1. Introduction
Neurology has become fundamental in diagnosing and managing central nervous system (CNS) disorders. Neuroradiology plays a major role in imaging the brain, spine, and associated structures to detect abnormalities such as tumors, infections, vascular lesions, and degenerative conditions. Neuro-oncology, on the other hand, uses radiation techniques to treat these pathologies, particularly in CNC disorders [
1].
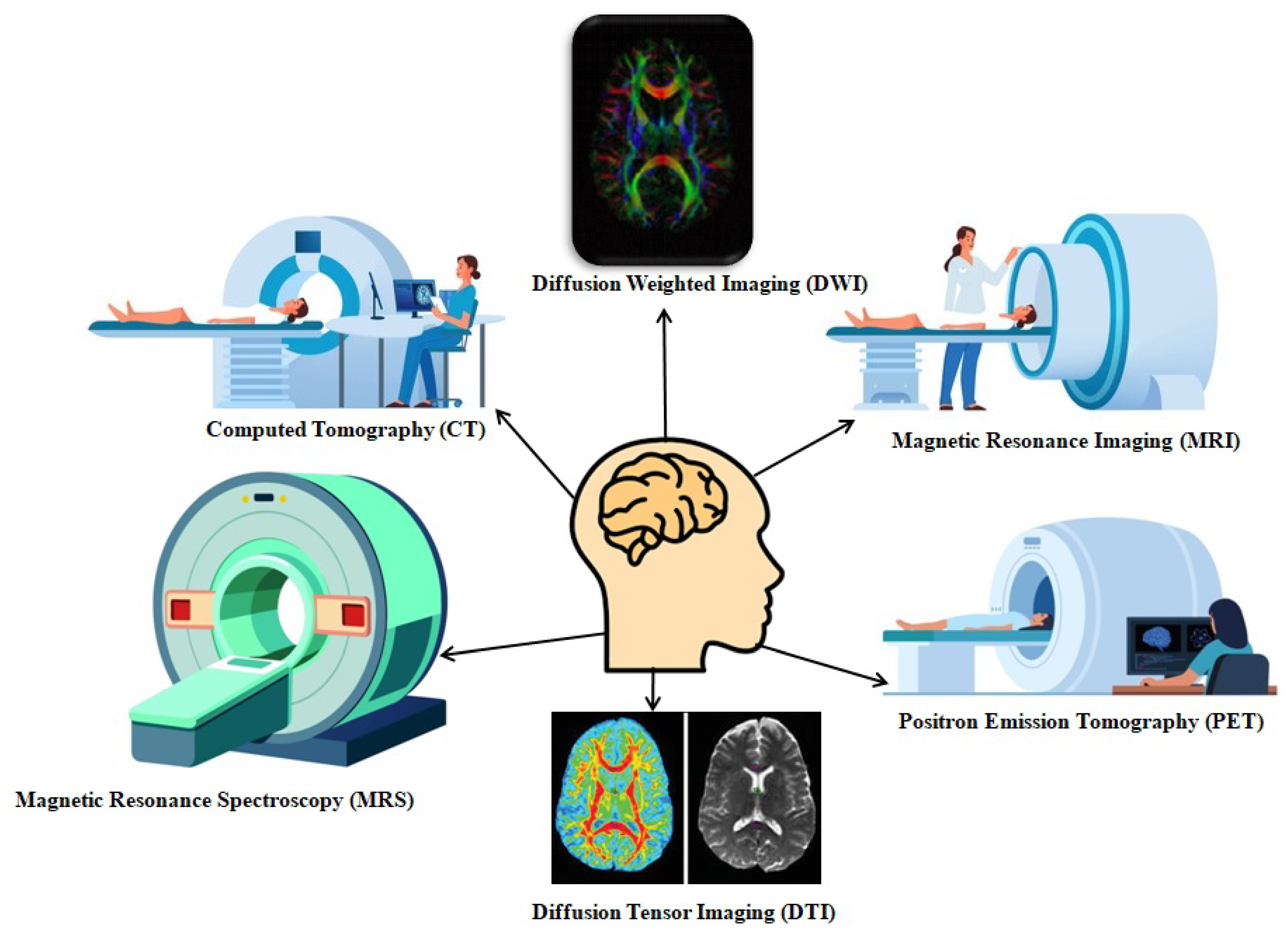
Neuroradiology has evolved from basic X-ray to computed tomography (CT) and magnetic resonance imaging (MRI) for diagnosis, which enables accurate visualization and understanding of the depth of intracranial and spinal pathologies. These inventions allow radiologists and neurologists to only visualize the structural anatomy; to overcome this, functional imaging modalities, like functional MRI (fMRI), diffusion tensor imaging (DTI), and molecular techniques like positron emission tomography (PET), have been invented to assess function and metabolism. During this transition in radiology, radiotherapy has also evolved from 2D planning to high-precision techniques, such as stereotactic radiosurgery (SRS) and image-guided radiotherapy (IGRT), and image-guided stereotactic radiosurgery, enabling the safe delivery of high doses to complex CNS targets.
CNS disorders, such as malignant tumors (such as glioblastoma), neurodegenerative disease (such as Alzheimer’s), and inflammatory disease (such as multiple sclerosis), indicate the potential of imaging and targeted treatment. Precise imaging in radiotherapy enables accurate diagnosis, better definition of tumor margins, measurement of radiation throughout a target, and estimation of tumor response at subsequent time points during follow-up. Good-quality imaging can also diminish complications by sparing normal tissues and more definitely and effectively irradiating the desired site.
These advanced inventions are encouraged by close collaboration betweenradiologists, radiation oncologists, neurologists, neurosurgeons, and medical physicists. Interdisciplinary teamwork has enabled comprehensive care strategies, encompassing early detection, treatment planning, and follow-up. This synergy continues to influence personalized and adaptive neuro-oncologic interventions.
2. Imaging Techniques in Neuroradiology
2.1. Computed Tomography (CT) and Magnetic Resonance Imaging (MRI)
CT and MRI are the primary imaging modalities in neuroradiology, which have been extensively used to diagnose a wide range of intracranial and spinal disorders, as shown in
Figure 1. CT imaging uses basic X-ray technology combined with a computer algorithm to generate cross-sectional images. They are particularly important in emergencies due to their speed and availability [
1]. Because of their high sensitivity, they are used to detect intracranial hemorrhages such as subdural, epidural, and intraparenchymal bleeds, as well as skull fractures, bone lesions, and pathological calcifications, including those seen in infections like neurocysticercosis and tumors such as oligodendrogliomas. In contrast, MRI employs powerful magnetic fields to generate radiofrequency pulses in the body by aligning hydrogen atoms, thus producing superior soft tissue contrast images, which are essential in analyzing brain tumors, infections like abscesses and encephalitis, demyelinating diseases such as multiple sclerosis, and spinal cord abnormalities, including lesions and disc herniations. It offers detailed insights into various sequences, such as T1, T2, FLAIR, SWI, GRE, and post-contrast imaging, enabling precise identification and characterization of neurological pathologies [
2].
2.2. Functional MRI (fMRI)
Functional magnetic resonance imaging (fMRI) measures regional brain activity by examining blood oxygen level-dependent (BOLD) signals associated with neuronal activation. Particularly for patients undergoing tumorresection or epilepsy operations when lesions are near important brain regions, this technique is crucial to pre-surgical planning. fMRI helps precisely identify the motor cortex (such as areas for hand, face, and limb movements) and language regions, including Broca’s and Wernicke’s areas, as well as visual andsensory cortices, based on the activity being performed. In addition to task-based fMRI, resting-state fMRI (rs-fMRI) has gained clinical significance for patients who are unable to perform tasks, as it provides valuable insights intothe intrinsic functional connectivity of the brain [
3,
4]. Thus, fMRI helps neurosurgeons in avoiding critical functional areas during surgery, thereby safeguarding neurological function and enhancing post-operative outcomes in neuro-oncology and epilepsy treatment.
2.3. Perfusion Imaging
Perfusion imaging is a crucial method in neuroradiology and neuro-oncology that offers detailed insights into cerebral hemodynamics by measuring blood flow, blood volume, and mean transit time within brain tissues. It plays a major role in the diagnosis, grading, and treatment of brain tumors, particularly gliomas, highlighting abnormal perfusion that often correlates with tumor aggressiveness and angiogenesis. CT perfusion (CTP), dynamic susceptibility contrast MRI (DSC-MRI), and arterial spin labeling (ASL) MRI are commonly used techniques that aid in distinguishing high-grade from low-grade tumors, thus identifying tumor recurrence versus post-treatment effects and assessing treatment response. Perfusion imaging helps guide revascularization techniques and detect ischemic penumbra in stroke and vascular diseases. Perfusion imaging improves diagnosis accuracy, directs biopsy and surgical planning, and helps customize treatment in neuro-oncologic care when combined with anatomical imaging and molecular approaches.
2.4. Diffusion-Weighted Imaging (DWI) and Diffusion Tensor Imaging (DTI)
DWI and DTI are advanced MRI techniques that provide important insights into the microstructure of the brain. DWI is a highly sensitive technique for detecting the early onset of ischemic stroke, often detecting cytotoxic edema within 30 min by measuring the Brownian motion of water molecules. Additionally, it is used to identify brain abscesses, which often demonstrate restricted diffusion because of their viscous purulent material, and to evaluate tumor cellularity, which helps in differentiating high-gradefromlow-gradegliomas. As an expansion of DWI, DTI allows for the imaging of white matter fiber tracts via tractography and measures directional diffusion. This is especially crucial when planning surgery for lesions close to critical circuits, such as the arcuate fasciculus, optic radiation, and corticospinal tract [
5,
6]. DTI is also utilized to assess neurodevelopmental or connection diseases such as autism and schizophrenia, multiple sclerosis, and traumatic brain damage.
