Are Self-Reported Sensations During tACS Linked to Cortical Excitability Measured by Transcranial Magnetic Stimulation? A Pilot Study in an Older Adult Sample
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Transcranial Alternating Current Stimulation (tACS)
2.3. Stimulation Sensations
2.4. Motor Thresholding
2.5. Data Analysis
3. Results
3.1. Participant Characteristics
3.2. Reported Sensations During tACS
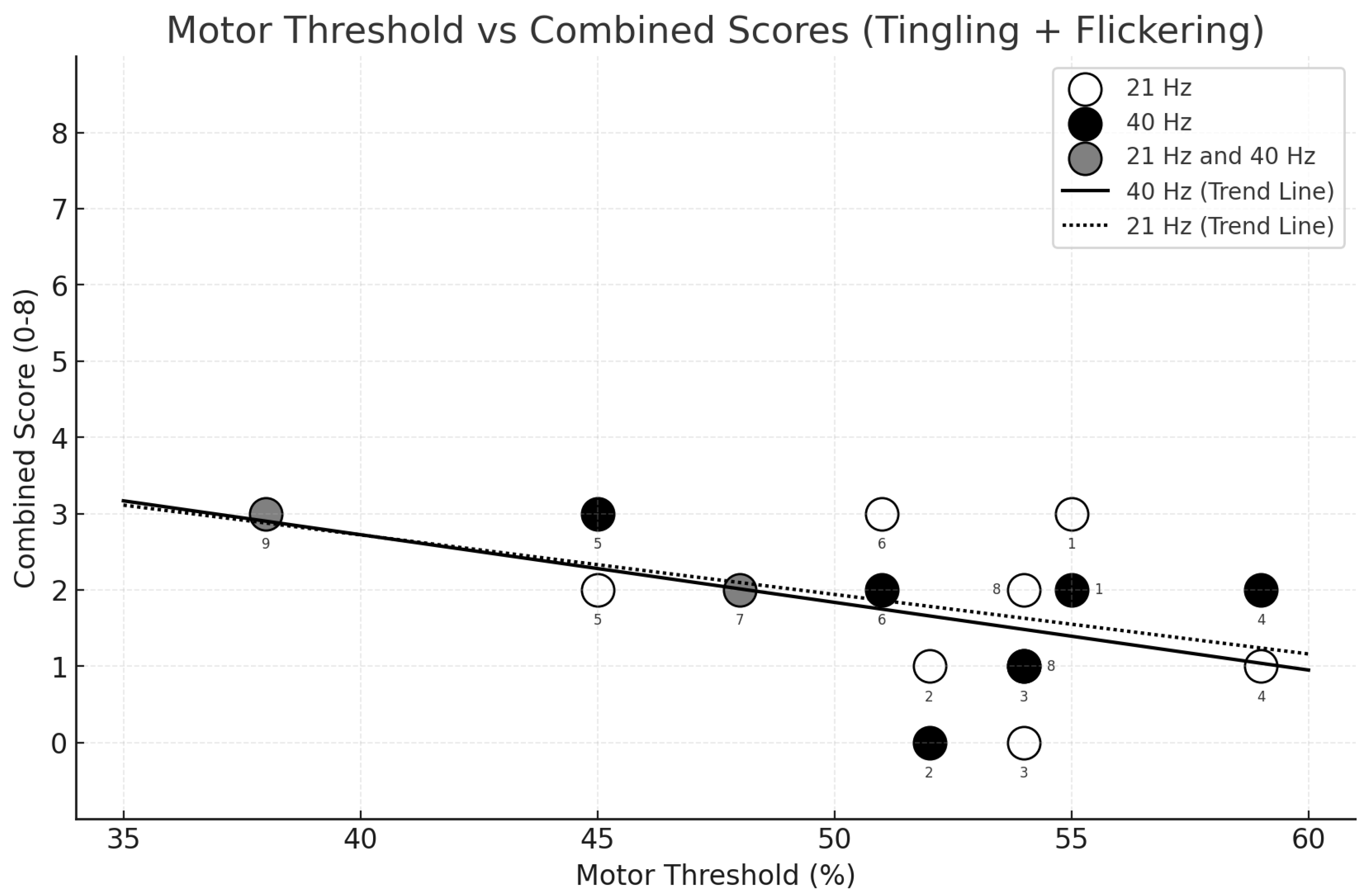
3.3. Relationship Between Sensation Intensity and Motor Threshold
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Frohlich, F.; Riddle, J. Conducting double-blind placebo-controlled clinical trials of transcranial alternating current stimulation (tACS). Transl. Psychiatry 2021, 11, 284. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matsumoto, H.; Ugawa, Y. Adverse events of tDCS and tACS: A review. Clin. Neurophysiol. Pract. 2017, 2, 19–25. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Raco, V.; Bauer, R.; Olenik, M.; Brkic, D.; Gharabaghi, A. Neurosensory effects of transcranial alternating current stimulation. Brain Stimul. 2014, 7, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Schutter, D.J.; Hortensius, R. Retinal origin of phosphenes to transcranial alternating current stimulation. Clin. Neurophysiol. 2010, 121, 1080–1084. [Google Scholar] [CrossRef] [PubMed]
- Turi, Z.; Ambrus, G.G.; Janacsek, K.; Emmert, K.; Hahn, L.; Paulus, W.; Antal, A. Both the cutaneous sensation and phosphene perception are modulated in a frequency-specific manner during transcranial alternating current stimulation. Restor. Neurol. Neurosci. 2013, 31, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Evans, I.D.; Palmisano, S.; Loughran, S.P.; Legros, A.; Croft, R.J. Frequency-dependent and montage-based differences in phosphene perception thresholds via transcranial alternating current stimulation. Bioelectromagnetics 2019, 40, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Thiele, C.; Zaehle, T.; Haghikia, A.; Ruhnau, P. Amplitude modulated transcranial alternating current stimulation (AM-TACS) efficacy evaluation via phosphene induction. Sci. Rep. 2021, 11, 22245. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kar, K.; Krekelberg, B. Transcranial electrical stimulation over visual cortex evokes phosphenes with a retinal origin. J. Neurophysiol. 2012, 108, 2173–2178. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Laakso, I.; Hirata, A. Computational analysis shows why transcranial alternating current stimulation induces retinal phosphenes. J. Neural Eng. 2013, 10, 046009. [Google Scholar] [CrossRef] [PubMed]
