cai, C.; Zhang, C.; Shi, Y. Corrosion mechanism of amorphous alloy strengthened stainless steel composite fabricated by selective laser melting. Corros. Sci. 2020, 163, 108241. [Google Scholar] [CrossRef]
- Kroll, R.; Kearns, P.; Usman, B.J.; Zhou, X.; Engelberg, D.L. A novel approach to determine cathodic passivation characteristics and semiconducting properties of pure aluminium 99.5 wt% and aluminium alloy 7075-T6 with an electrochemical pen electrode. Corros. Sci. 2023, 211, 110898. [Google Scholar] [CrossRef]
- Liu, M.; Cheng, X.; Li, X.; Pan, Y.; Li, J. Effect of Cr on the passive film formation mechanism of steel rebar in saturated calcium hydroxide solution. Appl. Surf. Sci. 2016, 389, 1182–1191. [Google Scholar] [CrossRef]
- Shi, J.; Ming, J.; Wu, M. Electrochemical behavior and corrosion products of Cr-modified reinforcing steels in saturated Ca(OH)2 solution with chlorides. Cem. Concr. Compos. 2020, 110, 103587. [Google Scholar] [CrossRef]
- Song, G.; Atrens, A.; John, D.S.; Wu, X.; Nairn, J. The anodic dissolution of magnesium in chloride and sulphate solutions. Corros. Sci. 1997, 39, 1981–2004. [Google Scholar] [CrossRef]
- Lamaka, S.V.; Shchukin, D.G.; Andreeva, D.V.; Zheludkevich, M.L.; Möhwald, H.; Ferreira, M.G.S. Sol-Gel/Polyelectrolyte Active Corrosion Protection System. Adv. Funct. Mater. 2008, 18, 3137–3147. [Google Scholar] [CrossRef]
- Song, G.-L.; Unocic, K.A. The anodic surface film and hydrogen evolution on Mg. Corros. Sci. 2015, 98, 758–765. [Google Scholar] [CrossRef]
