Neurorehabilitation as a Cornerstone of the Brain Health Plan
Abstract
1. Introduction
2. Characteristics of Neurorehabilitation
2.1. Multidimensional Impairment Profile and Interdisciplinary Management
2.2. Heterogeneous Disease Severity Spectrum
2.3. Extended Temporal Treatment Requirements
2.4. Evidence-Based Therapeutic Principles and Technological Integration
2.5. Therapeutic Objectives and Clinical Outcomes Framework
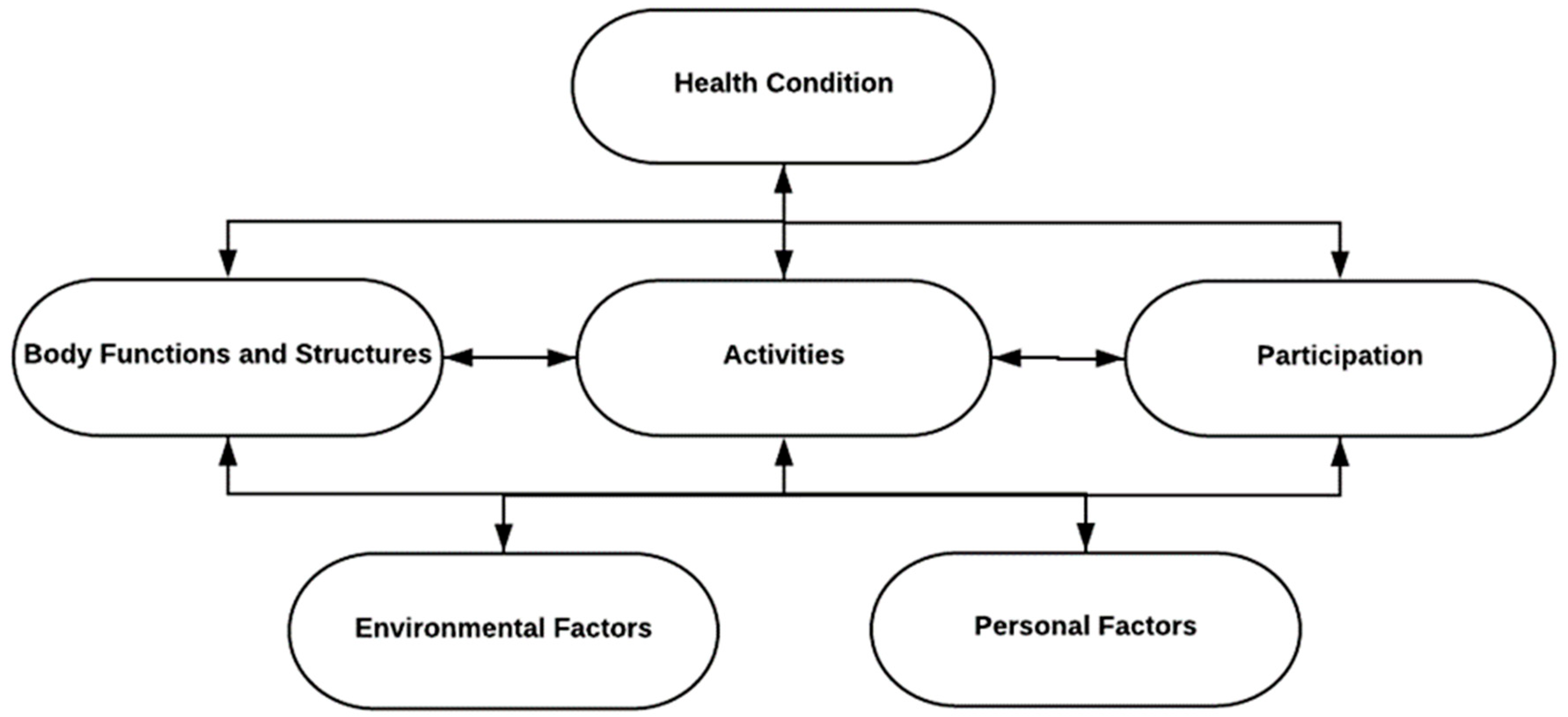
3. Integration of the ICF Model in Rehabilitation Practice and Brain Health Strategy
3.1. Application in Neurorehabilitation
3.2. ICF Model Integration with Brain Health and Neurorehabilitation
3.3. Scientific Integration and Policy Implications
4. Prevention Strategies in Neurological Rehabilitation
4.1. Hierarchical Prevention Framework
- (1)
- Primary prevention aims to prevent disease onset before pathological processes develop, thereby achieving incidence reduction. This approach particularly targets modifiable risk factors through promotion of healthy, neuroprotective lifestyle behaviors. Primary prevention strategies focus on population-level interventions that address established risk factors for neurological conditions.
