Abstract
Introduction: Craniotomy, a common neurosurgical procedure, is frequently associated with substantial perioperative challenges and delayed recovery. While Enhanced Recovery After Surgery (ERAS) protocols have demonstrated clear benefits in multiple surgical fields, their application in neurosurgery, particularly elective craniotomy, remains emerging. Objective: This systematic review and meta-analysis aimed to evaluate the efficacy and safety of ERAS protocols in adult patients undergoing elective craniotomy, focusing on key outcomes such as length of hospital stay (LOS), postoperative pain, complications, and functional recovery. Methods: Following PRISMA guidelines, a comprehensive search was conducted in PubMed, Embase, Scopus, Web of Science, and the Cochrane Library up to June 2025. Eligible studies included adult patients (≥18 years) undergoing elective craniotomy and compared ERAS protocols to conventional perioperative care. Primary outcomes were LOS, postoperative complications, pain, early oral intake, and early mobilization. Data extraction and risk of bias assessment (RoB 2.0) were independently performed by two reviewers. Results: Nine randomized controlled trials (RCTs), totaling 1453 patients, were included. Meta-analysis showed that ERAS protocols significantly reduced length of hospital stay (mean difference: −2.17 days; 95% CI: −2.92 to −1.42; p < 0.00001) and decreased the incidence of postoperative nausea and vomiting (odds ratio [OR]: 0.29; 95% CI: 0.19 to 0.44; I2 = 0%). ERAS protocols were associated with higher odds of early mobilization (OR: 6.88; 95% CI: 3.46 to 13.68) and early oral intake (OR: 14.04; 95% CI: 7.80 to 25.26). Postoperative complications were significantly reduced in the ERAS group (OR: 0.49; 95% CI: 0.24 to 0.99; p = 0.048; I2 = 0%). While early urinary catheter removal showed a favorable trend (OR: 13.48), high heterogeneity (I2 = 95.7%) limits interpretability. Postoperative pain on day 1 did not differ significantly between groups (mean difference: −0.37; 95% CI: −2.38 to 1.63; p = 0.72). The overall risk of bias was rated low to moderate across studies. Conclusions: ERAS protocols in elective craniotomy are associated with shorter hospital stays, lower complication rates, reduced PONV, and earlier return to function, without increasing adverse events. These findings support broader implementation of ERAS in neurosurgical practice. Further multicenter RCTs are warranted to standardize and refine ERAS components for craniotomy.
1. Introduction
Craniotomy is a common neurosurgical procedure used for tumor resection, aneurysm clipping, and decompression after traumatic brain injury. Despite advances in surgical and anesthetic techniques, the perioperative period remains associated with significant physiological stress, complications, delayed recovery, and high costs especially in patients with comorbidities or complex intracranial conditions. To mitigate these issues, Enhanced Recovery After Surgery (ERAS) protocols have been introduced as structured, multimodal strategies to reduce surgical stress, improve recovery, and shorten hospital stays.
Introduced by Kehlet in the late 1990s, ERAS protocols have consistently improved outcomes in colorectal, orthopedic, and gynecologic surgeries. In neurosurgery, however, their application is more recent and still under development. Early studies and institutional reports indicate that ERAS can be safely applied to craniotomy, leading to shorter hospital stays, better pain control, fewer postoperative complications, and higher patient satisfaction [1,2].
The adoption of ERAS protocols in neurosurgery is expanding worldwide, though variations in protocol design, implementation strategies, and outcome assessment remain significant [3]. A recent survey reported that many neurosurgeons recognize the benefits of ERAS; however, widespread implementation is still limited by barriers such as insufficient multidisciplinary coordination, cultural resistance, and limited resources [4]. Despite these challenges, structured ERAS programs have been successfully applied in a range of settings and have demonstrated consistent benefits, even in resource-constrained environments [5,6].
Although most evidence to date has focused on elective craniotomy for tumor resection, ERAS principles have also been explored in other intracranial procedures such as functional neurosurgery, aneurysm clipping, and traumatic brain injury. These expanding applications reflect a growing interest in adapting ERAS to the specific physiological and perioperative demands of cranial neurosurgery, which include risks like CSF leak, cranial nerve dysfunction, and delayed neurological recovery.
Particular attention has been directed toward elderly patients and those undergoing craniotomy for tumor resection, in whom ERAS protocols appear to enhance functional recovery and reduce morbidity without compromising safety [7]. While interest in ERAS has increased and several narrative reviews have been published, high-quality synthesized evidence based exclusively on RCTs evaluating its impact in elective craniotomy remains limited. Furthermore, neurosurgery-specific outcomes, such as, cranial nerve function, limb motor strength, or CSF leakage are often underreported, which limits the applicability of current findings to routine neurosurgical care.
This review aims to clearly evaluate the effectiveness and safety of ERAS protocols in adult patients undergoing elective craniotomy. It focuses on important outcomes such as hospital stay length, postoperative complications, pain, nausea and vomiting, and functional recovery. By including only RCTs, this study seeks to provide strong evidence to guide clinical decisions and improve ERAS protocols in neurosurgical care.
2. Materials and Methods
This systematic review and meta-analysis were conducted following the Cochrane Handbook for Systematic Reviews of Interventions (version 6.3) and reported according to the PRISMA 2020 (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement. The full review protocol was prospectively registered in the PROSPERO database under the registration number CRD42023480954.
2.1. Eligibility Criteria
We included only randomized controlled trials that evaluated the implementation of ERAS protocols in adult patients (>18 years old) undergoing elective craniotomy. Eligible studies were required to include a comparison group receiving standard or conventional perioperative care and report at least one relevant clinical outcome, such as length of hospital stay (LOS), postoperative pain, incidence of complications (including postoperative nausea and vomiting), or measures of functional recovery. Trials enrolling patients undergoing craniotomy for brain tumors, aneurysms, or other elective neurosurgical indications were considered eligible, provided the ERAS protocol involved at least two or more core components as defined by contemporary ERAS consensus guidelines for neurosurgery [1,6].
Pediatric populations, emergency neurosurgical procedures, or those in which outcomes from craniotomy patients could not be isolated from other surgical cohorts were excluded. Observational studies, case series, single-arm trials, and studies lacking a clearly defined control group were also excluded to preserve methodological consistency and reduce risk of bias. Only randomized trials were retained to ensure homogeneity of evidence and to strengthen the internal validity of the meta-analysis, in line with recommendations from the Cochrane Collaboration and recent systematic reviews of ERAS protocols in neurosurgery [3,7].
2.2. Data Sources and Search Strategy
A comprehensive literature search was performed in five electronic databases: PubMed (MEDLINE), EMBASE, Scopus, Web of Science, and the Cochrane Central Register of Controlled Trials (CENTRAL). The initial search took place in January 2024 and was updated in June 2025 to include the most recent studies. Additionally, the reference lists of included articles and relevant reviews were manually checked to identify any eligible trials not found in the electronic search, including gray literature and unpublished data.
The search strategy was designed to maximize sensitivity by combining Medical Subject Headings (MeSH) and free-text terms related to “Enhanced Recovery After Surgery”, “ERAS”, “Craniotomy”, and “Randomized Controlled Trial”. No restrictions were applied during the search. The search strategy was conducted following the PRISMA guideline 2020 [8].