2.5. Magnetic Resonance Spectroscopy (MRS)
MRI is a non-invasive imaging technique that evaluates the biochemical composition of brain tissue, helping in both diagnosis and treatment planning. It measures important brain metabolites such as lactate, found in necrotic or ischemic tissue; myo-inositol, frequently elevated in Alzheimer’s disease; creatine (Cr), a marker of energy metabolism; choline (Cho), elevated in tumors due to increased membrane turnover; and N-acetyl aspartate (NAA), decreased in neuronal loss. In clinical settings, MRS is used to diagnose metabolic or mitochondrial diseases and to grade tumors, where a high Cho/NAA ratio indicates a high-grade glioma that distinguishes radiation necrosis from tumor recurrence. MRS offers a metabolic profile that enhances anatomic imaging and aids in diagnosis refinement, especially in instances that are unclear or complex [
7,
8].
2.6. Positron Emission Tomography (PET)
Positron emission tomography (PET) uses radiolabeled tracers to image the brain’s metabolic and molecular processes. Due to the strong background glucose uptake in normal cortical tissue, the most widely used tracer, [18F] FDG (fluorodeoxyglucose), has limited use in the brain but is helpful for general oncologic imaging. To address this, amino acid tracers such as [11C] methionine (MET), [18F] fluoroethyl-tyrosine (FET), and [18F] fluorodopa (FDOPA) offer much improved tumor-to-background contrast. These tracers are highly effective for glioma identification, grading, and recurrence assessment [
9,
10]. PET imaging is increasingly being utilized to plan radiotherapy, direct biopsy sites, and monitor treatment response. Presently, it is an essential component of individualized neuro-oncology care, particularly for malignancies that are difficult to differentiate using conventional imaging alone.
2.7. Hybrid Imaging (PET-CT and PET-MRI)
PET-CT and PET-MRI are hybrid imaging modalities that integrate functional and structural data in a single session, thus enhancing diagnostic accuracy and treatment planning. PET-CT is a fusion of two principles between PET and CT, that is, the metabolic insights of PET imaging and the anatomical clarity of CT imaging, which are commonly used for tumor detection, radiotherapy planning, and systemic metastasis monitoring. PET-MRI, representing an advanced imaging modality, provides exceptional soft tissue contrast and significantly reduces the radiation exposure compared to PET-CT, as it uses the principles of MRI rather than CT. It is particularly useful in the assessment of brain tumors, as it uses the simultaneous acquisition of metabolic data and high-resolution anatomical images. PET-MRI is clinically used for glioma characterization, grading, assessment of treatment effects, such as pseudo progression versus true recurrence, and pediatric brain tumor imaging, further highlighting its minimized radiation doses. Hybrid imaging is constantly evolving, and it is turning into a vital tool for the thorough evaluation and treatment of CNS tumors [
11,
12].
2.8. Role of SPECT in Brain Imaging
In neuroradiology and neuro-oncology, single-positron emission computed tomography (SPECT) plays a crucial role in brain imaging. It provides both structural and functional evaluation by using the three-dimensional assessment of cerebral blood flow and receptor activity. Radiotracers like Tc-99m HMPAO and Tc-99m Tetrofosmin are used to identify perfusion irregularities, where SPECT helps neuro-oncologists to diagnose and grade brain cancers, particularly gliomas. It helps in distinguishing high-grade and low-grade tumors based on the intensity of perfusion. Additionally, by comparing vascularity and tumor metabolism before and after chemotherapy or radiation therapy, SPECT plays a crucial role in monitoring treatment response. Distinguishing post-radiation necrosis from tumor recurrence, which appears in scans, plays a crucial role in the treatment. Additionally, by identifying areas with reduced perfusion, SPECT aids in diagnosing neurologic conditions, including dementia and epilepsy. By combining anatomical imaging modalities such as MRI or CT, SPECT improves diagnosis accuracy and supports integrated decision-making for patient care.
3. Neuroradiological Applications in CNS Tumors
3.1. Gliomas and Glioblastoma
Neuroradiology is essential in diagnosing, grading, formulating treatment plans for, and conducting follow-ups for gliomas and glioblastoma multiforme (GBM). For example, multiparametric MRI T1-weighted post-contrast, T2-FLAIR, DWI, and perfusion imaging, as well as MR spectroscopy, help assess the degree of tumor and edema, as well as angiogenesis and tumor cellularity. Compare that to the FDG-PET; PET imaging of amino acids (18F) FET, (11C) MET, and (18F) FDOPA provides better tumor-to-background ratios. These also help in the more accurate delineation of the malignancy after radiotherapy and differentiate better between genuine tumor growth and pseudo-progression [
13,
14].
3.2. Brain Metastases
Malignancies of the lungs and breasts, and melanoma, often have a greater risk of brain metastases. Contrast-enhanced MRI is the most effective imaging technique for identifying and displaying lesions, particularly those in the cortical and posterior fossa regions. The FLAIR, SWI, T2, and T1 post-contrast sequences are noteworthy. PET-CT provides metabolic information using FDG or amino acid tracers, particularly in distinguishing between necrotic radiation tissue and active tumor tissue [
15,
16]. Imaging is crucial for shaping treatment plans for SRS and WBRT.
3.3. Epilepsy
MRI is widely used to localize structural abnormalities like mesial temporal sclerosis (MTS), cortical dysplasia, and hippocampal atrophy in epilepsy, which are resistant to drugs. DTI, which is an advanced imaging modality, helps visualize white matter tract disruption, while fMRI images functional regions like the language or motor cortex, which is important for surgical planning. Localizing seizure foci in MRI-negative epilepsy is aided by PET and SPECT [
17,
18].
3.4. Role of Imagingin Radiotherapy Planning
MRI is a critical tool in radiotherapy planning, supporting and, in certain instances, replacing conventional CT. As noted by De Pietro et al. [
19], MRI offers superior contrast of soft tissues, which allows for more precise delineation of tumors and lower inter-observer variability, especially in intricate areas like the brain, head and neck, prostate, and pelvis. This accuracy facilitates tighter margins and better sparing of surrounding organs at risk.
Beyond anatomical imaging, functional MRI methods like diffusion-weighted imaging and dynamic contrast-enhanced sequences provide information related to tumor biology and heterogeneity. These advances have paved the way toward biologically guided radiotherapy that will allow treatment to be customized not only to the anatomy of the patient but also to parameters of the patient’s functional and metabolic characteristics.
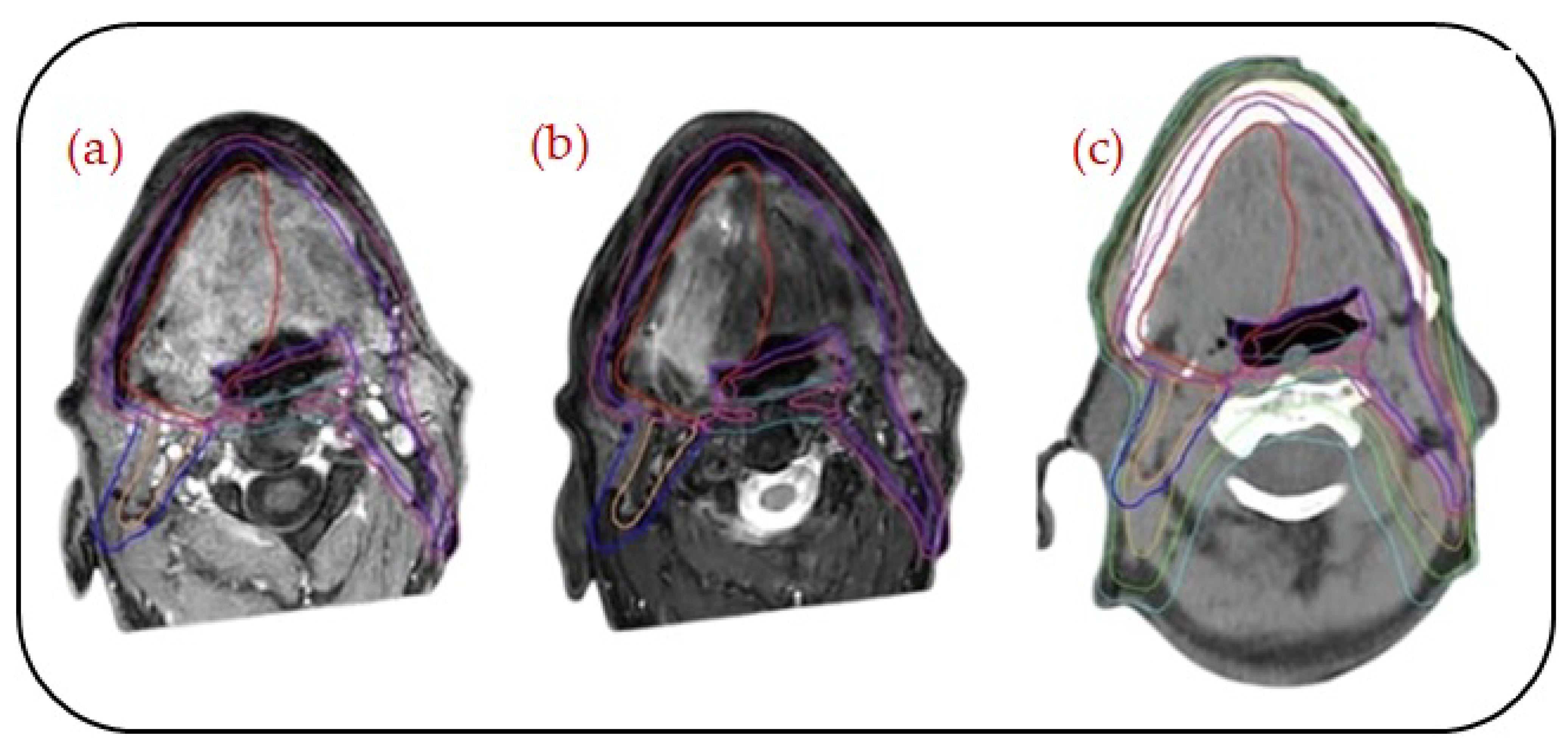
MRI integration is also at the heart of adaptive radiotherapy. MR-Linac systems provide real-time visualization during treatment so that on-table plan adjustments can be made according to daily anatomical changes, as presented in
Figure 2 and
Figure 3. This feature improves personalization and accuracy and, quite possibly, therapeutic outcomes, and it reduces toxicity. The geometric distortion, protocol standardization, and integration of CT-based dose calculations are also important. Nevertheless, MRI is creating a new paradigm in “head to toe” radiotherapy planning, with increasing evidence supporting its function in precision oncology [
20,
21,
22].