- (2)
- Secondary prevention encompasses early disease detection and prompt therapeutic intervention to favorably influence the disease trajectory and hinder progression. This approach emphasizes diagnostic screening protocols and timely treatment initiation to minimize long-term functional consequences and disability development.
- (3)
- Tertiary prevention seeks to prevent complications of established neurological conditions, alleviate symptom burden, and maintain quality of life and functional independence. Rehabilitation interventions exemplify tertiary prevention strategies, including physiotherapy and occupational therapy, designed to prevent complications such as falls, spasticity development, or contracture formation.
4.2. Rehabilitation as Primary Prevention Platform
4.3. Preventive Measures in Neurorehabilitation: A Brain Health Perspective
4.3.1. Cerebrovascular Disease Management
4.3.2. Physical Activity and Motor Function Restoration
4.3.3. Nutritional Interventions
4.3.4. Sleep Health Management
4.3.5. Mental Health and Psychological Support
4.3.6. Cognitive and Social Integration
5. Future Directions and Technological Integration
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- World Health Organization. Rehabilitation. Fact Sheet. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/rehabilitation (accessed on 15 January 2025).
- Cieza, A.; Causey, K.; Kamenov, K.; Hanson, S.W.; Chatterji, S.; Vos, T. Global estimates of the need for rehabilitation based on the global burden of disease study 2019: A systematic analysis for the global burden of disease study 2019. Lancet 2020, 396, 2006–2017. [Google Scholar] [CrossRef]
- Alvsåker, K.; Hanoa, R.; Gran, J.M.; Högvall, L.M.; Sogn, C.J.F.; Bech, H.C.; Olasveengen, T. Impact of rehabilitation in the neurointensive care unit on long-term survival in patients with traumatic brain injury. Acta Anaesthesiol. Scand. 2025, 69, e70026. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cuccurullo, S.J.; Fleming, T.K.; Zinonos, S.; Cosgrove, N.M.; Cabrera, J.; Kostis, J.B.; Greiss, C.; Ray, A.R.; Eckert, A.; Scarpati, R.; et al. Stroke Recovery Program with Modified Cardiac Rehabilitation Improves Mortality, Functional & Cardiovascular Performance. J. Stroke Cerebrovasc. Dis. 2022, 31, 106322. [Google Scholar] [CrossRef]
- Hou, W.H.; Ni, C.H.; Li, C.Y.; Tsai, P.S.; Lin, L.F.; Shen, H.N. Stroke rehabilitation and risk of mortality: A population-based cohort study stratified by age and gender. J. Stroke Cerebrovasc. Dis. 2015, 24, 1414–1422. [Google Scholar] [CrossRef] [PubMed]
- Kabboul, N.; Tomlinson, G.; Francis, T.; Grace, S.; Chaves, G.; Rac, V.; Daou-Kabboul, T.; Bielecki, J.M.; Alter, D.A.; Krahn, M. Comparative effectiveness of the core components of cardiac rehabilitation on mortality and morbidity: A systematic review and network meta-analysis. J. Clin. Med. 2018, 7, 514. [Google Scholar] [CrossRef]
- Lahtinen, A.; Leppilahti, J.; Harmainen, S.; Sipila, J.; Antikainen, R.; Seppanen, M.L.; Willig, R.; Vähänikkilä, H.; Ristiniemi, J.; Rissanen, P.; et al. Geriatric and physically oriented rehabilitation improves the ability of independent living and physical rehabilitation reduces mortality: A randomised comparison of 538 patients. Clin. Rehabil. 2014, 29, 892–906. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Tabassum, D.; Baig, S.S.; Moyle, B.; Redgrave, J.; Nichols, S.; McGregor, G.; Evans, K.; Totton, N.; Cooper, C.; et al. Effect of Exercise Interventions on Health-Related Quality of Life After Stroke and Transient Ischemic Attack: A Systematic Review and Meta-Analysis. Stroke 2021, 52, 2445–2455. [Google Scholar] [CrossRef] [PubMed]
- Bruce, J.; Mazuquin, B.; Canaway, A.; Hossain, A.; Williamson, E.; Mistry, P.; Lall, R.; Petrou, S.; E Lamb, S.; Rees, S.; et al. Exercise versus usual care after non-reconstructive breast cancer surgery (UK PROSPER): Multicentre randomised controlled trial and economic evaluation. BMJ 2021, 375, e066542. [Google Scholar] [CrossRef] [PubMed]
- Graven, C.; Brock, K.; Hill, K.D.; Cotton, S.; Joubert, L. First year after stroke an integrated approach focusing on participation goals aiming to reduce depressive symptoms. Stroke 2016, 47, 2820–2827. [Google Scholar] [CrossRef]