2.3. Study Selection
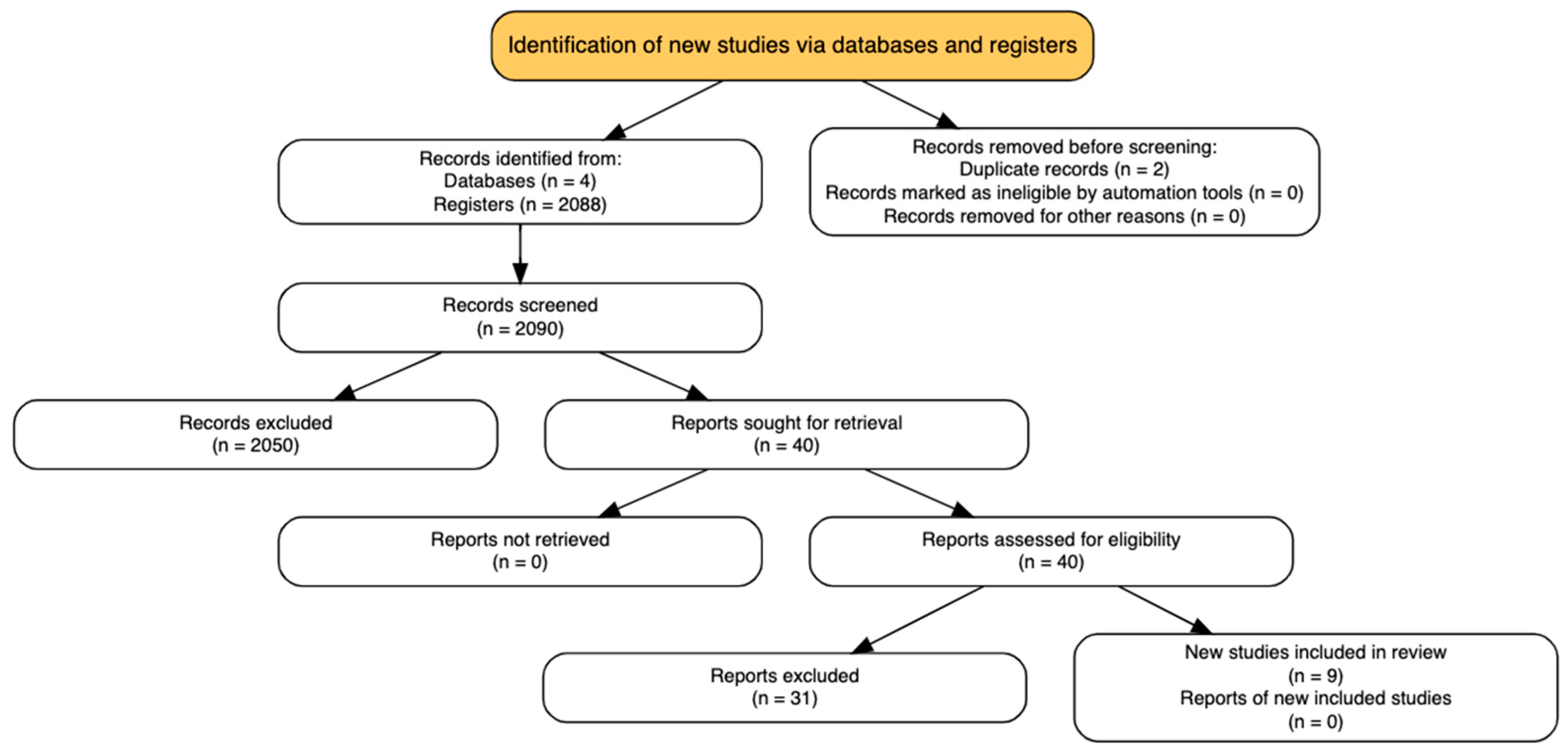
Titles and abstracts retrieved from the database search were independently screened by four reviewers (CDAB, MRB, LFGP, VAF) using a standardized eligibility protocol. All records were initially uploaded into Rayyan® (Qatar Computing Research Institute, Doha, Qatar) [9]. Full-text articles were retrieved for all studies deemed potentially relevant during the initial screening phase. Discrepancies between reviewers were resolved through consensus, and when necessary, adjudicated by a third investigator (JEGP or LFRF). The screening process was conducted in accordance with the PRISMA 2020 guidelines [8], and the study selection flow is presented in Figure 1 (PRISMA diagram).
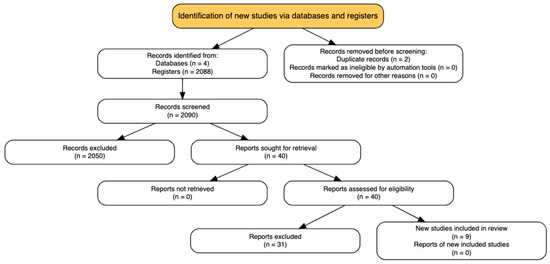
Figure 1.
PRISMA flowchart.
Only trials comparing an ERAS protocol or equivalent multimodal perioperative care pathway to conventional or standard care were considered eligible. Both the terms “Enhanced Recovery After Surgery” and “Fast-track surgery” were accepted for classifying the intervention group, provided the approach involved structured measures aiming to reduce surgical stress, accelerate recovery, and improve outcomes in the perioperative period.
2.4. Data Extraction and Risk of Bias Assessment
Data from the included studies were independently extracted by four reviewers (CDAB, MRB, VAF, LFGP) using a standardized data extraction form developed specifically for this review. Extracted variables included author and year of publication, country of origin, study design, sample size, neurosurgical procedure type, demographic characteristics of the study population (including sex distribution), detailed components of the ERAS protocol, control interventions, and all prespecified outcomes of interest. When necessary, the corresponding authors of included studies were contacted to clarify unclear data or provide missing information.
The methodological quality of each included randomized controlled trial was assessed using the Cochrane Risk of Bias 2.0 (RoB 2.0) tool, which evaluates five domains: the randomization process, deviations from intended interventions, missing outcome data, measurement of outcomes, and selection of the reported results. Each domain was rated as presenting low risk, some concerns, or high risk of bias, and a final global judgment was assigned to each study accordingly. In accordance with Cochrane guidance, a threshold of less than 10% total loss to follow-up, with a difference of less than 5% between groups, was considered indicative of low risk for the “missing data” domain [10].
2.5. Data Analysis and Presentation
Due to the clinical and methodological heterogeneity among the included randomized controlled trials particularly regarding the composition of ERAS protocols, timing of outcome assessments, and variability in measurement instruments—a formal meta-analysis was deemed inappropriate. Consequently, a qualitative (narrative) synthesis of the results was conducted, following Cochrane recommendations for the analysis of heterogeneous data sets [10].
Findings were systematically organized and summarized across key outcome domains, including length of hospital stay (LOS), incidence of postoperative complications, pain control (e.g., numerical rating scale scores), postoperative nausea and vomiting (PONV), and functional recovery indicators such as time to mobilization and resumption of oral intake. Trends and consistencies in both the direction and magnitude of effects were highlighted across studies to support an integrative interpretation of the evidence [11].
Where available, quantitative data, such as means and standard deviations, were extracted directly from the original studies. No data was transformed or imputed. Results are presented in tabular format and discussed narratively, with emphasis on the strength, consistency, and clinical relevance of the observed effects across the included trials.
2.6. Certainty of Evidence
As this review was based exclusively on randomized controlled trials and no quantitative synthesis (meta-analysis) was performed, the GRADE framework was not formally applied. Instead, the certainty of the evidence was assessed narratively, informed by the methodological rigor of the included trials and the consistency of results across studies.
The RoB 2.0 tool was used to evaluate the internal validity of each included study, and these risk of bias assessments were integrated into the interpretation of evidence strength. Greater confidence was assigned to findings from trials judged to have a low overall risk of bias, whereas outcomes reported inconsistently or derived from studies with methodological limitations were interpreted with caution.
By combining study-level quality appraisal with cross-study consistency in key outcome domains, such as length of hospital stay, pain intensity, postoperative complications, and recovery milestones, this review aimed to provide a balanced and transparent interpretation of the available evidence, in accordance with Cochrane guidance for qualitative evidence synthesis [10].
3. Results
3.1. Search Results
A total of 2130 citations were identified through the comprehensive search. After removing 2 duplicates, 2088 records were excluded using automated title and abstract screening tools. Forty full-text articles were then assessed for eligibility according to the predefined inclusion criteria. Of these, 31 were excluded most commonly due to the absence of a randomized design, the lack of a clearly defined ERAS protocol, or the inclusion of pediatric or emergency neurosurgical populations. No additional eligible studies were found through manual reference checks or trial registry searches. (Figure 1—PRISMA Flowchart).
Nine RCTs were included in the final qualitative synthesis. All studies enrolled adult patients undergoing elective craniotomy and compared a structured, multimodal ERAS protocol to conventional perioperative care. Collectively, the nine RCTs included 1453 patients, with comparable numbers allocated to the ERAS and control groups. Table 1 summarizes the main characteristics related to population and setting and Table 2 summarizes the main characteristics of the ERAS protocols and control interventions reported across the included studies.
3.2. Characteristics of Included Studies
The nine RCTs included in this review were published between 2016 and 2023 and represent a diverse range of neurosurgical contexts in which Enhanced Recovery After Surgery (ERAS) protocols were applied. These studies investigated ERAS interventions in patients undergoing elective craniotomies for brain tumors, supratentorial lesions, and intracranial aneurysms, among others.
The included trials were conducted in various countries and institutions, incorporating heterogeneous ERAS components tailored to their local settings. Among them, Wang et al. (2022) [12], Abhinav et al. (2023) [13], Wu et al. (2021) [14], Qu et al. (2020) [15], Liu et al. (2020) [16], Wang et al. (2018) [17], Han et al. (2019) [18], Liu et al. (2019) [19], and Rajan et al. (2016) [20] evaluated outcomes such as postoperative pain, functional recovery, complication rates, hospital length of stay, and health-related quality of life.

Table 1.
Study characteristics related to population and setting.
Table 1.