4. Principles and Modalities of Neuro-Oncology
Whether used as a main treatment or as an adjuvant, neuro-oncology is essential in the management of malignancies of the central nervous system (CNS). Treatments have become more conformal because of improvements in radiotherapy techniques, which preserve important brain structures while giving malignancies efficient dosages. The main ideas of CNS radiobiology are covered in this section, along with some clinically useful modalities (
Table 1 and
Table 2).
Table 1.
Comparison of the different treatment modalities of neuro-oncology.
Table 1.
Comparison of the different treatment modalities of neuro-oncology.
| Modality | Tumors Treated | Tumor Volume Range | Precision | Advantage | Disadvantage |
|---|
| Gamma Knife [23,24] | Vestibular Schwannoma
Meningioma
Brainmetastases
Pituitary adenoma
AVMs
Trigeminal neuralgia | Ideal: <3 cm.
Max: ~4 cm | Overall Assessment
~0.3 mm | Ultra-precise SRS for small brain lesions | Radiation exposure, source replacement, prolonged radiation, decay source, high cost |
Cyber Knife
[25,26] | Brain metastases
Spinal tumors
Skull-base lesions
Some gliomas and recurrent tumors | Up to ~60 cc (large volumes require fractionation) | ~0.5–1 mm | Frameless, spinal + brain, motion tracking | Longer treatment time, costlier |
LINAC-based SRS/FSRT
[27,28] | Glioblastoma
Post-operative cavities
Meningiomas
Brainstem gliomas
Re-irradiation cases | Small to large (1–150 cc), highly adaptable | ~1–2 mm | Widely available, versatile | Less precision, higher normal tissue dose |
| ZAP-X [25] | Brain metastases
Acoustic neuromas
Meningiomas
Pituitary adenomas | Up to 3–4 cm | ~0.5 mm | Vault-free, compact, brain-only SRS | New tech, limited availability |
Table 2.
Summary of radiobiological considerations.
Table 2.
Summary of radiobiological considerations.
| Modality | Fractionation | Biologically Effective Dose (BED) | USC/Modified USC |
|---|
| SRS | Single fraction
(e.g., 1 × 20 Gy) | High BED
(e.g., 100–120 Gy10) | Very high USC due to high dose per fraction and steep dose gradients |
| SBRT | Hypofractionation (e.g., 5 × 6 Gy) | Moderate to high BED
(e.g., ~90 Gy10) | USC considers reoxygenation and partial recovery between fractions |
| Conventional RT | Conventional fractionation
(e.g., 25 × 2 Gy) | Standard BED
(~60 Gy10) | Modified USC accounts for tumor repopulation, repair, redistribution, and reoxygenation |
4.1. Radiobiological Considerations
Due to the brain’s limited capacity for regeneration, radiotherapy of the central nervous system is subject to unique radiobiological properties. Major glial and vascular elements of the CNS are more sensitive to radiation than neurons, which are predominantly post-mitotic and somewhat radioresistant. There are potential acute, early delayed, and late radiation consequences; late effects (necrosis or cognitive decline) may be the most dose-limiting.
The Biological Effective Dose (BED) plays an essential role in evaluating the cumulative effects of radiotherapy, especially in hypo-fractionated regimes. Dose limits, derived from normal tissue complication probability (NTCP) models based on organs-at-risk (OAR), set safe limits for the cochlea, brainstem, optic system, and hippocampal regions. The introduction of new techniques, such as hippocampal-sparing radiotherapy, makes it even more pertinent to evaluate neurocognitive outcomes with tumor control.
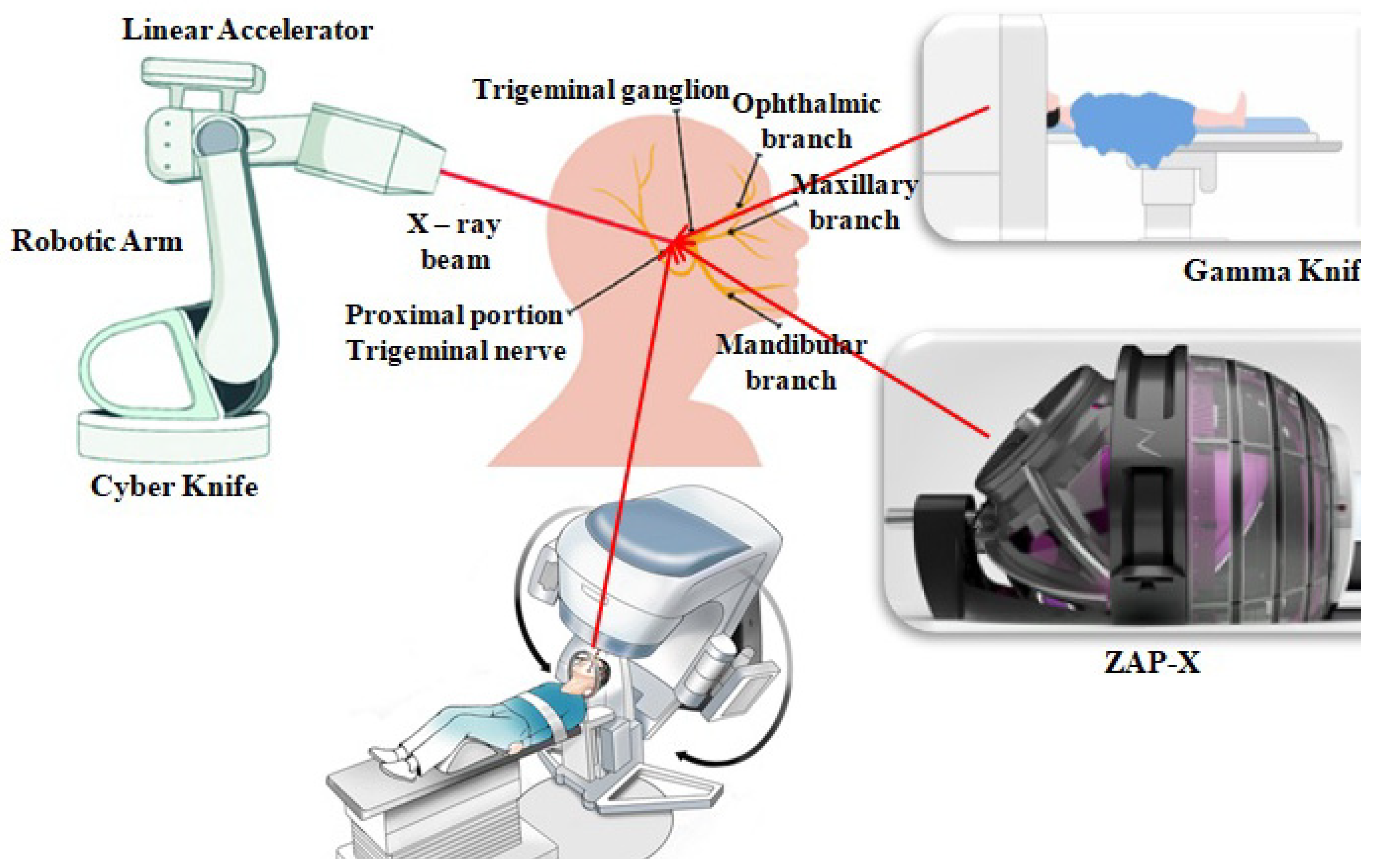
4.2. Stereotactic Radiosurgery (SRS) and Stereotactic Radiotherapy (SRT)
Stereotactic radiosurgery (SRS) is a high-end technique in radiotherapy that delivers a very high dose of radiation in a single fraction with submillimeter precision, whereas stereotactic radiotherapy (SRT) typically delivers the radiation with the same accuracy but across multiple fractions. For small, well-demarcated lesions, like brain metastases and arteriovenous malformations (AVMs),acoustic neuromas, and residual or recurrent gliomas, these techniques are preferred.
Three main platforms are used:
- ❖
Gamma Knife: A cobalt-60-based radiation therapy unit specifically intended for intracranial SRS. The equipment provides higher precision and is optimal for treating deeply seated tumors.
- ❖
Cyber Knife: A robot that integrates image-guided radiotherapy with real-time targeting. It is non-isocentric and hence able to conform to irregular tumor shapes and mobile area lesions.