- Prabhu, N.V.; Maiya, A.G.; Prabhu, N.S. Impact of cardiac rehabilitation on functional capacity and physical activity after coronary revascularization: A scientific review. Cardiol. Res. Pract. 2020, 2020, 1236968. [Google Scholar] [CrossRef]
- Ru, X.; Dai, H.; Jiang, B.; Li, N.; Zhao, X.; Hong, Z.; He, L.; Wang, W. Community-based rehabilitation to improve stroke survivors’ rehabilitation participation and functional recovery. Am. J. Phys. Med. Rehabil. 2017, 96, e123–e129. [Google Scholar] [CrossRef] [PubMed]
- Cramer, S.C.; Dodakian, L.; Le, V.; See, J.; Augsburger, R.; McKenzie, A.; Zhou, R.J.; Chiu, N.L.; Heckhausen, J.; Cassidy, J.M.; et al. Efficacy of home-based telerehabilitation vs in-clinic therapy for adults after stroke: A randomized clinical trial. JAMA Neurol. 2019, 76, 1079–1087. [Google Scholar] [CrossRef]
- Seijas, V.; Maritz, R.; Fernandes, P.; Bernard, R.M.; Lugo, L.H.; Bickenbach, J.; Sabariego, C. Rehabilitation delivery models to foster healthy ageing-a scoping review. Front. Rehabil. Sci. 2024, 5, 1307536. [Google Scholar] [CrossRef]
- Lannin, N.A.; Crotty, M.; Cameron, I.D.; Chen, Z.; Ratcliffe, J.; Morarty, J.; Turner-Stokes, L. Outcome ABI Group. Cost efficiency of inpatient rehabilitation following acquired brain injury: The first international adaptation of the UK approach. BMJ Open. 2024, 14, e094892. [Google Scholar] [CrossRef]
- Oddy, M.; da Silva Ramos, S. The clinical and cost-benefits of investing in neurobehavioural rehabilitation: A multi-centre study. Brain Inj. 2013, 27, 1500–1507. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shields, G.E.; Wells, A.; Doherty, P.; Heagerty, A.; Buck, D.; Davies, L.M. Cost-effectiveness of cardiac rehabilitation: A systematic review. Heart 2018, 104, 1403–1410. [Google Scholar] [CrossRef] [PubMed]
- Candio, P.; Violato, M.; Luengo-Fernandez, R.; Leal, J. Cost-effectiveness of home-based stroke rehabilitation across Europe: A modelling study. Health Policy 2022, 126, 183–189. [Google Scholar] [CrossRef]
- Sokolov, A.A.; Serino, A. SWISSNEUROREHAB–Technologie-assistiertes Continuum of Care in der Schweizer Neurorehabilitation. Neurol. Rehabil. 2024, 30, S28–S29. [Google Scholar] [CrossRef]
- Mehrholz, J.; Thomas, S.; Kugler, J.; Pohl, M.; Elsner, B. Electromechanical-assisted training for walking after stroke. Cochrane Database Syst. Rev. 2020, 10, CD006185. [Google Scholar] [CrossRef]
- Mehrholz, J.; Pohl, M.; Platz, T.; Kugler, J.; Elsner, B. Electromechanical and robot-assisted arm training for improving activities of daily living, arm function, and arm muscle strength after stroke. Cochrane Database Syst. Rev. 2018, 9, CD006876. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Mustafaoglu, R.; Rossi, S.; Cavdar, F.A.; Agyenkwa, S.K.; Pang, M.Y.C.; Straudi, S. Non-invasive Brain Stimulation Techniques for the Improvement of Upper Limb Motor Function and Performance in Activities of Daily Living After Stroke: A Systematic Review and Network Meta-analysis. Arch. Phys. Med. Rehabil. 2023, 104, 1683–1697. [Google Scholar] [CrossRef] [PubMed]
- Cantone, M.; Lanza, G.; Ranieri, F.; Opie, G.M.; Terranova, C. Editorial: Non-invasive Brain Stimulation in the Study and Modulation of Metaplasticity in Neurological Disorders. Front. Neurol. 2021, 12, 721906. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The International Classification of Functioning, Disability and Health: ICF; WHO: Geneva, Switzerland, 2001. [Google Scholar]
- World Health Organization. How to Use the ICF: A Practical Manual for Using the International Classification of Functioning, Disability and Health (ICF); Exposure Draft for Comment; WHO: Geneva, Switzerland, 2013. [Google Scholar]
- Leonardi, M.; Lee, H.; Kostanjsek, N.; Fornari, A.; Raggi, A.; Martinuzzi, A.; Yáñez, M.; Almborg, A.H.; Fresk, M.; Besstrashnova, Y.; et al. 20 Years of ICF-International Classification of Functioning, Disability and Health: Uses and Applications around the World. Int. J. Environ. Res. Public Health 2022, 19, 11321. [Google Scholar] [CrossRef] [PubMed]
- Lexell, J.; Brogårdh, C. The use of ICF in the neurorehabilitation process. NeuroRehabilitation 2015, 36, 5–9. [Google Scholar] [CrossRef]
- World Health Organization. Brain Health. Available online: https://www.who.int/health-topics/brain-health#tab=tab_1 (accessed on 15 January 2025).