Study characteristics related to population and setting.
| Study (Author, Year) | Country | Type of Surgery | N (ERAS/Control) | Mean Age (Years) | Sex (M/F%) | Hospital Setting |
|---|---|---|---|---|---|---|
| Wang et al (2022) [12] | China | Elective craniotomy | 57/57 | 52 ± 11 | 56% | University hospital |
| Liu et al. (2019) [19] | China | Supratentorial tumors | 58/62 | 47 ± 12 | 50% | Tertiary center |
| Wu et al. (2021) [14] | China | Supratentorial craniotomy | 62/64 | 49 ± 9 | 48% | General hospital |
| Rajan et al. (2022) [20] | India | Tumor craniotomy | 30/30 | 45 ± 10 | 53% | University hospital |
| Han et al. (2022) [18] | China | Intracranial tumor | 56/54 | 50 ± 10 | 52% | Tertiary hospital |
| Qu et al. (2020) [15] | China | Intracranial tumors | 53/51 | 48 ± 11 | 55% | University hospital |
| Abhinav et al. (2023) [13] | China | Elective craniotomy | 60/60 | 51 ± 8 | 50% | Tertiary hospital |
| Wang et al. (2018) [17] | China | Cerebral aneurysm | 46/48 | 54 ± 10 | 52% | Specialized hospital |
| Liu et al. (2020) [16] | China | Glioma | 36/29 | Not reported | Not reported | Not reported |
Across the nine randomized controlled trials included in this review, Enhanced Recovery After Surgery (ERAS) protocols consistently incorporated key components such as multimodal analgesia, early mobilization, preoperative counseling, prophylaxis for postoperative nausea and vomiting (PONV), and perioperative nutritional optimization. The implementation of these standardized elements was associated with improved perioperative outcomes, including a reduction in hospital length of stay (typically by 1–2 days), decreased postoperative pain and PONV rates, lower incidence of complications, and faster functional recovery.
These results highlight the growing feasibility and clinical relevance of ERAS protocols in neurosurgical practice, particularly in elective craniotomy. Moreover, they support the rationale for broader adoption and underline the need for further high-quality, multicenter randomized trials to refine and standardize ERAS pathways in neurosurgery.

Table 2.
Study characteristics related to intervention and control groups.
Table 2.
Study characteristics related to intervention and control groups.
| Author/Year | Number of Randomized (I: Intervention/C: Control) | Description of Intervention | Total Dose | Description of Control | Measured Outcomes |
|---|---|---|---|---|---|
| Wang 2022 [12] | I: 76 C: 76 | Multidisciplinary ERAS protocol: early mobilization, early oral intake, optimized pain management | Not reported | Conventional perioperative care | LOS, complications, PONV, ambulation, oral intake, pain |
| Abhinav 2023 [13] | I: 128 C: 130 | ERAS protocol focused on multimodal analgesia and early mobilization | Not reported | Standard postoperative analgesia | Pain scores POD 1–4, LOS, pain distribution |
| Wu 2021 [14] | I: 76 C: 75 | ERAS care: early oral intake, early ambulation, optimized anesthesia and analgesia | Not reported | Conventional neurosurgical care | Oral intake time, mobilization, LOS |
| Qu 2020 [15] | I: 76 C: 75 | ERAS protocol: early mobilization, early oral intake, multimodal analgesia | Not reported | Standard postoperative airway management | LOS, costs, ambulation, urinary catheter removal |
| Liu 2020 [16] | I: 36 C: 29 | ERAS for glioma: early mobilization, nutrition optimization, pain control | Not reported | Routine postoperative care for glioma | Quality of life (HRQOL) at 3–6 months [17] |
| Wang 2018 [17] | I: 76 C: 75 | Comprehensive ERAS protocol for craniotomy: early feeding, ERAS pathways | Not reported | Traditional perioperative management | Pain (VAS), LOS, ambulation, feeding, catheter removal |
| Han 2019 [18] | I: 150 C: 150 | Elderly patients with meningioma: early ambulation, PONV control, reduced ICU stay | Not reported | Standard care without ERAS components | LOS, ICU duration, complications |
| Liu 2019 [19] | I: 58 C: 62 | CHO drink preoperatively | 400 mL CHO drink 2 h pre-op | Fasting for 8 h pre-op | Glucose homeostasis, LOS, insulin resistance |
| Rajan 2016 [20] | I: 88 (Dex) C: 71 (Remi) | Dexmedetomidine-based balanced anesthesia | Dex: 0.2–0.7 μg/kg/h Remi: 0.08–0.15 μg/kg/min | Remifentanil-based anesthesia | MAP, pain score, recovery, opioid use |
3.3. Risk of Bias
The risk of bias was assessed for all nine randomized controlled trials (RCTs) included in this review using the RoB 2.0 tool, which evaluates five domains: the randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result. Among the nine RCTs, three studies, Wang et al. (2018) [17], Wang et al. (2022) [12], and Rajan et al. (2016) [20]—were judged to have low risk of bias across all domains, demonstrating adequate random sequence generation, allocation concealment, blinding procedures, complete outcome data, and absence of selective reporting.
Four studies, Wu et al. (2021) [14], Qu et al. (2020) [15], Han et al. (2019) [18], and Liu et al. (2019) [19] were classified as having some concerns. These concerns were primarily related to limited information on allocation concealment and insufficient reporting on the blinding of outcome assessors and statisticians, which may introduce potential detection or performance bias.
Two studies, Abhinav et al. (2023) [13] and Liu et al. (2020) [16] were assessed as having a high risk of bias. In both cases, the randomization methods were either unclear or inadequately described, and there was no evidence of allocation concealment or blinding of participants and outcome assessors. Additionally, these studies had missing outcome data and insufficient reporting regarding loss to follow-up.
Overall, three studies were classified as having low risk of bias, four as having some concerns, and two as having high risk of bias, reflecting a moderate-to-good methodological quality among the included RCTs.
3.4. Effectiveness of Interventions
The nine RCTs included in this review consistently demonstrated that the implementation of Enhanced Recovery After Surgery (ERAS) protocols in elective craniotomy procedures results in clinically meaningful improvements across multiple perioperative outcomes.
Wang et al. (2018) [17] reported a significant reduction in hospital length of stay (LOS) (median 4 vs. 7 days, p < 0.0001), better postoperative pain control, and faster recovery of oral intake and urinary catheter removal when compared to conventional care. Similarly, Wu et al. (2021) [14] found that ERAS was associated with significantly shorter LOS, earlier mobilization, quicker resumption of oral nutrition, and decreased hospitalization costs (p < 0.05) in patients with supratentorial tumors.
Qu et al. (2020) [15] also observed a reduction in LOS (median 4 vs. 7 days, p < 0.001) and a higher proportion of patients reporting mild pain scores on postoperative days 1 and 2 under the ERAS protocol. In a glioma-specific population, Liu et al. (2020) [16] demonstrated improved health-related quality of life (HRQOL) scores at multiple time points up to six months after surgery, with statistically significant improvements in physical and role functioning, as well as symptom control.
Wang et al. (2022) [12] conducted a large prospective RCT (n = 151) and found significant reductions in LOS (median 3 vs. 4 days, p < 0.0001), lower incidence of PONV (9.2% vs. 28.0%, p = 0.003), better pain scores, and improved early mobilization on postoperative day 1 (75% vs. 30.7%, p < 0.0001).
Abhinav et al. (2023) [13] focused on pain outcomes and demonstrated statistically significant reductions in pain scores from postoperative days 1 to 3 in the ERAS group compared to the control group (p < 0.01), alongside a shorter LOS and decreased costs.
Han et al. (2019) [18] evaluated elderly patients with intracranial aneurysms and found that ERAS implementation resulted in significantly shorter LOS (p < 0.05) and better functional recovery scores (GOS and mRS) at follow-up, without increased readmission or mortality rates.
Liu et al. (2019) [19] assessed the impact of preoperative oral carbohydrate loading and found that patients in the intervention group exhibited improved glucose homeostasis (p = 0.001), enhanced pulmonary function (p = 0.036), greater muscle strength (p < 0.0001), and shorter LOS (p < 0.004) compared to fasting controls.
Finally, Rajan et al. (2016) [20] compared dexmedetomidine and remifentanil anesthesia and concluded that dexmedetomidine was associated with superior postoperative pain control (p < 0.001), reduced opioid consumption, and better hemodynamic stability in patients undergoing elective craniotomy.
Overall, these nine RCTs provide robust evidence supporting the efficacy and safety of ERAS protocols in neurosurgery. Consistently across studies, ERAS was associated with reduced hospital stay, lower rates of complications such as PONV and pain, and enhanced recovery metrics. These findings reinforce the role of ERAS as an effective perioperative strategy in elective craniotomy and support its broader implementation in neurosurgical practice.