- ❖
Linac-based SRS: Contemporary linear accelerators with multi-leaf collimators (MLCs) and on-board imaging offer SRS/SRT capability. They provide more flexibility for treating more than one lesion or larger lesions.
These modalities are associated with high rates of local control and low toxicity if planned well, particularly when imaging guidance is utilized.
4.3. Intensity-Modulated Radiotherapy (IMRT) and Volumetric-Modulated Arc Therapy (VMAT)
IMRT is a technique in radiotherapy used to precisely shape radiation doses around complex tumor geometries using inverse planning algorithms. This is particularly useful for treating tumors near sensitive structures like gliomas, which are adjacent to the optic chiasm or brain stem, since IMRT allows for differential dose painting through intensity modulations, which target aggressive regions with higher doses.
VMAT is an improved version of IMRT that uses a continuous arc to deliver radiation, reducing treatment time while maintaining treatment plan quality. It can simultaneously change dose rate, gantry speed, and MLC position. Craniospinal irradiation (CSI) for medulloblastoma uses the VMAT technique and often provides excellent homogeneity and sparing of OARs like the kidneys and heart.
4.4. Image-Guided Radiotherapy (IGRT)
IGRT enhances the accuracy of delivering doses by making sure that daily patient positioning aligns with planning data through daily imaging. This is particularly important in neuro-oncology, where there is little room for mistakes.
Imaging modalities utilized in IGRT are as follows:
- ❖
Two-dimensional kV imaging: This is suitable for alignment of bony anatomy in situations with minimal soft tissue motion.
- ❖
Cone-beam CT (CBCT): This offers 3D volumetric imaging at the start of every session.
- ❖
Four-dimensional MRI-guided radiotherapy (4D MR-Linac): This enables 4D visualization of tumors and critical structures during treatment, a first for brain tumors.
By reducing planning target volume (PTV) margins, IGRT decreases radiation to nearby brain tissues, thus reducing neurotoxicity and enabling dose escalation within certain areas.
4.5. Proton and Heavy Ion Therapy
Proton therapy is a desirable modality for selected CNS tumors, especially in children and in tumors near sensitive structures. The physical properties of protons allow for dose deposition with the Bragg peak, such that maximum energy is deposited at the target site with a sharp dose fall-off, sparing the nearby normal tissues distal to the tumor.
Key advantages include
- ❖
Reduced integral dose to normal brain tissue;
- ❖
Reduced risk of neuroendocrine and neurocognitive impairments in children;
- ❖
Successful targeting of tumors close to the skull base or optic nerves;
- ❖
Diminished or mitigated secondary tumors.
Heavy ion therapy, as with carbon ion therapy, involves favorable dose deposition with higher Linear Energy Transfer (LET) for greater Relative Biological Effectiveness (RBE) outcomes. It is potentially useful for radioresistant tumors such as chordomas or recurrent gliomas. It is, however, limited due to infrastructure and cost.
4.6. Pediatric Neuro-Oncology
Radiotherapy for children demands special attention due to the long-term risk of neuro developmental, endocrine, and intellectual sequelae. A category of pediatric CNS tumors, such as medulloblastoma, ependymoma, and gliomas, typically requires radiation as part of multimodal therapy, as shown in
Table 3. Significant techniques in pediatric radiotherapy include the following:
- ❖
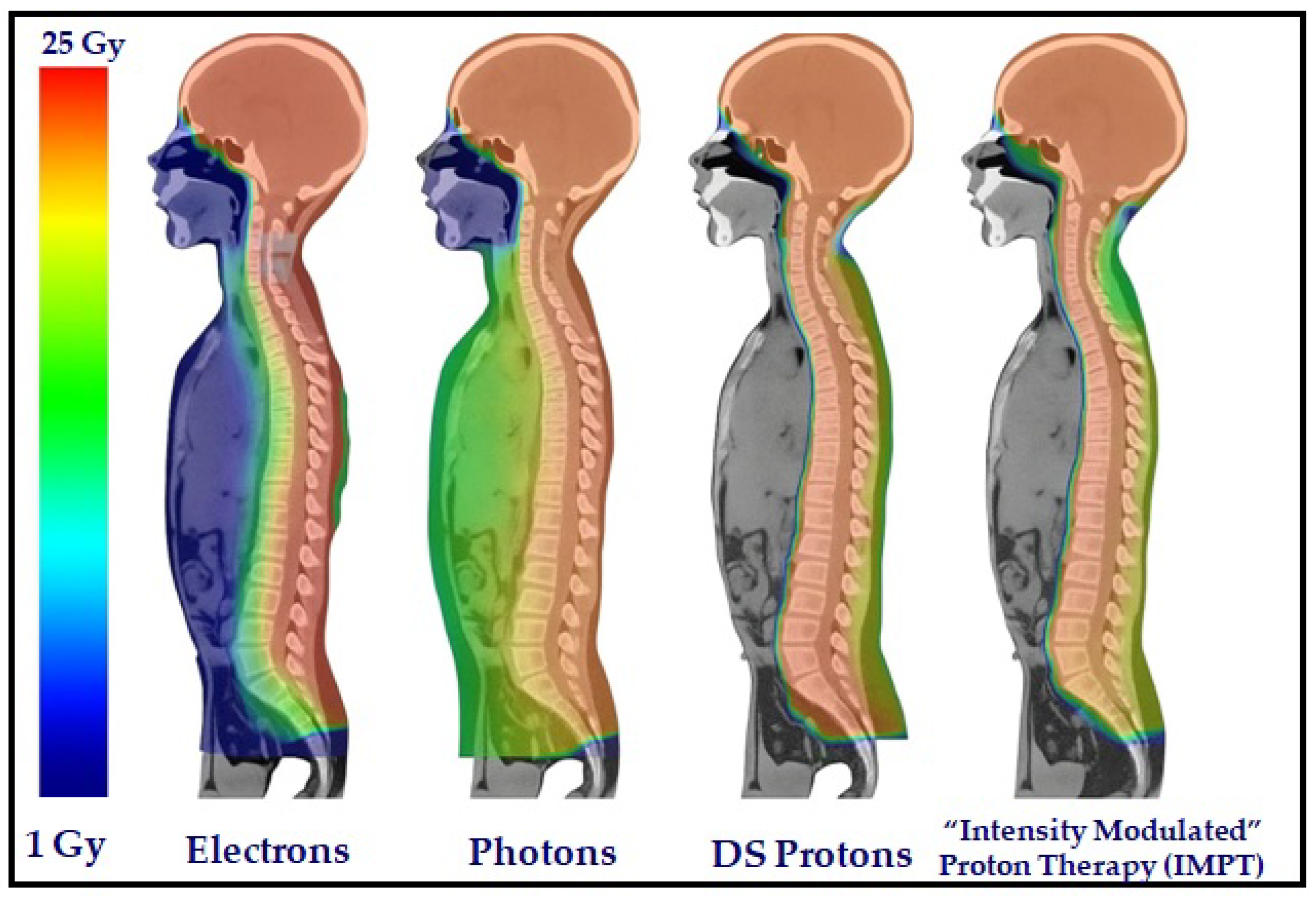
Craniospinal irradiation (CSI): This is the gold standard for medulloblastoma because it tends to spread through the CSF. Proton CSI considerably reduces doses at the bone marrow, heart, and gut organs, as compared with photon techniques (
Figure 4).
- ❖
Sparing of the hippocampus and temporal lobe: This is essential for the cognitive outcomes of long-term survivors.
- ❖
Follow-up of neurocognitive tests: This is essential for the early detection of cognitive function decline.
There is ongoing research on identifying biomarkers and imaging tools (like DTI and fMRI) that foretell and monitor post-therapy brain development.
Neuro-oncology is presently an imaging, planning, and delivery technology-supported precision science, as shown in
Figure 5. Through CNS-selective radiobiology information and the latest modalities, such as SRS, IMRT, proton therapy, and image guidance, physicians can treat with maximal sparing of essential neural function. With greater emphasis on survivorship and quality of life, more so in children, the future of neuro-oncology fabrication will involve greater individualization, technological integration, and cross-disciplinary research.
Figure 4.
Dose distributions for an 11-year-old male patient from the CSI technique, applying electrons, photons, DS protons, and spot-scanning IMPT [
29].
Figure 4.
Dose distributions for an 11-year-old male patient from the CSI technique, applying electrons, photons, DS protons, and spot-scanning IMPT [
29].
Figure 5.
Schematic representation of neuro-oncology.
Figure 5.
Schematic representation of neuro-oncology.
Table 3.
Summary of clinical trial data and long-term follow-up results of proton therapy in pediatric neuro-oncology, highlighting tumor types, survival outcomes, neurocognitive, and functional effects, and key findings from recent studies.
Table 3.
Summary of clinical trial data and long-term follow-up results of proton therapy in pediatric neuro-oncology, highlighting tumor types, survival outcomes, neurocognitive, and functional effects, and key findings from recent studies.
| Study | Tumor Type | Survival Rates | Neurocognitive Outcomes | Key Findings |
|---|
| St. Jude (2024) | Craniopharyngioma | High survival | Improved IQ and adaptive behavior | Proton therapy is superior to photon therapy [30] |
| Mayo Clinic (2024) | Various brain tumors | 93% at 2 years | Reduced endocrine and cognitive side effects | Effective disease control with minimal toxicity [31] |
| Le Reun et al. (2025) | Anaplastic ependymoma | 82.2% OS, 63.5% PFS at 5 years | Not specified | High local control and survival rates [32] |
| Indelicato et al. (2019) | Low-grade glioma | 92% OS, 84% PFS at 5 years | Not specified | Effective disease control with minimal toxicity [33] |
| Yock et al. (2016) | Medulloblastoma | High survival | Reduced late toxicities | Supports use of proton therapy in pediatric medulloblastoma [34] |
5. Integrating Imaging in Radiotherapy Planning
The use of modern imaging modalities plays a critical role in the proper and accurate planning of CNS malignancy radiation. The physician can now more precisely outline target volumes while safely sparing pertinent brain structures and adjust treatment accordingly with dynamic biological and anatomical changes using progressively more advanced neuroimaging instruments. The following section provides insight into the contribution of multimodal imaging to more accurate treatment outcomes.