- Xu, J.; Liu, L.; Li, Z.; Munroe, P.; Xie, Z.H. Niobium addition enhancing the corrosion resistance of nanocrystalline Ti5Si3 coating in H2SO4 solution. Acta Mater. 2014, 63, 245–260. [Google Scholar] [CrossRef]
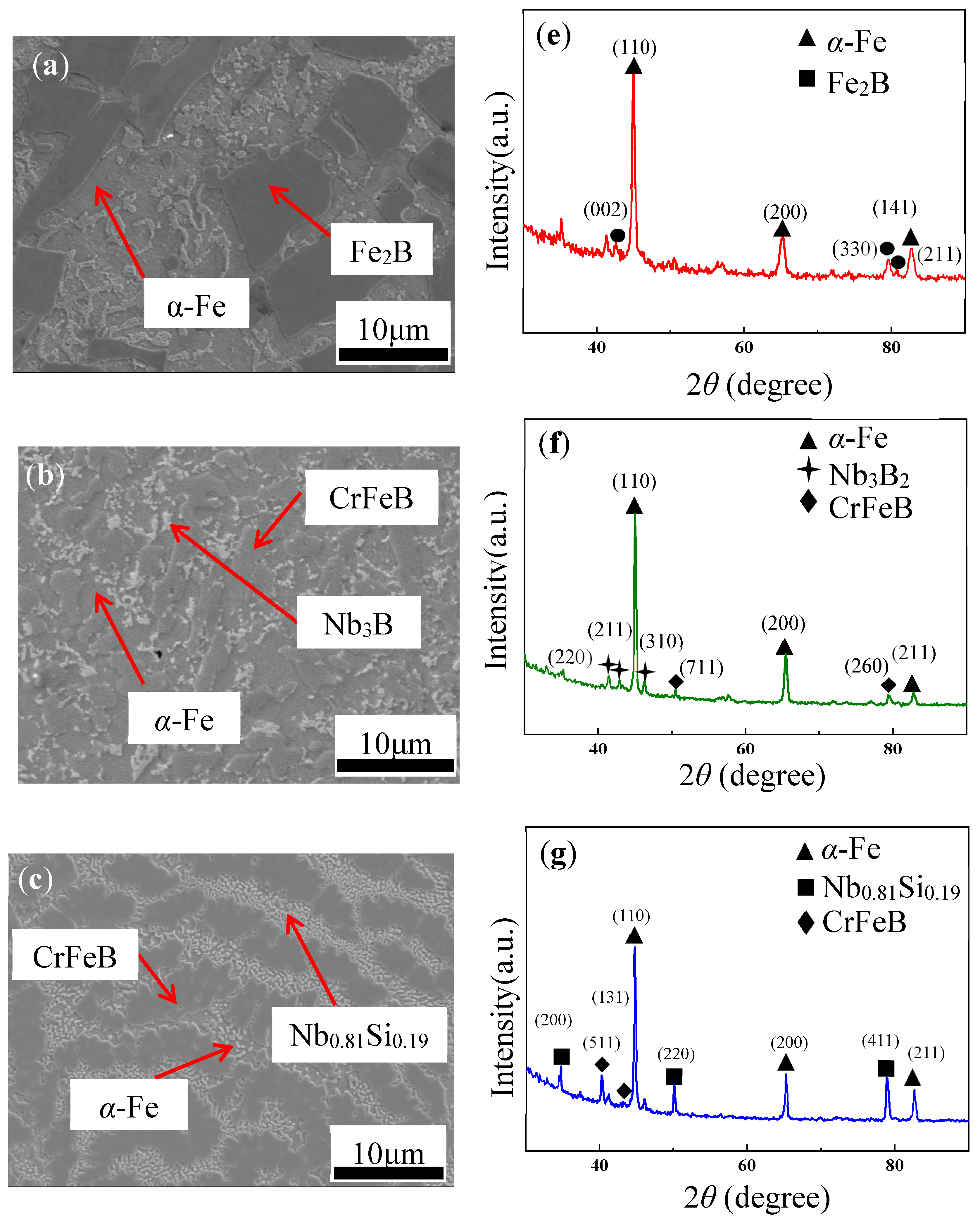
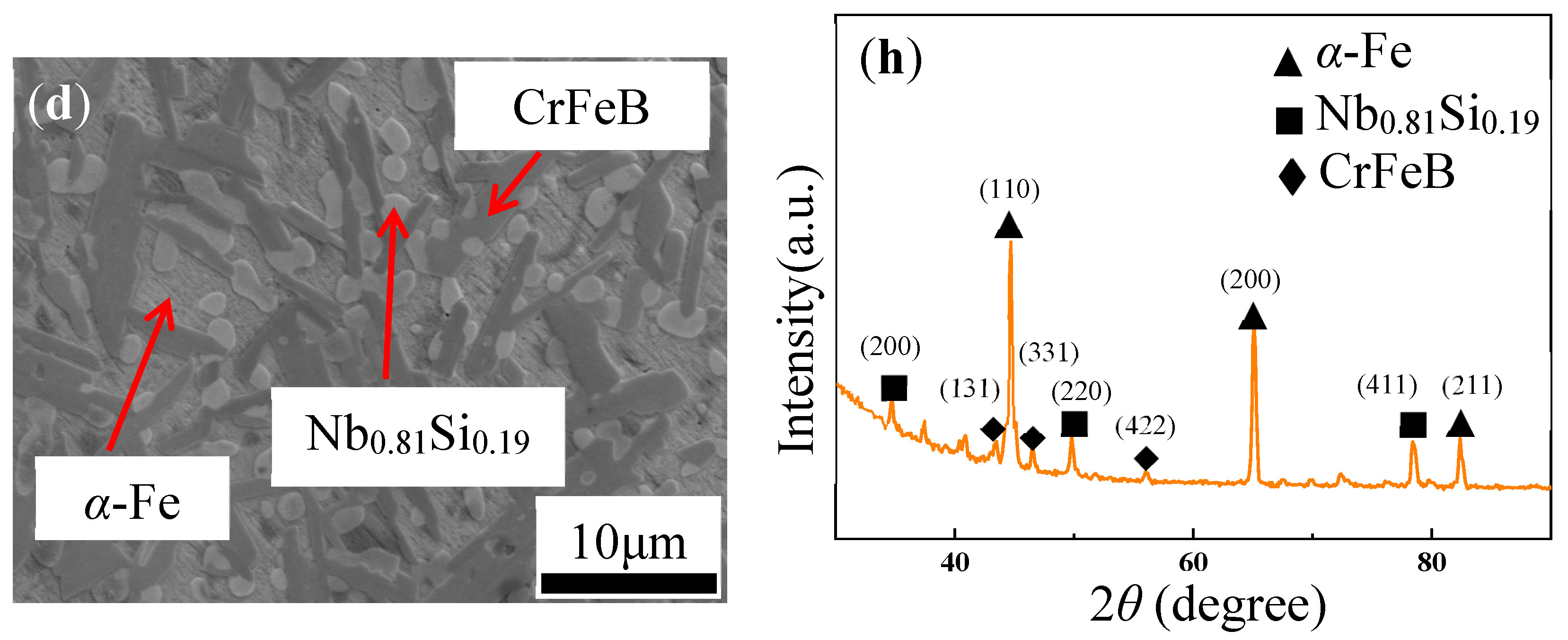
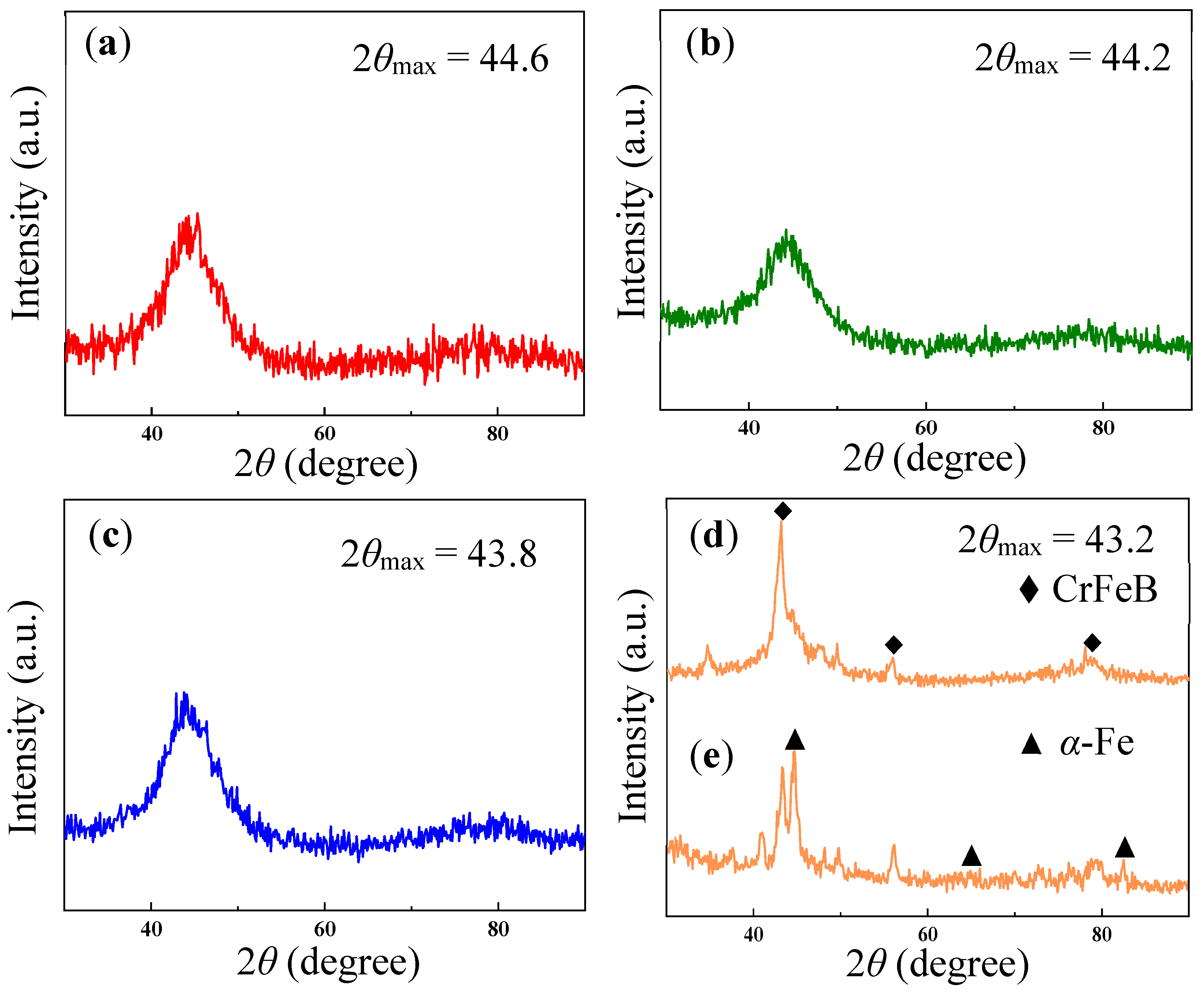
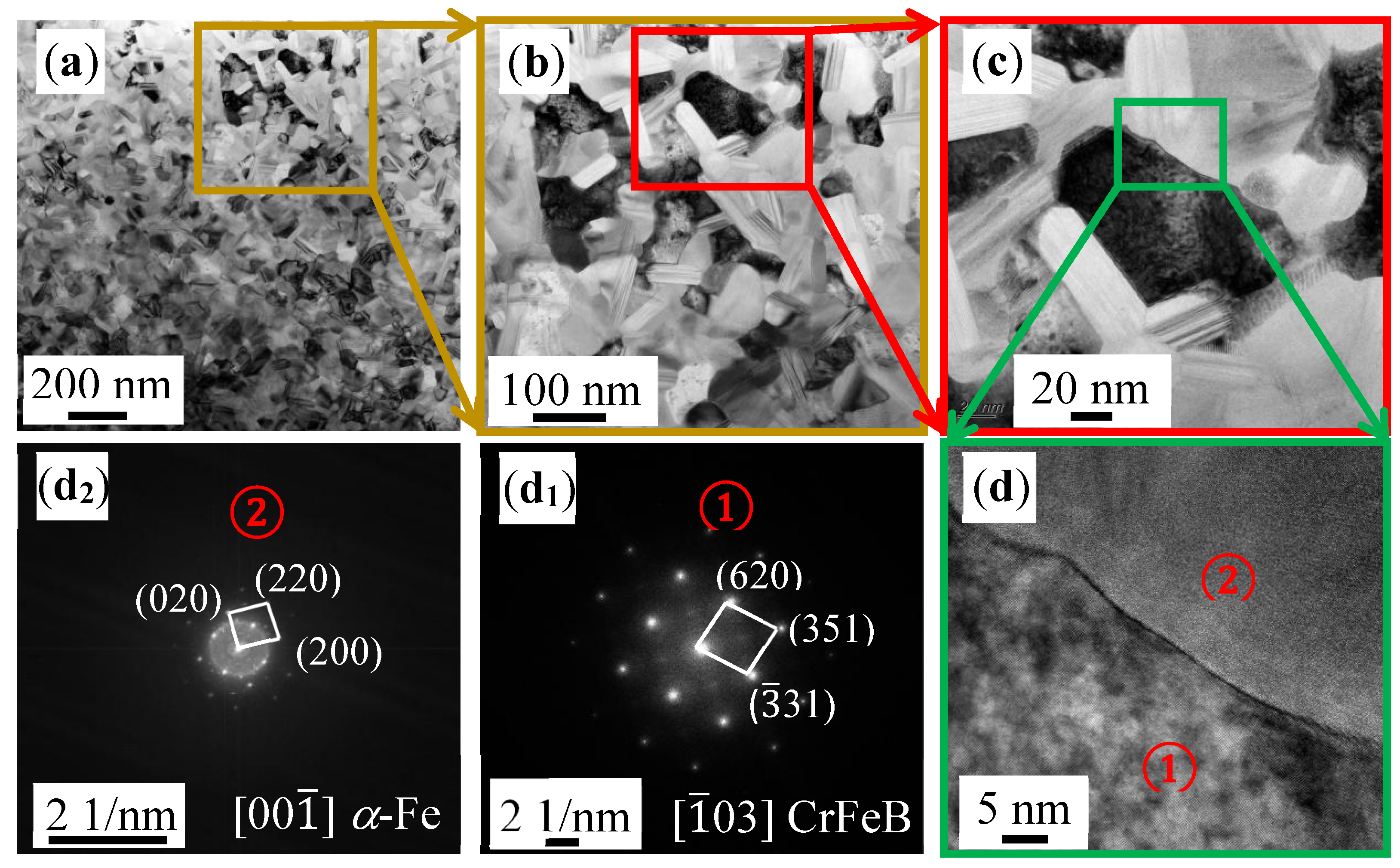

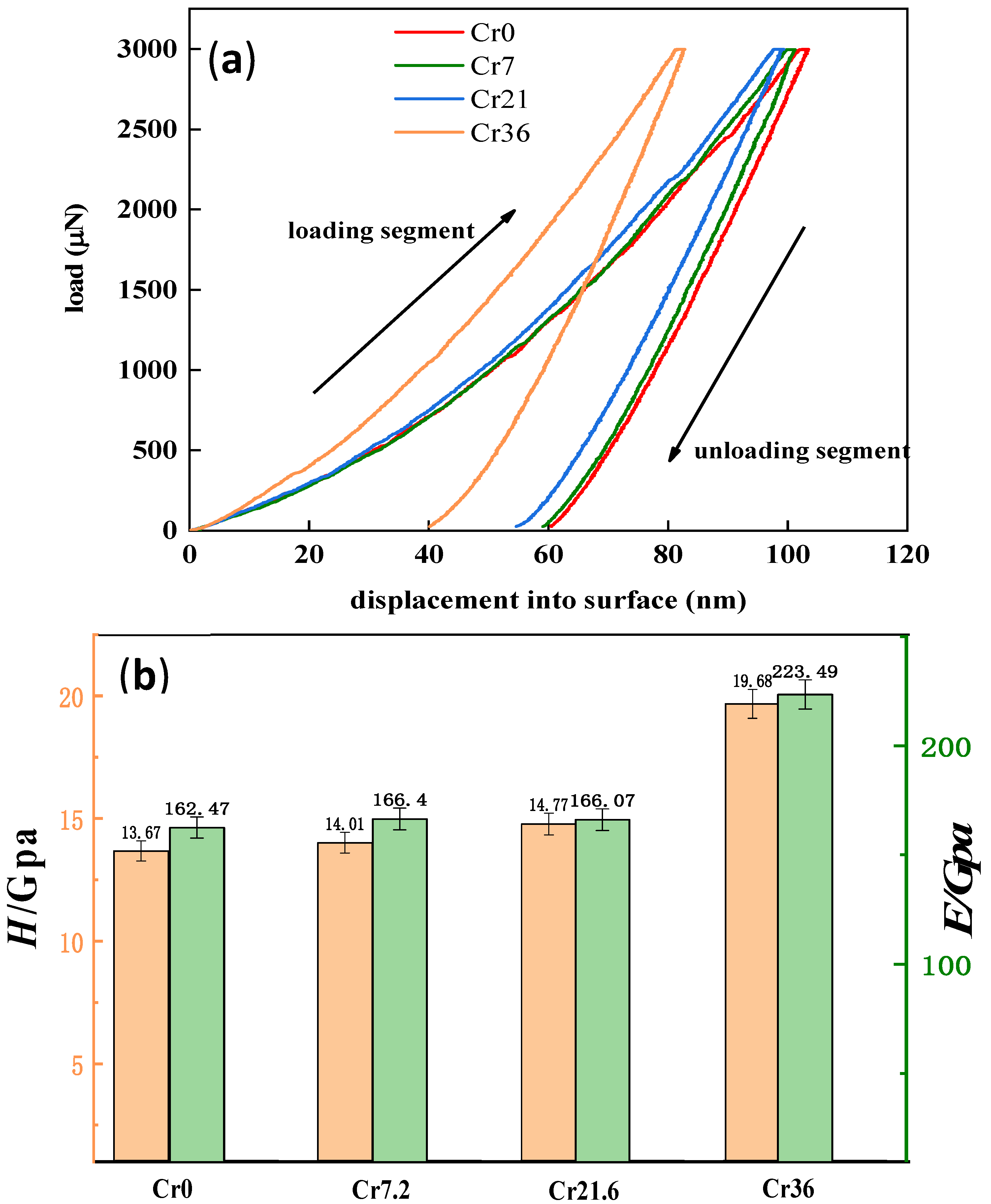
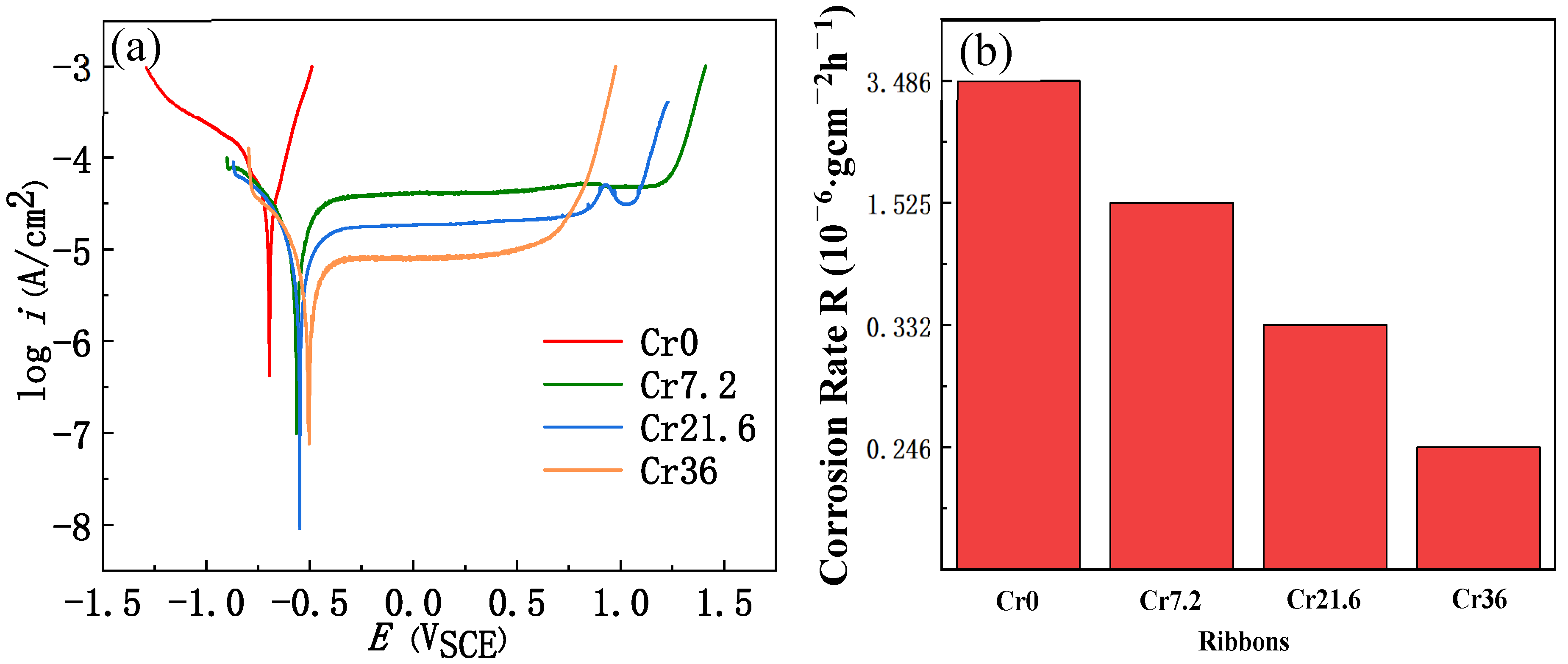