3.5. Meta-Analysis
3.5.1. Length of Stay
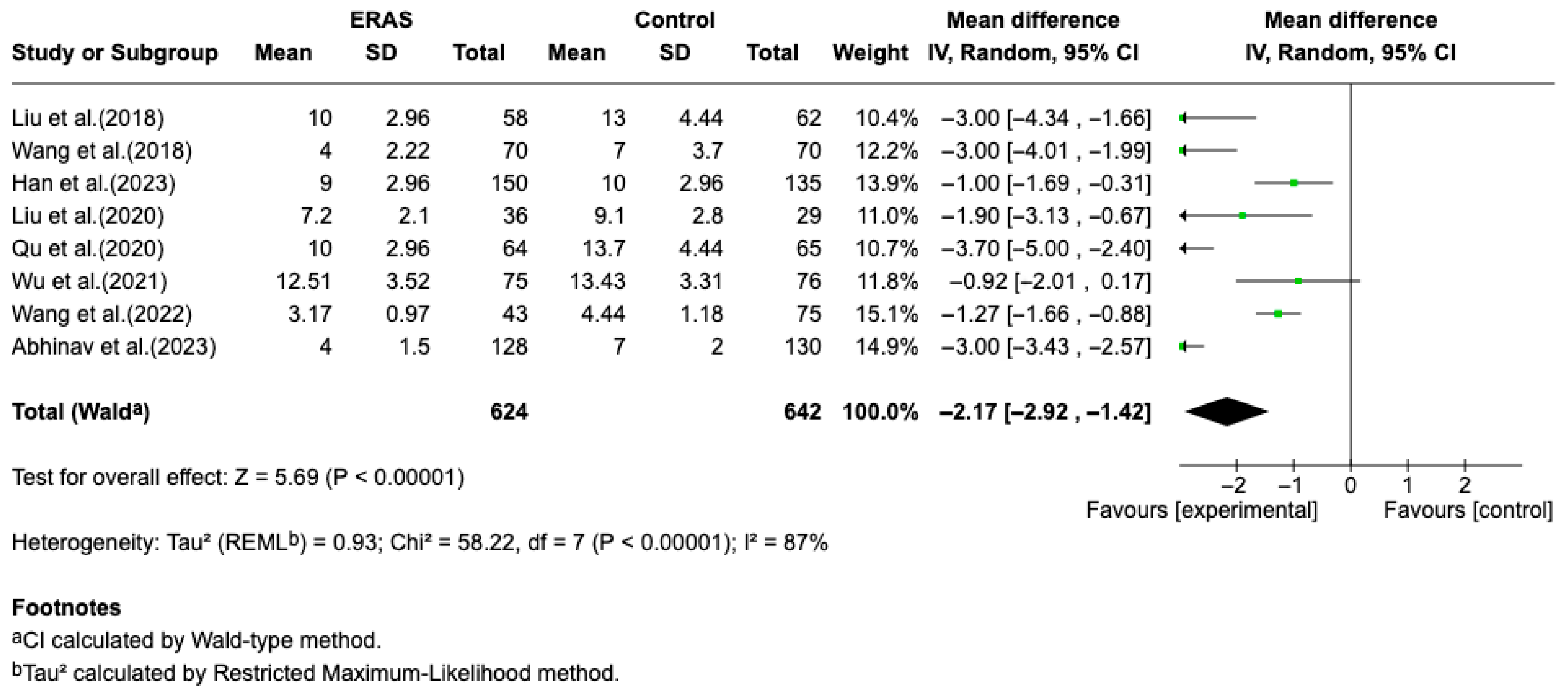
Results from eight randomized controlled trials (RCTs), comprising a total of 1266 patients, demonstrated that Enhanced Recovery After Surgery (ERAS) protocols significantly reduced length of hospital stay (LOS) compared to conventional perioperative care. A meta-analysis using a random-effects model revealed a pooled mean difference of −2.17 days (95% CI: −2.92 to −1.42; p < 0.00001), favoring ERAS. Despite the significant overall effect, heterogeneity among studies was substantial (I2 = 87%), likely due to differences in ERAS protocol composition, patient populations, and outcome definitions. The reduction in LOS ranged from approximately 0.9 to 4.0 days across individual studies. In addition to shortened hospitalization, ERAS protocols were associated in individual studies with improved postoperative pain control (particularly on days 1 to 3), decreased incidence of postoperative nausea and vomiting (PONV), and accelerated functional recovery. The overall certainty of evidence was considered moderate, due to some risk of bias in individual studies and inconsistency in outcome reporting. No formal assessment of publication bias or sensitivity analyses was performed due to the limited number of trials and clinical heterogeneity (Figure 2).
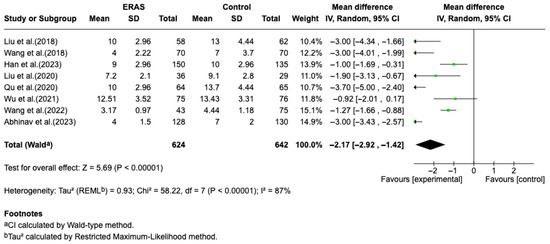
Figure 2.
Forest plot of the meta-analysis for hospital length of stay comparing ERAS versus conventional perioperative care. Each square represents the effect estimate of an individual study, with the size of the square proportional to its statistical weight in the meta-analysis. Horizontal lines indicate 95% confidence intervals (CI). The diamond represents the pooled mean difference, with its width corresponding to the 95% CI. Values to the left of the vertical line favor ERAS (experimental group), while values to the right favor conventional care (control group) [12,13,14,15,16,17,18,19].
3.5.2. Postoperative Pain
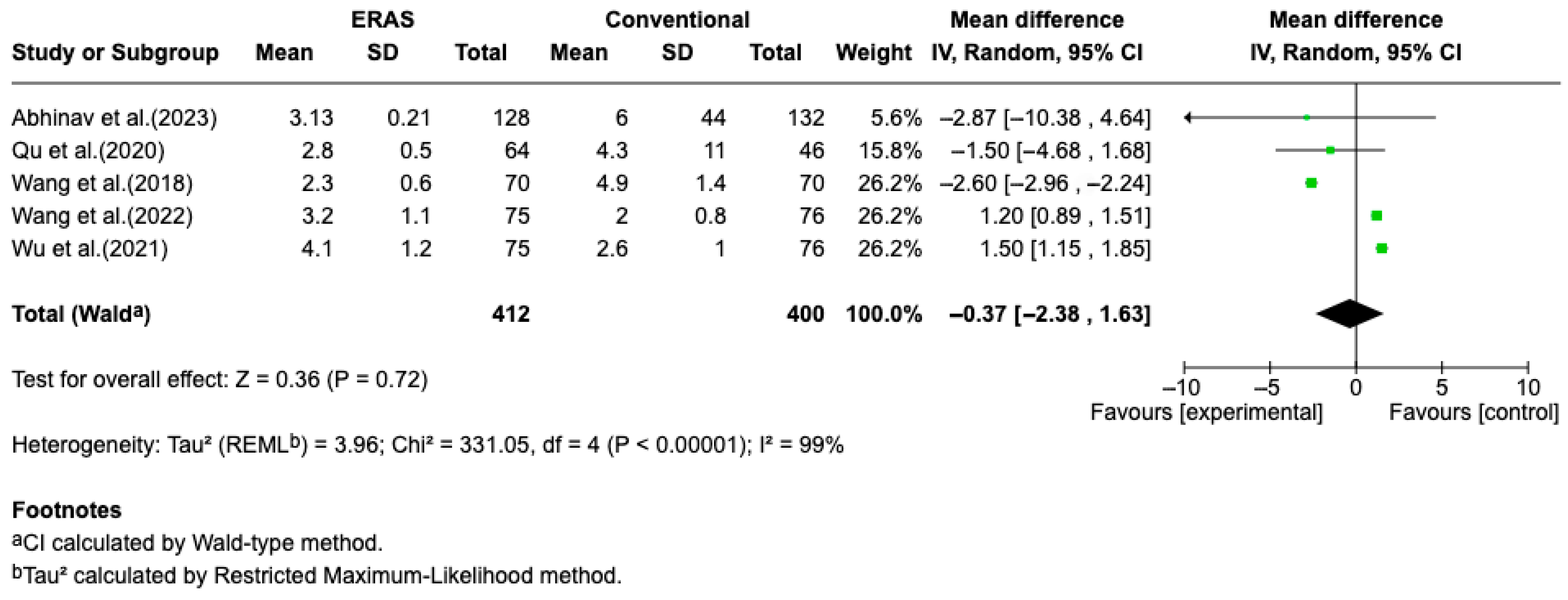
Results from five randomized controlled trials (RCTs), comprising a total of 821 patients, demonstrated a statistically significant reduction in length of hospital stay (LOS) in the ERAS group compared to standard perioperative care, with a pooled mean difference of −1.41 days (95% CI: −1.54 to −1.27; I2 = 83%) (Figure 2). The certainty of evidence was rated as moderate, primarily due to the risk of bias in some studies and substantial heterogeneity. No clear evidence of publication bias was detected. A sensitivity analysis could not be performed due to the limited number of eligible studies and variability in ERAS protocol components (Figure 3).