- Bassetti, C.L.A.; Endres, M.; Sander, A.; Crean, M.; Subramaniam, S.; Carvalho, V.; Di Liberto, G.; Franco, O.H.; Pijnenburg, Y.; Leonardi, M.; et al. The European Academy of Neurology Brain Health Strategy: One brain, one life, one approach. Eur. J. Neurol. 2022, 29, 2559–2566. [Google Scholar] [CrossRef]
- Feigin, V.L.; Vos, T.; Nichols, E.; Owolabi, M.O.; Carroll, W.M.; Dichgans, M.; Deuschl, G.; Parmar, P.; Brainin, M.; Murray, C. The global burden of neurological disorders: Translating evidence into policy. Lancet Neurol. 2020, 19, 255–265. [Google Scholar] [CrossRef]
- GBD 2021 Nervous System Disorders Collaborators. Global, regional, and national burden of disorders affecting the nervous system, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Neurol. 2024, 23, 344–381. [Google Scholar] [CrossRef]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- Livingston, G.; Huntley, J.; Liu, K.Y.; Costafreda, S.G.; Selbæk, G.; Alladi, S.; Ames, D.; Banerjee, S.; Burns, A.; Brayne, C.; et al. Dementia prevention, intervention, and care: 2024 report of the Lancet standing Commission. Lancet 2024, 404, 572–628. [Google Scholar] [CrossRef] [PubMed]
- Di Legge, S.; Koch, G.; Diomedi, M.; Stanzione, P.; Sallustio, F. Stroke prevention: Managing modifiable risk factors. Stroke Res. Treat. 2012, 2012, 391538. [Google Scholar] [CrossRef]
- Schäffer, E.; Schara-Schmidt, U.; Schnieder, M. Präventive Neurologie–nie zu früh und nie zu spät. DGNeurologie 2025, 8, 120–128. [Google Scholar]
- Grisold, W.; Dodick, D.W.; Guekht, A.; Lewis, S.L.; Wijeratne, T. World Brain Day 2024: A focus on brain health and prevention. Lancet Neurol. 2024, 23, 863–864. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.Y.; Shu, J.H.; Hsu, H.C.; Liang, Y.; Chang, S.T.; Kao, C.L.; Leu, H.B. The Impact of Rehabilitation Frequencies in the First Year after Stroke on the Risk of Recurrent Stroke and Mortality. J. Stroke Cerebrovasc. Dis. 2017, 26, 2755–2762. [Google Scholar] [CrossRef] [PubMed]
- Yoo, D.Y.; Choi, J.K.; Baek, C.Y.; Shin, J.B. Impact of intensive rehabilitation on long-term prognosis after stroke: A Korean nationwide retrospective cohort study. Medicine 2022, 101, e30827. [Google Scholar] [CrossRef]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.-P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Billinger, S.A.; Arena, R.; Bernhardt, J.; Eng, J.J.; Franklin, B.A.; Johnson, C.M.; MacKay-Lyons, M.; Macko, R.F.; Mead, G.E.; Roth, E.J.; et al. American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Lifestyle and Cardiometabolic Health; Council on Epidemiology and Prevention; Council on Clinical Cardiology. Physical activity and exercise recommendations for stroke survivors: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014, 45, 2532–2553. [Google Scholar] [PubMed]
- Saunders, D.H.; Greig, C.A.; Mead, G.E. Physical activity and exercise after stroke: Review of multiple meaningful benefits. Stroke 2014, 45, 3742–3747. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.; Williams, G.; Sherrington, C.; Pilli, K.; Chagpar, S.; Auchettl, A.; Beard, J.; Gill, R.; Vassallo, G.; Rushworth, N.; et al. The effect of physical activity on health outcomes in people with moderate-to-severe traumatic brain injury: A rapid systematic review with meta-analysis. BMC Public Health 2023, 23, 63. [Google Scholar] [CrossRef] [PubMed]