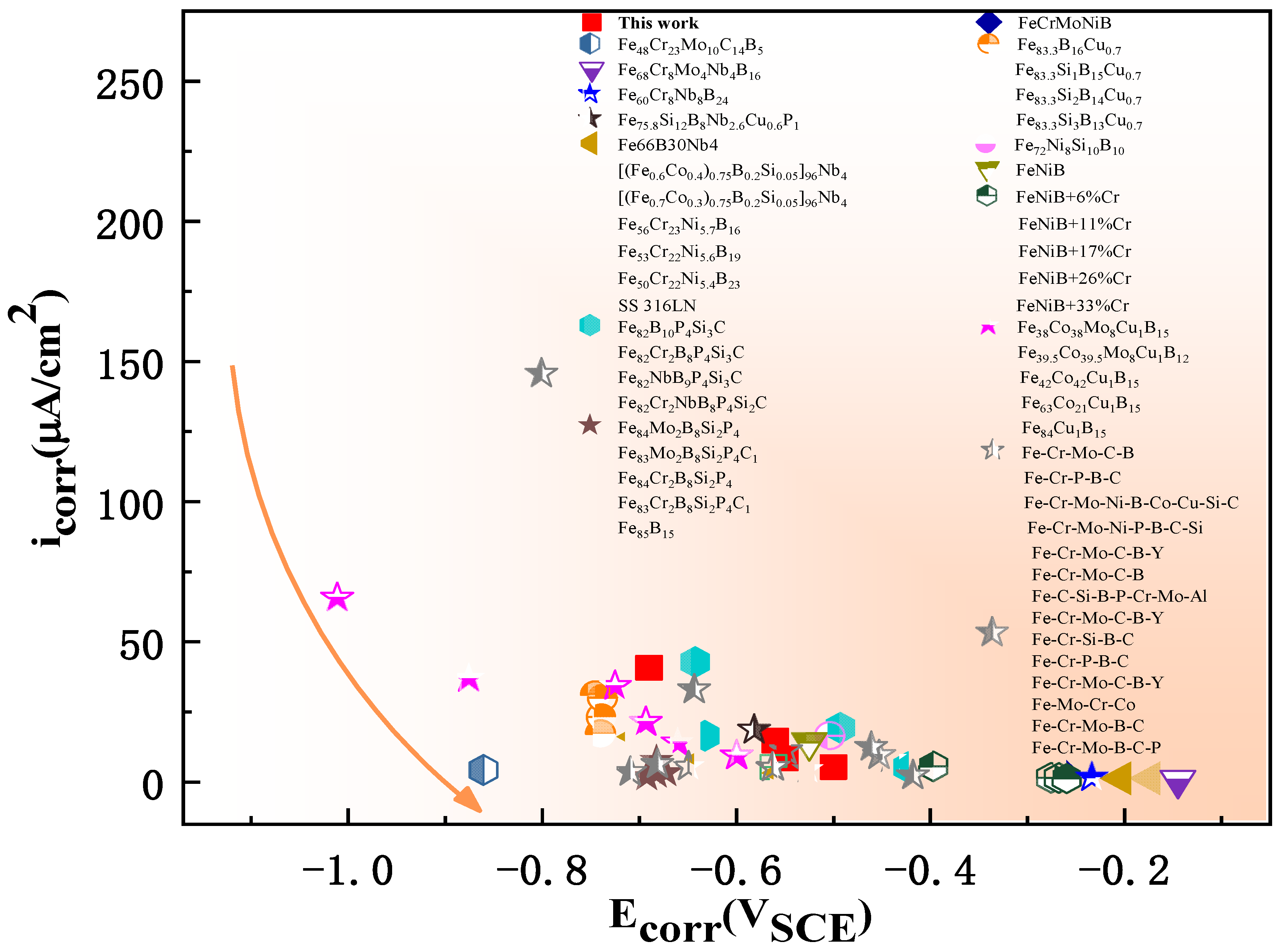
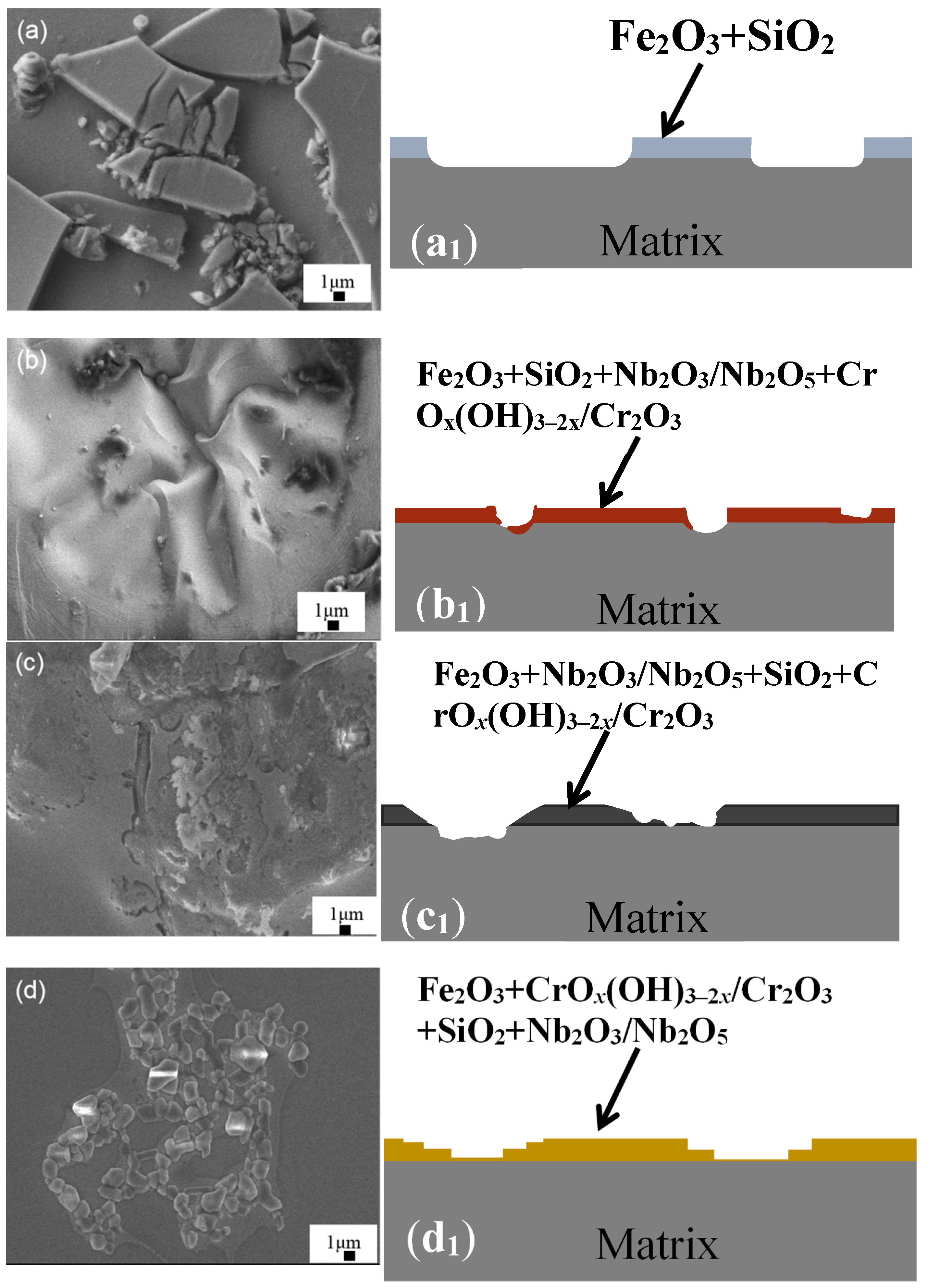

| Ribbon | Tx (K) | Ts (K) | Tm (K) | Tl (K) | ΔTl (K) | ΔT (K) | Trx | ΔHrc (ΔHc/ΔHm) |
|---|---|---|---|---|---|---|---|---|
| x = 0 | 882 | 1492 | 1394 | 1541 | 147 | 49 | 0.57 | 0.18 |
| x = 7.2 | 916 | 1580 | 1406 | 1619 | 213 | 39 | 0.57 | 0.23 |
| x = 21.6 | 933 | 1459 | 1438 | 1482 | 44 | 23 | 0.63 | 0.52 |
| x = 36 | - | 1554 | 1512 | 1615 | 103 | 61 | - | - |
| Ribbon | Ecorr (V) | icorr (μA·cm−2) | Epit (V) | CR (μm·y−1) |