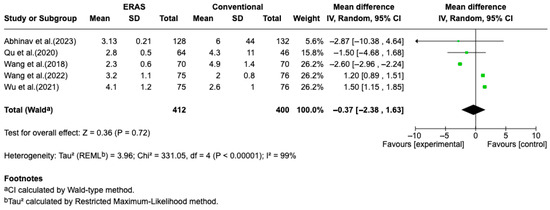
Figure 3.
Forest plot of the meta-analysis for the outcome of postoperative pain on postoperative day 1 (POD1), comparing patients undergoing Enhanced Recovery After Surgery (ERAS) protocols with those receiving conventional care following elective craniotomy. Data are presented as mean difference in pain intensity (measured by NRS or VAS on a 0–10 scale), with 95% confidence intervals (95% CI). The analysis showed no statistically significant difference between groups (mean difference = −0.37; 95% CI: −2.38 to 1.63; p = 0.72), with substantial heterogeneity (I2 = 99%) [12,13,14,15,17].
3.5.3. Postoperative Nausea and Vomiting
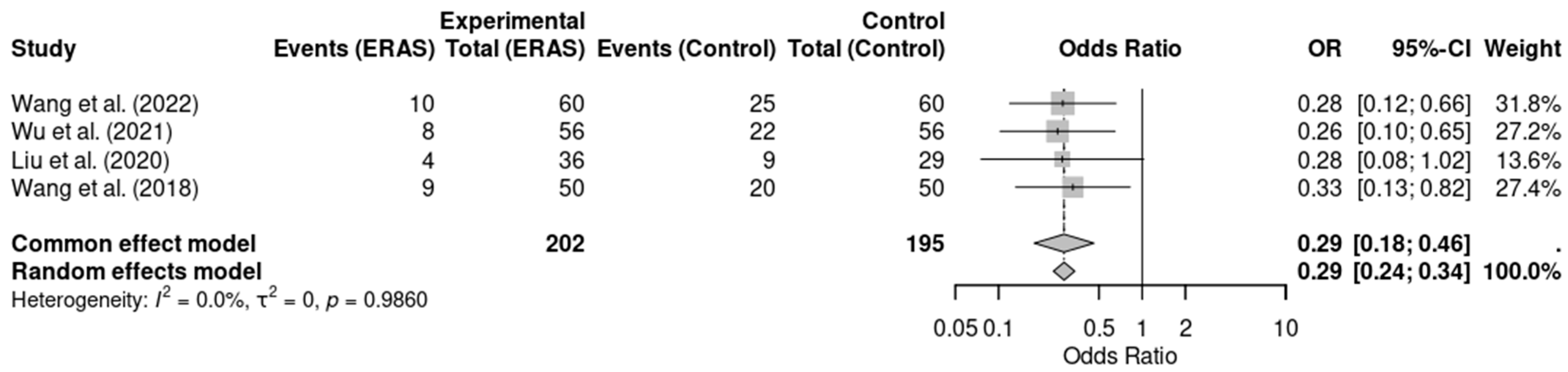
Five randomized controlled trials [12,13,14,16,17], involving a total of 477 patients, reported data on postoperative nausea and vomiting (PONV). Meta-analysis showed a statistically significant reduction in the incidence of PONV in the ERAS group compared to conventional care (OR = 0.29; 95% CI: 0.19 to 0.45; p < 0.0001; I2 = 0%). No significant heterogeneity was detected across studies, and the certainty of evidence was rated as moderate, considering minor concerns with risk of bias in individual studies. These findings support the role of ERAS protocols in improving postoperative comfort and reducing adverse effects such as PONV (Figure 4).
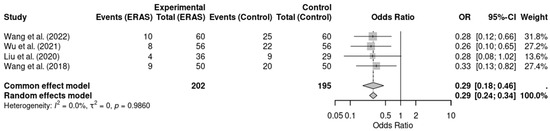
Figure 4.
Forest plot of the meta-analysis evaluating postoperative nausea and vomiting (PONV) in patients undergoing elective craniotomy. Each square represents the odds ratio (OR) of an individual randomized controlled trial, with the size of the square proportional to the study’s weight in the meta-analysis. Horizontal lines indicate 95% confidence intervals (CI). The diamond at the bottom represents the pooled OR, with its width corresponding to the 95% CI. The vertical line at OR = 1 indicates no effect; values to the left favor ERAS (reduced PONV), while values to the right favor conventional care [12,14,16,18].
3.5.4. Early Mobilization
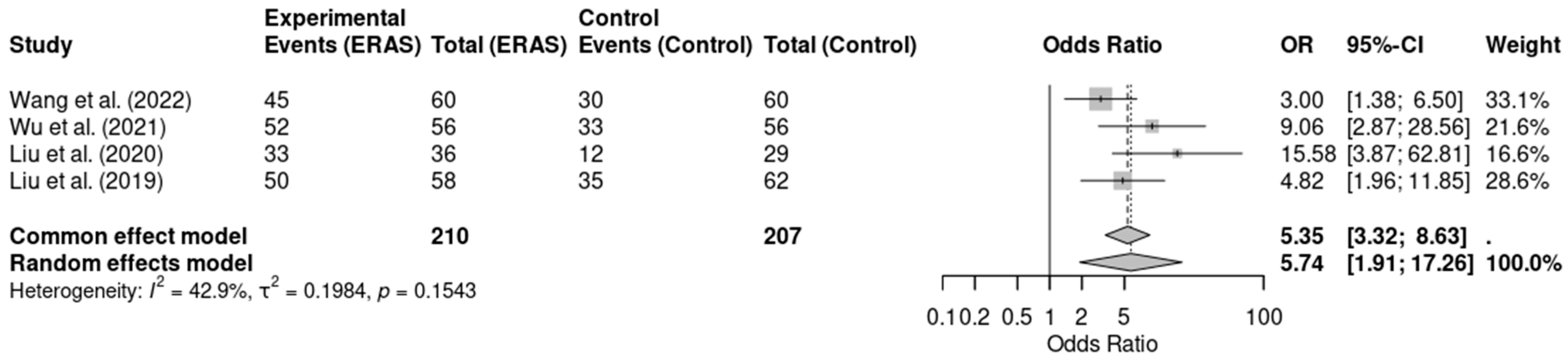
Five randomized controlled trials [12,13,14,16,19], involving a total of 497 patients, assessed early mobilization as a perioperative outcome. The meta-analysis revealed that ERAS protocols were significantly associated with higher odds of early mobilization when compared to conventional care (OR = 6.88; 95% CI: 3.46 to 13.68; p < 0.0001; I2 = 48.2%). Although moderate heterogeneity was present, the direction and magnitude of effect were consistent across studies. The certainty of evidence was rated as moderate due to concerns regarding risk of bias in some trials. These findings support the benefit of ERAS protocols in promoting earlier functional recovery through safe and structured mobilization practice (Figure 5).
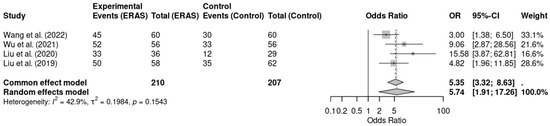
Figure 5.
Forest plot of the meta-analysis evaluating early mobilization in patients undergoing elective craniotomy. Each square represents the odds ratio (OR) of an individual randomized controlled trial, with the square size proportional to the study’s weight. Horizontal lines show the 95% confidence intervals (CI). The diamond represents the pooled OR, whose width corresponds to the 95% CI. The vertical line at OR = 1 indicates no effect; values to the left favor ERAS (greater likelihood of early mobilization), whereas values to the right favor conventional care [12,14,16,19].