5.1. Role of MRI, fMRI, and DTI in Defining Target Volumes
Magnetic resonance imaging (MRI), the corner stone of brain tumor visualization, is still used due to its superior soft tissue contrast. Contrast-enhancing tumor margins can be defined with the help of T1-weighted images, with T2 and FLAIR sequences providing insight into edema and non-enhancing margins of the tumor. These series allow for the definition of Gross Tumor Volume (GTV) and Clinical Target Volume (CTV) [
35].
Functional MRI (fMRI) provides critical information by localizing eloquent brain areas for speech, motor, and cognitive functions. The functional maps are combined with planning CT or MRI scans so that avoidance regions can be outlined for radiotherapy. It is extremely helpful if tumors lie within or very close to regions like Broca’s area or the motor cortex.
Diffusion tensor imaging (DTI), a continuation of diffusion-weighted imaging, is used for visualization of white matter tracts, like the corticospinal tract and the arcuate fasciculus, and optic radiation. These tissues on conventional imaging typically cannot be visualized but must be avoided in radiotherapy to preserve the patient’s neurological function. Tractography with DTI-based imaging enables 3D visualization of tracts, enabling dose planning with conformal avoidance.
5.2. PET Imaging for Biological Target Definition
Positron emission tomography (PET) complements MRI with metabolic and molecular information. (18F) FET and (11C) MET, both amino acid PET traces, are particularly effective in delineating gliomas, including infiltrating regions that are not visible on conventional imaging. PET can distinguish tumor recurrence versus radiation necrosis or pseudo-progression, a challenging issue in high-grade gliomas after treatment.
In radiotherapy planning, the inclusion of PET within the workflow aids in outlining Biological Target Volumes (BTVs), which are metabolically active tumor volumes that need higher doses. Co-registration of PET with planning CT or MRI enables dose escalation for aggressive sub-volumes with the sparing of normal tissue, a technique referred to as dose painting [
36]. In addition to tumor bordering, (18F) FDG-PET is utilized in selected cases of brain metastasis and lymphoma for the assessment of systemic disease and CNS disease. Future developments of theranostics, such as (18F) DOTATATE PET, may be able to detect and treat CNS cancers with receptor-targeting radiopharmaceuticals.
5.3. Tumor Delineation in Eloquent Brain Areas
Tumors near eloquent regions of the brain, such as the speech, vision, and motor regions, are very difficult to treat. In such situations, fMRI-DTI combined imaging is mandatory. These imaging techniques enable individualized contouring strategies with maximal dose delivery to the tumor while minimizing the possibility of neurological deficiencies. Inverse planning techniques fine-tune plans for radiation so that the target, as well as organs at risk(OARs), may be specified with high accuracy with the use of functional imaging data. A glioma, for instance, near the motor cortex, may be defined as an OAR using fMRI activations and may have tough dose constraints applied. Such high-degree individualization of planning would be unachievable with anatomical imaging alone [
37].
5.4. Adaptive Radiotherapy Approaches
Adaptive radiotherapy (ART) is an emerging field whereby plans are modified during treatment based on changes of an anatomical or functional nature. It is of relevance in neuro-oncology, where edema, tumor shrinkage, and necrosis as a result of treatment can radically alter target volumes over time. MRI-ART involves serial imaging at intervals during the treatment course for the assessment of volume and positional changes. Advanced planning systems can re-contour and re-optimize plans in hours. Functional imaging (such as fMRI and PET) may be repeated at mid-treatment for reassessment of tumor activity or neural function, with resultant modification of the therapy plan as appropriate. Weekly or daily imaging with onboard MRI (on MR-Linacs) is further enhancing the clinical relevance of ART. Online adaptive planning is made possible with these systems, where the radiation plan is adapted in real-time before each fraction of treatment [
38].
5.5. Motion Correction and Artifact Management
Even with immobilization protocols, intracranial motion, most noticeable in awake patients or with involuntary motion, may compromise image quality and treatment precision. Physiological sources of variation, such as breathing, pulsating cerebrospinal fluid, and patient anxiety, contribute to variability.
Motion artifacts in MRI may blur the edges of tumors, particularly in long acquisition time sequences. Motion-corrected averaging, navigator echoes, and real-time monitoring systems (such as eye-tracking during visual cortex mapping) may be used to offset this issue. Motion correction is relevant in PET imaging due to long scan times and poor spatial resolution. Strategies involve respiratory gating and head fixation through software motion correction with fiducial markers or with internal anatomy.
In addition, geometric distortion of MRI, especially in images like echo-planar imaging (EPI) used in fMRI or DWI, needs to be corrected while performing image registration with CT or PET. Quality assurance protocols, distortion correction programs, and daily calibration all have roles in verifying co-registration accuracy [
39].
Integration of advanced neuroimaging modalities with radiotherapy planning has revolutionized CNS tumor treatment. Multimodality imaging enables the definitive definition of the tumor, the sparing of function, and the customization of the therapy technique with the patient’s unique anatomy and the biological phenotypes of the tumor. With hybrid imaging and analytics with artificial intelligence, radiotherapy workflows can be simplified and tumor control and patient quality of life maximized.
6. Neurotoxicity and Cognitive Preservation
Radiation therapy is a critical modality of CNS tumor treatment. Nevertheless, the radio-sensitivity of brain tissues mandates that there be a fine balance between tumor control and preservation of intellectual function. Neurotoxicity is of significant long-term interest, more notably in children and long-term surviving adult populations. The current section outlines mechanisms of radiation-induced brain injury and future directions toward minimizing its impact.
6.1. Radiation-Induced Brain Injury
Cranial radiation can cause structural and functional damage to the neural tissue, with presentation of neurocognitive decline. The damage is delayed and progressive in character, typically staged in three phases: in the first few weeks as acute, 1–6 months as early delayed, and months to years post-treatment as late delayed. These late effects are of utmost concern and can involve myelination, vascular injury, and neuroinflammation, with ultimate presentation of memory deficits, executive dysfunction, and poor quality of life. The hippocampus, involved with learning and memory, is of special concern. Several studies have indicated an association of higher doses of radiation to hippocampal sites with deteriorated performance of verbal memory [
40].
6.2. Hippocampal Avoidance Techniques
New technologies in delivering radiation, specifically with intensity-modulated radiotherapy (IMRT) and volumetric-modulated arc therapy (VMAT), allow for conformal dose sculpting around critical structures. Hippocampal-sparing whole-brain radiotherapy (HA-WBRT) is acknowledged as an effective way to prevent cognitive decline for those with cranial irradiation for brain metastases [
41]. Clinical trials such as RTOG 0933 have established the feasibility and intellectual benefit of this method. With strategic dose reductions in hippocampal locations with adequate coverage of the remainder of the brain, HA-WBRT offers a promising compromise of treatment efficacy and preservation of quality of life.
6.3. Role of fMRI in Sparing Cognitive Areas
Functional MRI (fMRI) is becoming a standard part of pre-treatment planning, particularly for tumors near eloquent brain sites. Using blood oxygen level-dependent (BOLD) imaging, fMRI allows for the localization of sites of language, memory, and motor function. Integration of fMRI information into radiotherapy planning can prevent high-dose irradiation of these functional regions [
42]. When planning irradiation of tumors close to Broca’s area or Wernicke’s area, fMRI planning ensures that these areas have the minimum dose, sparing the abilities of speech and comprehension.
In addition, combining fMRI with DTI-based tractography enables visualization of white matter tracts such as the arcuate fasciculus and corticospinal tracts, further improving the precision of treatment planning.
6.4. Neuroprotective Strategies
Besides technical dose adjustment, certain pharmacological and behavioral interventions have also been investigated as countermeasures for radiation-induced cognitive decline. Neuroprotective agents such as memantine, an NMDA receptor antagonist, are able to suppress cognitive decline while administered at the time of cranial irradiation. The Phase III trial RTOG 0614 demonstrated that the cognitive performance of patients on memantine was superior compared with the group on the placebo.
Antioxidants, anti-inflammatory substances, and inhibitors of microglial-activating agents continue to be investigated as preventors of neurotoxicity. Concurrent with these efforts, cognitive rehabilitation programs and neurofeedback therapies continue to be crafted as preventors of the loss of, or re-acquisition of, cognition after treatment [
43].
As it results in a reduced exit dose and high precision, proton therapy is gaining favor over conventional photons for greater sparing of normal tissues. It is thus extremely beneficial in pediatric neuro-oncology, where sparing of the brain is of the highest importance. Neurotoxicity remains of great relevance within neuro-oncology, more so with rising rates of survival. However, the convergence of high-precision imaging, precision radiotherapy, and drug therapy offers many choices for managing these effects. Progressive advances in hippocampal-sparing techniques, the integration of functional imaging within treatment planning, and research on neuroprotective therapy all point toward an evolutionary step forward in sustaining survival as much as quality of life in CNS disease patients [
44].