- Kolanu, N.D.; Ahmed, S.; Kerimkulova, M.K.; Stańczak, M.; Aguirre Vera, G.J.; Shaikh, N.; Addula, A.R.; Cheran, M.; Chilla, S.P.; Oliveira Souza Lima, S.R.; et al. Influence of Nutritional Interventions on Functional Outcomes in Stroke Rehabilitation: A Systematic Review. Cureus 2024, 16, e53711. [Google Scholar] [CrossRef] [PubMed]
- Vorster, A.; Krakow, K.; Bassetti, C.; Zutter, D. Sleep disorders in a rehabilitation setting for neurological patients. J. Sleep Res. 2021, 33, 528. [Google Scholar]
- Gottesman, R.F.; Lutsey, P.L.; Benveniste, H.; Brown, D.L.; Full, K.M.; Lee, J.-M.; Osorio, R.S.; Pase, M.P.; Redeker, N.S.; Redline, S.; et al. American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; and Council on Hypertension. Impact of Sleep Disorders and Disturbed Sleep on Brain Health: A Scientific Statement From the American Heart Association. Stroke 2024, 55, e61–e76. [Google Scholar] [PubMed]
- Lim, D.C.; Najafi, A.; Afifi, L.; LA Bassetti, C.; Buysse, D.J.; Han, F.; Högl, B.; Melaku, Y.A.; Morin, C.M.; I Pack, A.; et al. World Sleep Society Global Sleep Health Taskforce. The need to promote sleep health in public health agendas across the globe. Lancet Public Health 2023, 8, e820–e826. [Google Scholar] [CrossRef] [PubMed]
- Aben, I.; Denollet, J.; Lousberg, R.; Verhey, F.; Wojciechowski, F.; Honig, A. Personality and Vulnerability to Depression in Stroke Patients. Stroke 2002, 33, 2391–2395. [Google Scholar] [CrossRef] [PubMed]
- Turner-Stokes, L.; Hassan, N. Depression after stroke: A review of the evidence base to inform the development of an integrated care pathway. Part 1: Diagnosis, frequency and impact. Clin. Rehabil. 2002, 16, 231–247. [Google Scholar] [CrossRef]
- Krakauer, J.W.; Carmichael, S.T.; Corbett, D.; Wittenberg, G.F. Getting neurorehabilitation right: What can be learned from animal models? Neurorehabil. Neural Repair 2012, 26, 923–931. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Swiss Federation of Clinical Neuro-Societies. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krakow, K.; Rossi, P.; Sokolov, A.A.; Elstner, M.; Müri, R.M.; Zutter, D. Neurorehabilitation as a Cornerstone of the Brain Health Plan. Clin. Transl. Neurosci. 2025, 9, 50. https://doi.org/10.3390/ctn9040050
Krakow K, Rossi P, Sokolov AA, Elstner M, Müri RM, Zutter D. Neurorehabilitation as a Cornerstone of the Brain Health Plan. Clinical and Translational Neuroscience. 2025; 9(4):50. https://doi.org/10.3390/ctn9040050
Chicago/Turabian StyleKrakow, Karsten, Paolo Rossi, Arseny A. Sokolov, Matthias Elstner, Rene M. Müri, and Daniel Zutter. 2025. "Neurorehabilitation as a Cornerstone of the Brain Health Plan" Clinical and Translational Neuroscience 9, no. 4: 50. https://doi.org/10.3390/ctn9040050
APA StyleKrakow, K., Rossi, P., Sokolov, A. A., Elstner, M., Müri, R. M., & Zutter, D. (2025). Neurorehabilitation as a Cornerstone of the Brain Health Plan. Clinical and Translational Neuroscience, 9(4), 50. https://doi.org/10.3390/ctn9040050