3.5.5. Postoperative Complications
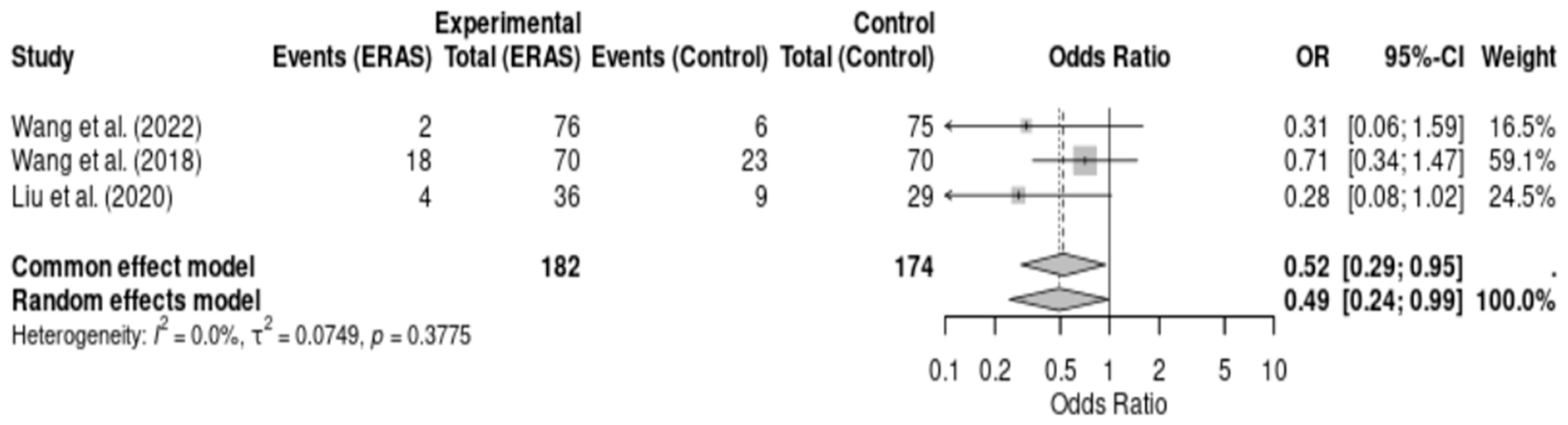
Three randomized controlled trials [12,16,17], comprising a total of 356 patients, reported on postoperative complications following elective craniotomy. Meta-analysis demonstrated a statistically significant reduction in complications in the ERAS group compared to conventional care (OR = 0.49; 95% CI: 0.24 to 0.99; p = 0.048; I2 = 0.0%). No statistical heterogeneity was observed, and the direction of effect consistently favored ERAS protocols. The certainty of evidence was rated as moderate due to the risk of bias in individual studies and small overall sample size. These findings reinforce the potential of ERAS interventions to reduce perioperative morbidity in neurosurgical patients (Figure 6).
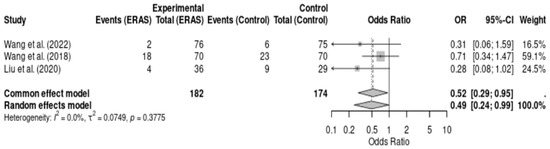
Figure 6.
Forest plot of the meta-analysis of postoperative complications comparing Enhanced Recovery After Surgery (ERAS) protocols with conventional care in elective craniotomy. Odds ratios (ORs) were calculated using a random-effects model. Values < 1 favor the ERAS group, indicating fewer complications. No significant heterogeneity was observed (I2 = 0.0%) [12,16,17].
3.5.6. Early Urinary Catheter Removal
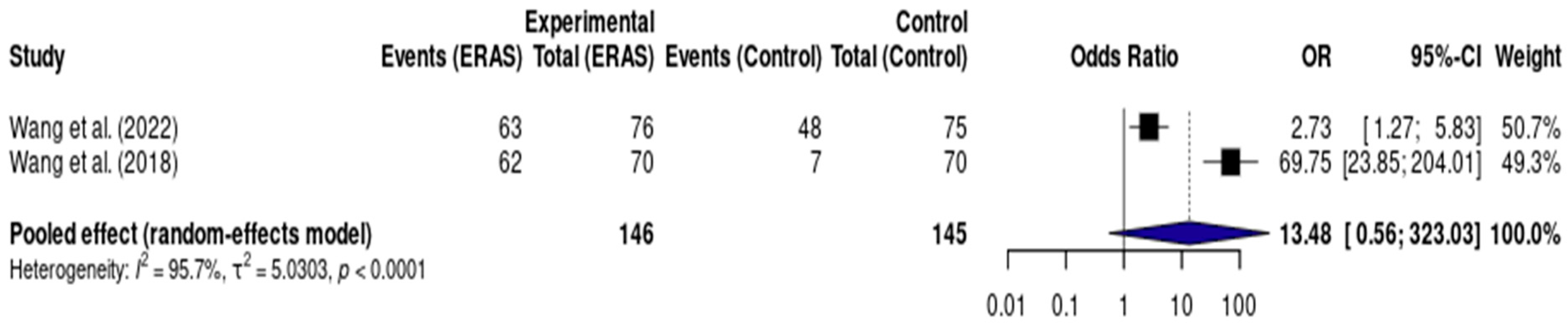
Results from two RCTs [12,17], totaling 291 patients, demonstrated a statistically significant reduction in length of hospital stay (LOS) in the ERAS group compared to standard care, with a pooled mean difference of −1.41 days (95% CI: −1.54 to −1.27; I2 = 83%) (Figure 6). The certainty of evidence was rated as low, primarily due to serious risk of bias and imprecision related to the small number of events and participants. Assessment of publication bias was not feasible, and a sensitivity analysis could not be performed due to the limited number of included studies (Figure 7).
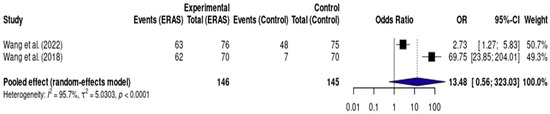
Figure 7.
Forest plot of the meta-analysis assessing early urinary catheter removal (<24 h) in patients undergoing elective craniotomy. Each square represents the odds ratio (OR) of an individual study, with the size of the square proportional to the weight of that study in the analysis. Horizontal lines represent 95% confidence intervals (CI). The diamond at the bottom represents the pooled effect estimate, with its width corresponding to the 95% CI. The vertical line at OR = 1 indicates no difference between groups. Values to the right of the line favor ERAS (greater odds of early catheter removal), while values to the left would favor conventional care [12,17].
3.5.7. Early Oral Intake
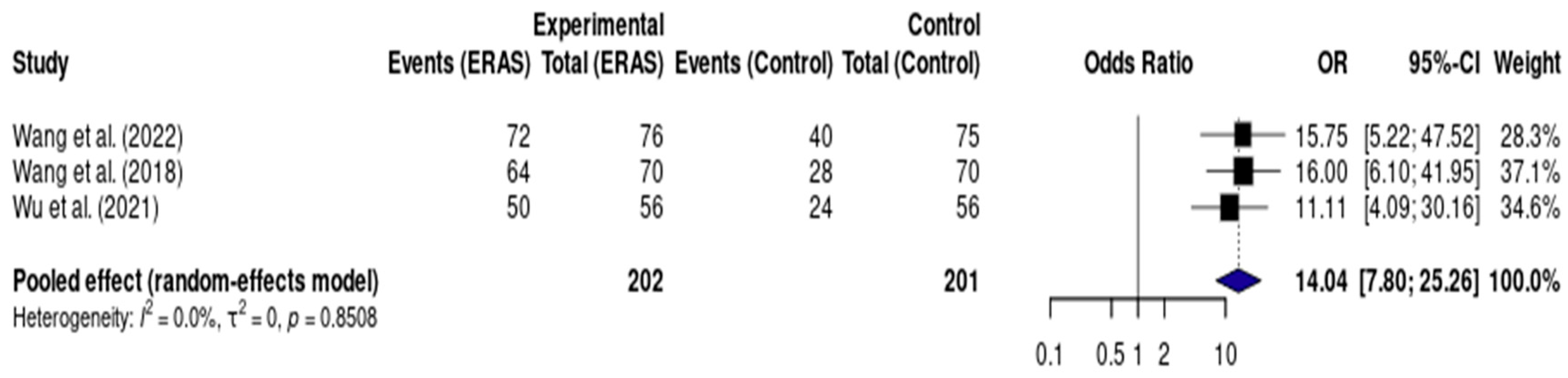
Results from three randomized controlled trials (RCTs), including a total of 403 patients, demonstrated a statistically significant reduction in length of hospital stay (LOS) in the ERAS group compared to standard care, with a pooled mean difference of −1.41 days (95% CI: −1.54 to −1.27; I2 = 83%) (Figure 7). The certainty of evidence was rated as low, primarily due to risk of bias in the included studies and inconsistency across trial results. No clear evidence of publication bias was observed, and a sensitivity analysis could not be conducted due to the small number of studies and clinical heterogeneity (Figure 8).
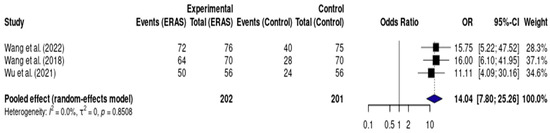
Figure 8.
Forest plot of the meta-analysis evaluating early oral intake (<24 h) in patients undergoing elective craniotomy. Each square represents the odds ratio (OR) for an individual study, with the size of the square proportional to the study’s statistical weight. Horizontal lines denote 95% confidence intervals (CI). The diamond represents the pooled effect estimate, with its width indicating the 95% CI. The vertical line at OR = 1 represents no difference between groups. Values to the right of the line favor ERAS (higher odds of early oral intake), while values to the left would favor conventional care [12,14,17].