7. Emerging Trends and Future Directions
The specialties of neuroradiology and neuro-oncology are being redefined at their very foundation by changes driven by technological advances and the need for personalized medicine. The future is promising, combining imaging information, molecular biomarkers, and computational simulation for more accurate diagnosis, treatment planning, and response monitoring. Several emerging trends are redefining the treatment of CNS disorders by physicians, as shown in
Table 4.
7.1. Artificial Intelligence and Radiomics
Artificial intelligence, and specifically machine learning and deep learning, is revolutionizing image analysis with the provision of automated segmentation, pattern recognition, and prediction of response to therapy. Radiomics, a research area that extracts quantitative features from imaging, offers an effective weapon with which imaging biomarkers that are potentially non-apparent to the naked eye can be discovered [
45]. Large datasets can be assessed with AI software to determine differences between tumor types, predict prognosis, and point toward individualized treatment options. For instance, computer-aided software facilitated by AI has shown the potential of defining glioma margins with greater accuracy than human contouring, which is important for the planning of surgery and radiotherapy dose targeting. Radiomics with clinical and genetic data can additionally predict molecular subtypes of glioblastoma without the need for invasive techniques and can provide valuable information.
7.2. Theranostic Approaches
The intersection of therapeutics and diagnostics under the umbrella of theranostics is yet another exciting area. Theranostics is being applied in neuro-oncology with radiolabeled therapeutics such as 68Ga-DOTATATE and 177Lu-DOTATATE for imaging and treatment of neuroendocrine neoplasms. These molecules bind with somatostatin receptors and can be used for targeted radionuclide therapy while allowing for specific imaging via PET.
While currently more common with extracranial cancers, theranostics in brain tumors has gained increasing recognition. Researchers have explored the option of using the same molecular tracers to visualize and treat gliomas or brain metastases, potentially providing personalized therapy guidance using receptor expression profile [
46,
47].
7.3. Functional Imaging for Response Prediction
Traditional imaging techniques are often unable to distinguish tumor progression from treatment reactions, such as pseudo-progression or radiation necrosis. Functional imaging techniques such as perfusion MRI, MR spectroscopy, and amino acid PET (such as FET or MET) provide early clues of the tumor microenvironment’s metabolic and vascular changes.
Such imaging biomarkers may be used as soon as the response to radio- and chemotherapy can be measured before anatomical changes are apparent [
48,
49]. Amino acid PET imaging, as an example, has a greater detection rate for true tumor progression of glioblastoma than contrast-enhanced MRI alone. Integration of such imaging biomarkers into treatment planning workflows can provide the flexibility of adaptively adjusting treatments based on biological responses.
7.4. Personalized Treatment Algorithms
The shift toward personalized oncology is influencing neuro-oncology protocols. Tumor genomics, radio genomics, and imaging phenotypes are being integrated to personalize treatment for individual patients. For example, the decision on whether it is beneficial to pursue dose escalation or concurrent chemotherapy can be informed by IDH mutation status and MGMT methylation patterns in gliomas.
Adaptive radiotherapy based on serial imaging and molecular profiling may allow for optimal muscle-sparing therapy [
50]. This strategy may be particularly useful in children and patients with long-anticipated survivals, where the conservation of neurocognitive function is important for an improved QOL.
In addition, clinical trials are increasingly incorporating image-based endpoints and predictive biomarkers to refine eligibility criteria and select patients who may derive the most benefit. This paradigm shift should be emphasized with the need to create harmonized imaging protocols and data-sharing structures to enable collaborative studies.
7.5. Integration of Multimodal Data and Decision Support
Future neuro-oncologic care would be best composed of multimodal platforms that combine structural, functional, and molecular imaging data with genetic and clinical information. These platforms would enable real-time decision-making through predictive algorithms and therapy simulators. Such tools can be employed to evaluate diverse scenarios, predict side effects, and offer optimal treatment protocols.
Federated learning networks and cloud systems have also been explored as means of information sharing while maintaining patient confidentiality. They can be utilized as the basis for constructing robust, generalize able AI models and learning databases for rare CNS tumors. The neuroradiology of the future is precision, integration, and innovation. Advances in technology with AI, functional imaging, and theranostics promise highly personalized therapy as shown in
Table 5 [
51,
52].
Multidisciplinary collaboration, as these technologies continue to emerge, will be essential in translating these tools from bench to bedside. These future advances all seek higher diagnostic precision, greater treatment efficacy, and enhanced patient outcomes in neuro-oncology.
7.6. Comparative Evaluation of Emerging Imaging Technologies in Neuroradiology and Neuro-Oncology
The integration of sophisticated imaging and computational science is changing the face of neuro-oncology and general CNS disease care as shown in
Table 6. Highly complex MRI technologies, such as diffusion, perfusion, and tractography, are currently modestly translational, particularly in glioblastoma and metastases to the brain, where they improve tumor delineation and monitoring. Hybrid PET/MRI offers a robust synergy between metabolic and structural information, but it is essentially relegated to research settings because of exorbitant costs and logistical constraints. Radiomics and radio genomics have potential as predictive and prognostic biomarkers, evolving the precision medicine paradigm. Their clinical use is held back at present by reproducibility problems and the absence of multicenter validations in large numbers. Likewise, AI-driven segmentation and adaptive planning improve workflow efficiency and minimize inter-observer variability but are held back from routine clinical use by regulatory, ethical, and data-related challenges. MR-guided radiotherapy (MR-Linac) is becoming one of the most advanced clinical modalities, with expanding use in prostate and head-and-neck malignancies and increasing potential for CNS indications, with real-time adaptive treatment. Lastly, new molecular and functional imaging technologies (e.g., MR fingerprinting, QSM, and ASL) are still in an early investigational phase but hold promising potential for the identification of early treatment response biomarkers and personalized therapy guidance. In short, although several modalities exhibit moderate evidence and translational readiness, mass uptake into clinical neuro-oncology will require standardization, cost minimization, and intensive multicenter validation.
8. Conclusions
Neuroradiology and neuro-oncology have undergone radical revisions over the past few decades, evolving from largely diagnosis- and treatment-supplementing specialties into highly specialized, interconnected fields. With advances in structural and functional imaging, doctors can more accurately stage and monitor CNS pathology, in particular, malignant brain tumors and degenerative diseases, than previously possible. The integration of anatomical imaging (MRI, CT) with functional and molecular techniques (fMRI, DTI, MR spectroscopy, and PET) allows for more accurate localization of disease with diminished radiation while being more sparing of eloquent cortex areas.
Concurrently, neuro-oncology has embraced precision-focused techniques such as stereotactic radiosurgery, IMRT, and particle therapy, with dose maximization to tumors and decreased collateral damage to surrounding neural tissue. The combination of imaging and therapy has borne fruit in image-guided radiotherapy and biologically guided treatment planning, further underlining the necessity of multidisciplinary collaboration between radiologists, radiation oncologists, physicists, and neurosurgeons. The growing emphasis on the protection of neurocognitive integrity and quality of life puts an added burden on individually specific approaches to therapy. This is especially important in long-survivor and pediatric populations. Radiomics, artificial intelligence, and theranostics hold much promise as future approaches to personalized patient care, enabling early diagnosis, real-time determination of response, and adaptation of treatment on a dynamic basis.
In brief, neuroradiology and neuro-oncology can no longer be separated as distinct areas; rather, they must be treated as connected pillars of modern neuro-oncologic care. Continuing efforts would ideally work toward greater integration of these through combined research endeavors, technological advances, and convergence of decision-making with data. With a growing focus on more personalized and biologically driven interventions, these fields shall continue to be at the forefront of patient results for those suffering from difficult CNS diseases.