|---|---|---|---|---|
| x = 0 | −0.69 | 40.72 | −0.60 | 473 |
| x = 7.2 | −0.56 | 14.83 | 1.21 | 172 |
| x = 21.6 | −0.55 | 8.43 | 1.04 | 98 |
| x = 36 | −0.50 | 5.28 | 0.68 | 61 |
| Ribbon | Rs(Ω·cm2) | Qp | Rp (kΩ·cm2) | C (μF·cm2) | Rct (kΩ·cm2) | Rtotal (kΩ·cm2) | |
|---|---|---|---|---|---|---|---|
| Y(10−5 Ω−1sncm−2) | n | ||||||
| x = 0 | 3.5 | 50.6 | 0.72 | 0.5 | 31,790 | 1.1 | 1.7 |
| x = 7.2 | 1.1 | 3.3 | 0.84 | 0.0 | 9.8 | 43.0 | 43.0 |
| x = 21.6 | 3.0 | 1.2 | 0.88 | 0.1 | 0.4 | 678.6 | 678.7 |
| x = 36 | 1.3 | 1.1 | 0.86 | 3.2 × 10−3 | 6.0 | 2410 | 2410 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Z.; Wang, Z.; Chen, Q.; Qi, Z.; Kim, K.B.; Wang, W. Role of Cr Element in Highly Dense Passivation of Fe-Based Amorphous Alloy. Materials 2023, 16, 6630. https://doi.org/10.3390/ma16206630
Song Z, Wang Z, Chen Q, Qi Z, Kim KB, Wang W. Role of Cr Element in Highly Dense Passivation of Fe-Based Amorphous Alloy. Materials. 2023; 16(20):6630. https://doi.org/10.3390/ma16206630
Chicago/Turabian StyleSong, Ziqi, Zhaoxuan Wang, Qi Chen, Zhigang Qi, Ki Buem Kim, and Weimin Wang. 2023. "Role of Cr Element in Highly Dense Passivation of Fe-Based Amorphous Alloy" Materials 16, no. 20: 6630. https://doi.org/10.3390/ma16206630
APA StyleSong, Z., Wang, Z., Chen, Q., Qi, Z., Kim, K. B., & Wang, W. (2023). Role of Cr Element in Highly Dense Passivation of Fe-Based Amorphous Alloy. Materials, 16(20), 6630. https://doi.org/10.3390/ma16206630