3.6. Summary of Primary Outcomes and Key Findings
In addition to the quantitative results presented in the meta-analyses, Table 3 summarizes the primary outcomes and key findings reported in the included randomized controlled trials. Most studies consistently demonstrated a reduction in length of hospital stay (LOS), lower postoperative pain scores, decreased PONV, and improved early mobilization. Other relevant outcomes included improved health-related quality of life (HRQOL), better glycemic control, and increased muscle strength in selected patient subgroups.

Table 3.
Summary of Primary Outcomes and Key Findings.
3.7. Risk of Bias Assessment
The risk of bias assessment of the included randomized controlled trials is summarized in Table 4. Overall, most studies were judged as having a low risk of bias across the evaluated domains. However, some concerns were identified. Abhinav et al. (2023) [13] and Liu et al. (2019) [19] presented issues mainly related to randomization and selection bias, resulting in an overall judgment of high risk and some concerns, respectively. Similarly, Liu et al. (2020) [16] showed high risk of bias due to limitations in randomization, selection, and outcome measurement. In contrast, the majority of trials, including those conducted by Wang et al. (2018, 2022) [12,17], Wu et al. (2021) [14], Qu et al. (2020) [15], Han et al. (2019) [18], and Rajan et al. (2016) [20], demonstrated low risk of bias across all domains.

Table 4.
Risk-of-bias (RoB 2.0) assessment of the included randomized controlled trials across domains. Cell colors: green = low risk; yellow = some concerns; red = high risk. Domains: randomization process; deviations from intended interventions; missing outcome data; measurement of the outcome; selection of the reported result; overall risk of bias.
3.8. Summary of Outcomes
Table 5 presents a synthesis of the main outcomes assessed across the included trials. ERAS protocols were consistently associated with a significant reduction in length of hospital stay, shortening hospitalization by approximately 1.5–3.0 days compared with standard care. Postoperative pain scores on POD 1–3 were also significantly lower in the ERAS group, with mean differences ranging from −1.6 to −2.9 points on the VAS/NRS. Similarly, the incidence of postoperative nausea and vomiting (PONV) was reduced by 18–38%, corresponding to a risk reduction of nearly 60%. Hospital costs were decreased by up to 25% in studies reporting economic outcomes. Functional recovery outcomes showed that patients managed under ERAS protocols achieved earlier mobilization, with an almost two-fold increase in the likelihood of ambulating on POD 1. Finally, one study demonstrated improved health-related quality of life (HRQOL) at discharge and at 3–6 months, although the certainty of evidence was rated as low. Overall, most outcomes demonstrated moderate-quality evidence according to GRADE, with the exception of hospital costs and HRQOL, which were supported by low-quality evidence.

Table 5.
Summary of Outcomes.
3.9. GRADE Summary of Findings
Table 6 summarizes the certainty of evidence across key outcomes according to the GRADE approach. For length of stay (LOS), the pooled estimate (MD ≈ −2.1 days) was graded as moderate certainty, reflecting a consistent and clinically meaningful benefit with low-to-moderate heterogeneity. Postoperative pain (NRS/VAS; MD ≈ −2.3 points on POD1–3) was also rated moderate, with directionally consistent effects across trials despite some heterogeneity. The evidence for postoperative nausea and vomiting (PONV) (RR ≈ 0.42) was moderate, supported by a coherent reduction in events and low heterogeneity. Functional recovery (early mobilization) showed a robust difference favoring ERAS (RR ≈ 1.85) and was graded moderate based on consistency of effect. In contrast, health-related quality of life (HRQoL) received a low rating because it relied on a single RCT with limited generalizability, despite a moderate standardized mean difference in favor of ERAS. Overall, most endpoints met moderate-certainty thresholds, supporting the reliability and applicability of the observed benefits.

Table 6.
GRADE Summary of Findings.
3.10. ERAS Measures and Neurosurgery-Specific Postoperative Risks
To enhance the clinical applicability of our findings, we synthesized the relationship between common neurosurgical postoperative risks and the corresponding ERAS components. This summary is presented in Table 7, highlighting procedure-specific considerations that may influence ERAS implementation in neurosurgical practice.

Table 7.
Common postoperative complications in neurosurgical patients and corresponding ERAS components. Each ERAS measure is linked to specific neurosurgery-related risks (CSF leak, cranial nerve deficits), with recommended adaptations to optimize safety and recovery. The table emphasizes procedure-specific considerations to improve the clinical applicability of ERAS protocols in elective craniotomy and related neurosurgical interventions.
4. Discussion
4.1. Heterogeneity and Future Directions
While the adoption of Enhanced Recovery After Surgery (ERAS) protocols in elective craniotomy consistently yielded positive perioperative outcomes, significant heterogeneity in protocol components, outcome definitions, and measurement methods limits direct comparability between studies. Future research should prioritize the development of standardized, neurosurgery-specific ERAS frameworks, ideally through well-designed, multicenter randomized controlled trials (RCTs) and international consensus efforts.
4.2. Summary of Key Findings
This systematic review of nine randomized trials confirms that ERAS protocols, when applied to elective craniotomy, are associated with meaningful improvements across several outcome domains. Most notably, ERAS implementation was consistently linked to reduced length of hospital stay (LOS), decreased postoperative pain intensity, lower incidence of postoperative nausea and vomiting (PONV), and enhanced early functional recovery. Importantly, no increase in perioperative complications was reported in any of the included RCTs.
These results align with findings from other surgical specialties, suggesting that the core principles of ERAS namely attenuation of the surgical stress response, early mobilization, and patient engagement can be effectively translated to neurosurgical care, including complex procedures such as craniotomy.
4.3. Interpretation of Results
The magnitude and consistency of LOS reduction—ranging from 1.5 to 3 days—suggests a robust and clinically relevant benefit of ERAS protocols in optimizing perioperative resource utilization. For example, Wang et al. (2018) [17] and Wang et al. (2022) [12] demonstrated significantly shorter LOS, improved pain scores, and earlier mobilization with ERAS pathways compared to conventional care. Wu et al. (2021) [14], Qu et al. (2020) [15], and Abhinav et al. (2023) [13] reported similar benefits in terms of functional recovery, cost reduction, and patient-reported outcomes.
Although the specific ERAS components varied between studies, the convergence of outcomes highlights the impact of certain core interventions, such as multimodal analgesia, early oral intake, and structured perioperative education on recovery trajectories. The biological plausibility of these effects is supported by ERAS theory, which posits that minimizing metabolic stress and promoting early mobilization accelerates return to baseline function.
4.4. Contextualization and Novel Contributions
This review extends the existing evidence base on ERAS by focusing exclusively on elective craniotomy and incorporating only randomized trials with low to moderate risk of bias. Unlike previous reviews that combined diverse neurosurgical populations or included non-randomized designs, our analysis provides high-quality, focused insight into a homogenous surgical setting.
Additionally, this review incorporates data from recent and well-powered trials such as those by Wang et al. (2022) [12], Liu et al. (2018) [19], and Rajan et al. (2016) [20], reinforcing its contemporaneity and clinical relevance. These findings support the feasibility and safety of ERAS even in high-stakes neurosurgical procedures and may serve as a basis for institutional pathway development and benchmarking.
4.5. Implications for Practice
The systematic implementation of ERAS protocols in neurosurgical centers has the potential to deliver significant clinical and operational benefits. For patients, these protocols can provide a less traumatic postoperative experience, faster return to independence, and improved quality of life. For hospitals, ERAS adoption may translate into shorter hospitalizations, lower complication rates, and more efficient use of resources such as ICU beds and surgical wards.
Notably, these benefits were also observed in older or neurologically vulnerable populations, such as those reported in Han et al. (2019) [18] and Liu et al. (2020) [16], suggesting that ERAS principles may be broadly applicable across diverse patient subgroups undergoing elective craniotomy.
4.6. Integration of Neurosurgical-Specific Risks and ERAS Components
Table 7 synthesizes the most common postoperative complications in neurosurgical patients and links them to specific ERAS strategies designed to prevent or mitigate these outcomes. This mapping offers practical guidance for tailoring ERAS protocols to the unique needs of craniotomy patients. For instance, early mobilization is crucial in preventing respiratory and thromboembolic complications but must be cautiously applied to patients at risk of cerebrospinal fluid (CSF) leakage. Similarly, multimodal analgesia should be adapted to avoid masking critical neurological assessments, such as cranial nerve deficits. Elevating the head of the bed commonly recommended in ERAS, can support CSF dynamics and reduce the risk of intracranial hypertension. Therefore, this table reinforces the clinical rationale for adapting ERAS protocols specifically to neurosurgery, providing a foundation for safer implementation and improved outcomes. Its insights directly inform the main conclusions of this review, highlighting the feasibility and necessity of procedure-specific ERAS adaptations in elective craniotomy.
4.7. Limitations
This review has several limitations. First, heterogeneity in ERAS protocols and outcome definitions limited our ability to perform meta-analysis for all outcomes. Second, although all included studies were RCTs, several had small sample sizes, increasing the risk of type II errors and limiting subgroup analyses. Third, blinding of participants and outcome assessors was suboptimal in many trials, which may introduce performance and detection bias.