Author Contributions
Conceptualization, V.P.; Writing—original draft preparation, V.P.; Investigation, S.V., D.P.S., and K.G.; Writing—review and editing, S.V., S.K., V.T., M.P., S.K.V., K.G., and G.N.; Supervision, S.K. and V.T.; Resources, R.K., D.A., M.P., and S.K.V.; Project administration, R.K., D.A., and S.K.V.; Visualization, D.P.S.; Software, G.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing does not apply to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Burger, P.C.; Vogel, F.S. The brain, tumors. In Surgical Pathology of the Central Nervous System and Its Coverings, 2nd ed.; Burger, P.C., Vogel, F.S., Eds.; Wiley: New York, NY, USA, 1982; pp. 223–266. [Google Scholar]
- Burger, P.C.; Vogel, F.S.; Green, S.B.; Strike, T.A. Glioblastoma multiforme and anaplastic astrocytoma, pathologic criteria and prognostic implications. Cancer 1985, 56, 1106–1111. [Google Scholar] [CrossRef] [PubMed]
- Kleihues, P.; Sobin, L.H. World Health Organization classification of tumors. Cancer 2000, 88, 2887. [Google Scholar] [CrossRef]
- Kleihues, P.; Ohgaki, H. Phenotype vs. genotype in the evolution of astrocytic brain tumors. Toxicol. Pathol. 2000, 28, 164–170. [Google Scholar] [CrossRef]
- Ricci, P.E. Imaging of adult brain tumors. Neuroimaging Clin. N. Am. 1999, 9, 651–669. [Google Scholar] [CrossRef]
- Felix, R.; Schörner, W.; Laniado, M.; Niendorf, H.P.; Claussen, C.; Fiegler, W.; Speck, U. Brain tumors, MR imaging with gadolinium-DTPA. Radiology 1985, 156, 681–688. [Google Scholar] [CrossRef]
- Kates, R.; Atkinson, D.; Brant-Zawadzki, M. Fluid-attenuated inversion recovery (FLAIR), clinical prospectus of current and future applications. Top. Magn. Reson. Imaging 1996, 8, 389–396. [Google Scholar] [CrossRef]
- Ercan, N.; Gultekin, S.; Celik, H.; Tali, T.E.; Oner, Y.A.; Erbas, G. Diagnostic value of contrast-enhanced fluid-attenuated inversion recovery MR imaging of intracranial metastases. AJNR Am. J. Neuroradiol. 2004, 25, 761–765. [Google Scholar]
- Singer, M.B.; Atlas, S.W.; Drayer, B.P. Subarachnoid space disease, diagnosis with fluid-attenuated inversion-recovery MR imaging and comparison with gadolinium-enhanced spin-echo MR imaging–blinded reader study. Radiology 1998, 208, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Maher, E.A.; Furnari, F.B.; Bachoo, R.M.; Rowitch, D.H.; Louis, D.N.; Cavenee, W.K.; DePinho, R.A. Malignant glioma, genetics and biology of a grave matter. Genes Dev. 2001, 15, 13111333. [Google Scholar] [CrossRef]
- Edelman, R.R.; Wielopolski, P.; Schmitt, F. Echo-planar MR imaging. Radiology 1994, 192, 600–612. [Google Scholar] [CrossRef]
- Castillo, M.; Mukherji, S.K. Diffusion-weighted imaging in the evaluation of intracranial lesions. Semin. Ultrasound CT MR 2000, 21, 405–416. [Google Scholar] [CrossRef]
- Schaefer, P.W.; Grant, P.E.; Gonzalez, R.G. Diffusion-weighted MR imaging of the brain. Radiology 2000, 217, 331–345. [Google Scholar] [CrossRef]
- Holodny, A.I.; Ollenschlager, M. Diffusion imaging in brain tumors. Neuroimaging Clin. N Am. 2002, 12, 107–124, x. [Google Scholar] [CrossRef]
- Akai, H.; Mori, H.; Aoki, S.; Masutani, Y.; Kawahara, N.; Shibahara, J.; Ohtomo, K. Diffusion tensor tractography of gliomatosis cerebri, fiber tracking through the tumor. J. Comput. Assist Tomogr. 2005, 29, 127–129. [Google Scholar] [CrossRef]
- Chen, S.Q.; Kang, Z.; Hu, X.Q.; Hu, B.; Zou, Y. Diffusion tensor imaging of the brain in patients with Alzheimer’s disease and cerebrovascular lesions. J. Zhejiang Univ. Sci. B 2007, 8, 242–247. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mukherjee, P.; Berman, J.I.; Chung, S.W.; Hess, C.P.; Henry, R.G. Diffusion tensor MR imaging and fiber tractography, theoretic underpinnings. AJNR Am. J. Neuroradiol. 2008, 29, 632–641. [Google Scholar] [CrossRef]
- Nimsky, C.; Grummich, P.; Sorensen, A.G.; Fahlbusch, R.; Ganslandt, O. Visualization of the pyramidal tract in glioma surgery by integrating diffusion tensor imaging in functional neuronavigation. Central Eur. Neurosurg. 2005, 66, 133–141. [Google Scholar] [CrossRef] [PubMed]
- De Pietro, S.; Di Martino, G.; Caroprese, M.; Barillaro, A.; Cocozza, S.; Pacelli, R.; Cuocolo, R.; Ugga, L.; Briganti, F.; Brunetti, A.; et al. The role of MRI in radiotherapy planning, a narrative review “from head to toe”. Insights Imaging 2024, 15, 255. [Google Scholar] [CrossRef]
- Reichert, M.; Morelli, J.N.; Runge, V.M.; Tao, A.; von Ritschl, R.; von Ritschl, A.; Padua, A.; Dix, J.E.; Marra, M.J.; Schoenberg, S.O.; et al. Contrast-Enhanced Three-Dimensional SPACE versus MP-RAGE for the Detection of Brain Metastases, Considerations with a 32-Channel Head Coil. Investig. Radiol. 2013, 48, 55–60. [Google Scholar] [CrossRef]
- Danieli, L.; Riccitelli, G.; Distefano, D.; Prodi, E.; Ventura, E.; Cianfoni, A.; Kaelin-Lang, A.; Reinert, M.; Pravatà, E. Brain Tumor-Enhancement Visualization and Morphometric Assessment, A Comparison of MPRAGE, SPACE, and VIBE MRI Techniques. AJNR Am. J. Neuroradiol. 2019, 40, 1140–1148. [Google Scholar] [CrossRef]
- Maziero, D.; Straza, M.W.; Ford, J.C.; Bovi, J.A.; Diwanji, T.; Stoyanova, R.; Paulson, E.S.; Mellon, E.A. MR-Guided Radiotherapy for Brain and Spine Tumors. Front. Oncol. 2021, 11, 626100. [Google Scholar] [CrossRef] [PubMed]
- Weisskoff, R.M.; Rosen, B.R. Noninvasive determination of regional cerebral blood flow in rats using dynamic imaging with Gd (DTPA). Magn. Reson. Med. 1992, 25, 211–212. [Google Scholar] [CrossRef]
- Aronen, H.J.; E Gazit, I.; Louis, D.N.; Buchbinder, B.R.; Pardo, F.S.; Weisskoff, R.M.; Harsh, G.R.; Cosgrove, G.R.; Halpern, E.F.; Hochberg, F.H. Cerebral blood volume maps of gliomas, comparison with tumor grade and histologic findings. Radiology 1994, 191, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Adler, J.R.A.; Chang, S.D.; Murphy, M.J.; Doty, J.; Geis, P.; Hancock, S.L. The CyberKnife, a frameless robotic system for radiosurgery. Stereotact. Funct. Neurosurg. 1999, 69, 124–128. [Google Scholar] [CrossRef]
- Sahgal, A.; Bilsky, M.; Chang, E.L.; Ma, L.; Yamada, Y.; Rhines, L.D.; Létourneau, D.; Foote, M.; Yu, E.; Larson, D.A.; et al. Stereotactic body radiotherapy for spinal metastases, current status. J. Neurosurg. Spine 2011, 14, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, J.P.; Brenner, D.J.; Orton, C.G. Point/Counterpoint. The linear quadratic model is inappropriate to model high dose per fraction effectsin radiosurgery. Med. Phys. 2009, 36, 3381–3384. [Google Scholar] [CrossRef]
- Benedict, S.H.; Yenice, K.M.; Followill, D.; Galvin, J.M.; Hinson, W.; Kavanagh, B.; Keall, P.; Lovelock, M.; Meeks, S.; Papiez, L.; et al. Stereotactic body radiation therapy, the report of AAPM Task Group 101. Med. Phys. 2010, 37, 4078–4101. [Google Scholar] [CrossRef] [PubMed]
- Stokkevåg, C.H.; Engeseth, G.M.; Ytre-Hauge, K.S.; Röhrich, D.; Odland, O.H.; Muren, L.P.; Petersen, J.B.B. Estimated Risk of Radiation-Induced Cancer Following Paediatric Cranio-Spinal Irradiation with Electron, Photon and Proton Therapy. Acta Oncol. 2014, 53, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- St. Jude Children’s Research Hospital. Proton Therapy Improves Neurocognitive Outcomes of Childhood Craniopharyngioma; St. Jude Children’s Research Hospital: Memphis, TN, USA, 2024; Available online: https://www.stjude.org/research/why-st-jude/scientific-report/2024/proton-therapy-improves-neurocognitive-outcomes-of-childhood-cra.html (accessed on 20 September 2025).
- Mayo Clinic. Proton Beam Therapy for Pediatric Cancers; Mayo Clinic: Rochester, MN, USA, 2024; Available online: http://www.mayoclinic.org/medical-professionals/pediatrics/news/proton-beam-therapy-for-pediatric-cancers/mac-20576795 (accessed on 20 September 2025).