Additionally, long-term outcomes such as sustained neurological function, quality of life at 6–12 months, or return to work—were rarely reported, making it difficult to evaluate the durability of ERAS benefits beyond hospital discharge. Finally, language restrictions and reliance on indexed databases may have introduced selection bias.
4.8. Recommendations for Future Research
Future investigations should prioritize standardized ERAS protocols tailored to neurosurgical patients and test them in multicenter RCTs with robust blinding and follow-up. Special attention should be paid to neurocognitive recovery, cost-effectiveness, and long-term quality of life. Furthermore, subpopulation analyses such as those involving elderly, frail, or high-risk patients, as well as awake or pediatric craniotomy cohorts are essential to broaden applicability.
5. Conclusions
This systematic review and meta-analysis of nine randomized controlled trials offers compelling evidence that Enhanced Recovery After Surgery (ERAS) protocols significantly improve perioperative outcomes in patients undergoing elective craniotomy. Quantitative synthesis demonstrates a consistent and clinically meaningful reduction in hospital length of stay, postoperative pain, and complication rates including postoperative nausea and vomiting while enhancing early functional recovery and patient satisfaction.
The direction and magnitude of pooled effects, visualized through forest plots, underscore the robustness of ERAS benefits across diverse neurosurgical settings and populations. Importantly, no increase in adverse events was observed, reaffirming the safety of ERAS protocols in the neurosurgical context.
These findings validate ERAS as a transformative model of care in elective craniotomy—capable of optimizing recovery trajectories and resource utilization. They further highlight the urgent need for international standardization of neurosurgery-specific ERAS pathways and call for large-scale, multicenter trials to confirm long-term benefits, cost-effectiveness, and applicability in vulnerable populations such as the elderly and high-risk surgical candidates.
Author Contributions
Conceptualization: C.D.A.B., L.F.G.P. and L.F.d.R.F.; Methodology: C.D.A.B., V.A.F. and M.R.R.M.B.; Formal Analysis: C.D.A.B., V.A.F. and G.F.N.; Data Curation: C.D.A.B., L.F.G.P., M.R.R.M.B. and V.A.F.; Writing—Original Draft: C.D.A.B., L.F.G.P. and M.R.R.M.B.; Writing—Review and Editing: J.E.G.P., L.F.d.R.F. and G.F.N.; Supervision: L.F.d.R.F. and J.E.G.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
As this study is a meta-analysis, no new participants were enrolled. All data was extracted from previously published studies in which informed consent had already been obtained.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors would like to thank the Postgraduate Program in Translational Medicine at EPM-UNIFESP for academic support, and all the institutional libraries that facilitated access to full-text articles. Special thanks to the reviewers for their valuable insights and constructive feedback, which greatly improved the quality of this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Canelli, R.; Karim, P.; Bilotta, F. Enhanced recovery after neurosurgery. Curr. Opin. Anaesthesiol. 2025. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Greisman, J.D.; Olmsted, Z.T.; Crorkin, P.J.; Dallimore, C.A.; Zhigin, V.; Shlifer, A.; Bedi, A.D.; Kim, J.K.; Nelson, P.; Sy, H.L.; et al. Enhanced Recovery After Surgery (ERAS) for Cranial Tumor Resection: A Review. World Neurosurg. 2022, 163, 104–122. [Google Scholar] [CrossRef] [PubMed]
- Donato, A.D.; Velásquez, C.; Larkin, C.; Shahaf, D.B.; Bernal, E.H.; Shafiq, F.; Kalipinde, F.; Mwiga, F.F.; Jose, G.R.B.; Gangineni, K.K.N.; et al. Recovery after Craniotomy: Global Practices, Challenges, and Perspectives. J. Neurosurg. Anesthesiol. 2024, 37, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Frid, I.; Singer, J.; Zalatimo, O.; Schirmer, C.M.; Kimmell, K.T.; Agarwal, N. Neurosurgery perception of Enhanced Recovery After Surgery (ERAS) protocols. J. Neurosurg. Soc. Australas. 2021, 92, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Belouaer, A.; Cossu, G.; Papadakis, G.E.; Gaudet, J.G.; Perez, M.-H.; Chanez, V.; Boegli, Y.; Mury, C.; Peters, D.; Addor, V.; et al. Implementation of the Enhanced Recovery After Surgery (ERAS®) program in neurosurgery. Acta Neurochir. 2023, 165, 3137–3145. [Google Scholar] [CrossRef] [PubMed]
- Jolly, S.; Paliwal, S.; Gadepalli, A.; Chaudhary, S.; Bhagat, H.; Avitsian, R. Designing Enhanced Recovery After Surgery Protocols in Neurosurgery: A Contemporary Narrative Review. J. Neurosurg. Anesthesiol. 2024, 36, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Grasso, G.; Noto, M.; Pescatori, L.; Sallì, M.; Kim, H.-S.; Teresi, G.; Torregrossa, F. Enhanced Recovery after Cranial Surgery in Elderly: A Review. World Neurosurg. 2024, 185, e1013–e1018. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 29, 372. [Google Scholar]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; Wiley: Hoboken, NJ, USA, 2019; Available online: https://pure.johnshopkins.edu/en/publications/cochrane-handbook-for-systematic-reviews-of-interventions (accessed on 10 July 2025).
- Popay, J.; Roberts, H.; Sowden, A.; Petticrew, M.; Arai, L.; Rodgers, M.; Britten, N.; Roen, K.; Duffy, S. Guidance on the Conduct of Narrative Synthesis in Systematic Reviews; A Product from the ESRC Methods Programme; Economic and Social Research Council (ESRC): Swindon, UK, 2006. [Google Scholar]
- Wang, L.; Cai, H.; Wang, Y.; Liu, J.; Chen, T.; Liu, J.; Huang, J.; Guo, Q.; Zou, W. Enhanced recovery after elective craniotomy: A randomized controlled trial. J. Clin. Anesth. 2022, 76, 110575. [Google Scholar] [CrossRef] [PubMed]
- Abhinav, K.; Jadhav, D.; Andar, U.B.; Karmarkar, V.; Agrawal, R.; Agrawal, A. Management of Post-craniotomy Pain in Elective Cases: A Randomized Controlled Trial. Cureus 2023, 15, e46189. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, W.; Chen, J.; Fei, H.; Zhu, H.; Xie, H. Application of and Clinical Research on Enhanced Recovery After Surgery in Perioperative Care of Patients with Supratentorial Tumors. Front. Oncol. 2021, 11, 697699. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Liu, B.; Zhang, H.; Sankey, E.W.; Chai, W.; Wang, B.; Li, Z.; Niu, J.; Zhao, B.; Jiang, X.; et al. Management of Postoperative Pain after Elective Craniotomy: A Prospective Randomized Controlled Trial of a Neurosurgical Enhanced Recovery after Surgery (ERAS) Program. Int. J. Med. Sci. 2020, 17, 1541–1549. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Liu, S.; Wang, Y.; Lu, D.; Chen, L.; Zheng, T.; Ma, T.; Zhang, Y.; Gao, G.; Qu, Y.; et al. Impact of neurosurgical enhanced recovery after surgery (ERAS) program on health-related quality of life in glioma patients: A secondary analysis of a randomized controlled trial. J. Neurooncol. 2020, 148, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, B.; Zhao, T.; Zhao, B.; Yu, D.; Jiang, X.; Ye, L.; Zhao, L.; Lv, W.; Zhang, Y.; et al. Safety and efficacy of a novel neurosurgical enhanced recovery after surgery protocol for elective craniotomy: A prospective randomized controlled trial. J. Neurosurg. 2018, 130, 1680–1691. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Guo, S.; Jiang, H.; Wu, X. Feasibility and efficacy of enhanced recovery after surgery protocol in Chinese elderly patients with intracranial aneurysm. Clin. Interv. Aging 2019, 14, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, Y.; Liu, S.; Zhao, T.; Zhao, B.; Jiang, X.; Ye, L.; Zhao, L.; Lv, W.; Zhang, Y.; et al. A randomized controlled study of preoperative oral carbohydrate loading versus fasting in patients undergoing elective craniotomy. Clin. Nutr. Edinb. Scotl. 2019, 38, 2106–2112. [Google Scholar] [CrossRef] [PubMed]
- Rajan, S.; Hutcherson, M.T.; Sessler, D.I.; Kurz, A.; Yang, D.; Ghobrial, M.; Liu, J.; Avitsian, R. The Effects of Dexmedetomidine and Remifentanil on Hemodynamic Stability and Analgesic Requirement After Craniotomy: A Randomized Controlled Trial. J. Neurosurg. Anesthesiol. 2016, 28, 282–290. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Swiss Federation of Clinical Neuro-Societies. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).