- Le Reun, E.; Kotov, I.; Leiser, D.; Pica, A.; Vazquez, M.; Calaminus, G.; Weber, D.C. Long-Term Outcomes and Quality of Life of Children with Intracranial Ependymoma Treated with Proton Therapy. Pediatr. Blood Cancer 2025, 72, e40254810. [Google Scholar] [CrossRef]
- Indelicato, D.J.; Rotondo, R.L.; Uezono, H.; Sandler, E.S.; Aldana, P.R.; Ranalli, N.J.; Beier, A.D.; Morris, C.G.; Bradley, J.A. Outcomes Following Proton Therapy for Pediatric Low-Grade Glioma. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 1014–1021. [Google Scholar] [CrossRef]
- Yock, T.I.; Yeap, B.Y.; Ebb, D.H.; Weyman, E.; Eaton, B.R.; Sherry, N.A.; Jones, R.M.; MacDonald, S.M.; Pulsifer, M.B.; Lavally, B. Long-Term Toxic Effects of Proton Radiotherapy for Pediatric Medulloblastoma. Lancet Oncol. 2016, 17, 1276–1285. [Google Scholar] [CrossRef]
- Aldawsari, A.M.; Al-Qaisieh, B.; Broadbent, D.A.; Bird, D.; Murray, L.; Speight, R. The role and potential of using quantitative MRI biomarkers for imaging guidance in brain cancer radiotherapy treatment planning: A systematic review. Phys. Imaging Radiat. Oncol. 2023, 27, 100476. [Google Scholar] [CrossRef]
- Stewart, P.A.; Hayakawa, K.; Farrell, C.L.; Del Maestro, R.F. Quantitative study of microvessel ultrastructure in human peritumoral brain tissue, evidence for a blood–brain barrier defect. J. Neurosurg. 1987, 67, 697–705. [Google Scholar] [CrossRef]
- Uematsu, H.; Maeda, M.; Sadato, N.; Matsuda, T.; Ishimori, Y.; Koshimoto, Y.; Yamada, H.; Kimura, H.; Kawamura, Y.; Matsuda, T.; et al. Vascular permeability, quantitative measurement with double-echo dynamic MR imaging, theory and clinical application. Radiology 2000, 214, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Law, M.; Zagzag, D.; Wu, H.H.; Cha, S.; Golfinos, J.G.; Knopp, E.A.; Johnson, G. Dynamic contrast-enhanced perfusion MR imaging measurements of endothelial permeability, differentiation between atypical and typical meningiomas. AJNR Am. J. Neuroradiol. 2003, 24, 1554–1559. [Google Scholar] [PubMed]
- Provenzale, J.M.; Wang, G.R.; Brenner, T.; Petrella, J.R.; Sorensen, A.G. Comparison of permeability in high-grade and low-grade brain tumors using dynamic susceptibility contrast MR imaging. AJR Am. J. Roentgenol. 2002, 178, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Roberts, H.C.; Roberts, T.P.; Brasch, R.C.; Dillon, W.P. Quantitative measurement of microvascular permeability in human brain tumors achieved using dynamic contrast-enhanced MR imaging, correlation with histologic grade. AJNR Am. J. Neuroradiol. 2000, 21, 891–899. [Google Scholar]
- Knopp, E.A.; Cha, S.; Johnson, G.; Mazumdar, A.; Golfinos, J.G.; Zagzag, D.; Miller, D.C.; Kelly, P.J.; Kricheff, I.I. Glial neoplasms, dynamic contrast-enhanced T2*-weighted MR imaging. Radiology 1999, 211, 791–798. [Google Scholar] [CrossRef]
- Sugahara, T.; Korogi, Y.; Kochi, M.; Ikushima, I.; Hirai, T.; Okuda, T.; Shigematsu, Y.; Liang, L.; Ge, Y.; Ushio, Y.; et al. Correlation of MR imagingdetermined cerebral blood volume maps with histologic and angiographic determination of vascularity of gliomas. AJR Am. J. Roentgenol. 1998, 171, 1479–1486. [Google Scholar] [CrossRef]
- Sugahara, T.; Korogi, Y.; Shigematsu, Y.; Hirai, T.; Ikushima, I.; Liang, L.; Ushio, Y.; Takahashi, M. Perfusion-sensitive MRI of cerebral lymphomas, a preliminary report. J. Comput. Assist. Tomogr. 1999, 23, 232–237. [Google Scholar] [CrossRef]
- Cha, S.; Pierce, S.; Knopp, E.A.; Johnson, G.; Yang, C.; Ton, A.; Litt, A.W.; Zagzag, D. Dynamic contrast-enhanced T2*-weighted MR imaging of tumefactive demyelinating lesions. AJNR Am. J. Neuroradiol. 2001, 22, 1109–1116. [Google Scholar]
- Chukwujindu, E.; Faiz, H.; AI-Douri, S.; Faiz, K.; De Sequeira, A. Role of artificial intelligence in brain tumour imaging. Eur. J. Radiol. 2024, 176, 111509. [Google Scholar] [CrossRef]
- Burkett, B.J.; Bartlett, D.J.; McGarrah, P.W.; Lewis, A.R.; Johnson, D.R.; Berberoğlu, K.; Pandey, M.K.; Packard, A.T.; Halfdanarson, T.R.; Hruska, C.B.; et al. A Review of Theranostics, Perspectives on Emerging Approaches and Clinical Advancements. Radiol. Imaging Cancer 2023, 5, e220157. [Google Scholar] [CrossRef]
- Zhu, L.; Jiang, Y.; Tian, H.; Yu, Y.; Gan, Y.; Li, H.; Yuan, M.; Huang, X.; Liu, X. Recent Advancement in MRI-Based Nanotheranostic Agents for Tumor Diagnosis and Therapy Integration. Int. J. Nanomed. 2025, 20, 10503–10540. [Google Scholar] [CrossRef]
- Ryan, J.T.; Nakayama, M.; Gleeson, I.; Mannion, L.; Geso, M.; Kelly, J.; Ng, S.P.; Hardcastle, N. Functional brain imaging interventions for radiation therapy planning in patients with glioblastoma, a systematic review. Radiat. Oncol. 2022, 17, 178. [Google Scholar] [CrossRef]
- Yang, C.; Sun, Y.; Jiang, M.; Fan, Y.; Hu, Y.; Zhang, Q.; Zhang, Y.; Wang, Y.; Jiang, X.; Wang, Z.; et al. FLAIR-based radiomics signature from brain-tumor interface for early prediction of response to EGFR-TKI therapy in NSCLC patients with brain metastasis. Front. Cell Dev. Biol. 2025, 13, 1525989. [Google Scholar] [CrossRef]
- Valizadeh, P.; Jannatdoust, P.; Pahlevan-Fallahy, M.-T.; Hassankhani, A.; Amoukhteh, M.; Bagherieh, S.; Ghadimi, D.J.; Gholamrezanezhad, A. Diagnostic accuracy of radiomics and artificial intelligence models in diagnosing lymph node metastasis in head and neck cancers, a systematic review and meta-analysis. Neuroradiology 2025, 67, 449–467. [Google Scholar] [CrossRef] [PubMed]
- Wagner, D.T.; Tilmans, L.; Peng, K.; Niedermeier, M.; Rohl, M.; Ryan, S.; Yadav, D.; Takacs, N.; Garcia-Fraley, K.; Koso, M.; et al. Artificial Intelligence in Neuroradiology, A Review of Current Topics and Competition Challenges. Diagnostics 2023, 13, 2670. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Shang, J.; Zhang, X.; Li, N. Advances in molecular imaging and targeted therapeutics for lymph node metastasis in cancer, a comprehensive review. J. Nanobiotechnol. 2024, 22, 783. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Kim, H.S.; Jo, Y.; Yoo, R.E.; Choi, S.H.; Nam, S.J.; Kim, J.H. Radiomics Prognostication Model in Glioblastoma Using Diffusion- and Perfusion-Weighted MRI. Sci. Rep. 2020, 10, 4250. [Google Scholar] [CrossRef]
- Pasquini, L.; Di Napoli, A.; Napolitano, A.; Lucignani, M.; Dellepiane, F.; Vidiri, A.; Villani, V.; Romano, A.; Bozzao, A. Glioblastoma Radiomics to Predict Survival, Diffusion Characteristics of Surrounding Nonenhancing Tissue to Select Patients for Extensive Resection. J. Neuroimaging 2021, 31, 1192–1200. [Google Scholar] [CrossRef]
- Ziegenfeuter, J.; Delbridge, C.; Bernhardt, D.; Gempt, J.; Schmidt-Graf, F.; Griessmair, M.; Thomas, M.; Meyer, H.S.; Zimmer, C.; Meyer, B.; et al. Sequential and Hybrid PET/MRI Acquisition in Follow-Up Examination of Glioblastoma Show Similar Diagnostic Performance. Cancers 2023, 15, 83. [Google Scholar] [CrossRef]
- Wardhana, D.P.W.; Maliawan, S.; Mahadewa, T.G.B.; Rosyidi, R.M.; Wiranata, S. Radiomic Features as Artificial Intelligence Prognostic Models in Glioblastoma, A Systematic Review and Meta-Analysis. Diagnostics 2024, 14, 2354. [Google Scholar] [CrossRef]
- Choi, Y.; Jang, J.; Kim, B.S.; Ahn, K.J. Pretreatment MR-Based Radiomics in Patients with Glioblastoma, A Systematic Review and Meta-Analysis of Prognostic Endpoints. Eur. J. Radiol. 2023, 168, 111130. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Choi, Y.S.; Ahn, S.S.; Chang, J.H.; Kang, S.G.; Kim, E.H.; Kim, S.H.; Lee, S.K. Radiomic MRI Phenotyping of Glioblastoma, Improving Survival Prediction. Radiology 2018, 289, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Snyder, K.C.; Siddiqui, S.M.; Parikh, P.; Thind, K. Adaptive Treatment Workflow and Dosimetric Evaluation of Intracranial Fractionated Stereotactic Radiosurgery on a Low-Field Magnetic Resonance-Linear Accelerator. Phys. Imaging Radiat. Oncol. 2025, 33, 100702. [Google Scholar] [CrossRef]
- Han, E.Y.; Wang, H.; Briere, T.M.; Yeboa, D.N.; Boursianis, T.; Kalaitzakis, G.; Pappas, E.; Castillo, P.; Yang, J. Brain Stereotactic Radiosurgery Using MR-Guided Online Adaptive Planning for Daily Setup Variation, an End-to-End Test. J. Appl. Clin. Med. Phys. 2022, 23, e13518. [Google Scholar] [CrossRef]
- Liu, C.; Li, M.; Xiao, H.; Li, T.; Li, W.; Zhang, J.; Teng, X.; Cai, J. Advances in MRI-Guided Precision Radiotherapy. Prec. Radiat. Oncol. 2022, 6, 75–84. [Google Scholar] [CrossRef]
- Jena, A.; Taneja, S.; Jha, A.; Damesha, N.K.; Negi, P.; Jadhav, G.K.; Verma, S.M.; Sogani, S.K. Multiparametric Evaluation in Differentiating Glioma Recurrence from Treatment-Induced Necrosis Using Simultaneous 18F-FDG-PET/MRI, A Single-Institution Retrospective Study. Am. J. Neuroradiol. 2017, 38, 899–907. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Swiss Federation of Clinical Neuro-Societies. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).