- Acharya, J.N.; Hani, A.; Cheek, J.; Thirumala, P.; Tsuchida, T.N. American Clinical Neurophysiology Society Guideline 2: Guidelines for Standard Electrode Position Nomenclature. J. Clin. Neurophysiol. 2016, 33, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Giustiniani, A.; Vallesi, A.; Oliveri, M.; Tarantino, V.; Ambrosini, E.; Bortoletto, M.; Masina, F.; Busan, P.; Siebner, H.R.; Fadiga, L.; et al. A questionnaire to collect unintended effects of transcranial magnetic stimulation: A consensus based approach. Clin. Neurophysiol. 2022, 141, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Corp, D.T.; Bereznicki, H.G.K.; Clark, G.M.; Youssef, G.J.; Fried, P.J.; Jannati, A.; Davies, C.B.; Gomes-Osman, J.; Stamm, J.; Chung, S.W.; et al. Large-scale analysis of interindividual variability in theta-burst stimulation data: Results from the ‘Big TMS Data Collaboration’. Brain Stimul. 2020, 13, 1476–1488. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kvašňák, E.; Orendáčová, M.; Vránová, J. Phosphene attributes depend on frequency and intensity of retinal tACS. Physiol. Res. 2022, 71, 561–571. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sadrzadeh-Afsharazar, F.; Douplik, A. Non-invasive transcranial alternating current stimulation of spatially resolved phosphenes. Front. Neurosci. 2023, 17, 1228326. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Boyle, M.R.; Fröhlich, F. EEG feedback-controlled transcranial alternating current stimulation. In Proceedings of the 6th International IEEE/EMBS Conference on Neural Engineering (NER), San Diego, CA, USA, 6–8 November 2013; pp. 140–143. [Google Scholar] [CrossRef]
- Ghobadi-Azbari, P.; Jamil, A.; Yavari, F.; Esmaeilpour, Z.; Malmir, N.; Mahdavifar-Khayati, R.; Soleimani, G.; Cha, Y.H.; Shereen, A.D.; Nitsche, M.A.; et al. fMRI and transcranial electrical stimulation (tES): A systematic review of parameter space and outcomes. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 107, 110149. [Google Scholar] [CrossRef] [PubMed]
- Rossini, P.M.; Burke, D.; Chen, R.; Cohen, L.G.; Daskalakis, Z.; Di Iorio, R.; Di Lazzaro, V.; Ferreri, F.; Fitzgerald, P.B.; George, M.S.; et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin. Neurophysiol. 2015, 126, 1071–1107. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Groppa, S.; Oliviero, A.; Eisen, A.; Quartarone, A.; Cohen, L.G.; Mall, V.; Kaelin-Lang, A.; Mima, T.; Rossi, S.; Thickbroom, G.W.; et al. A practical guide to diagnostic transcranial magnetic stimulation: Report of an IFCN committee. Clin. Neurophysiol. 2012, 123, 858–882. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| 21 Hz | 40 Hz | |
|---|---|---|
| Flickering lights | 8 (88.9%) | 7 (77.8%) |
| Tingling | 5 (55.6%) | 6 (66.7%) |
| Itching | 4 (44.4%) | 4 (44.4%) |
| Sleepiness | 3 (33.3%) | 5 (55.6%) |
| Trouble concentrating | 2 (22.2%) | 4 (44.4%) |
| Pressure on head from electrodes | 2 (22.2%) | 4 (44.4%) |
| Headache | 3 (33.3%) | 3 (33.3%) |
| Improved mood | 2 (22.2%) | 3 (33.3%) |
| Burning sensation | 1 (11.1%) | 2 (22.2%) |
| Other | 1 (11.1%) | 1 (11.1%) |
| Scalp pain | 1 (11.1%) | 1 (11.1%) |
| Neck pain | 2 (22.2%) | 0 |
| Tooth pain | 1 (11.1%) | 0 |
| Local redness | 0 | 1 (11.1%) |
| Worsening of mood | 0 | 0 |
| Dizziness | 0 | 0 |
| Ringing/buzzing noise | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Swiss Federation of Clinical Neuro-Societies. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seiler, A.; Park, J.; Frohlich, F. Are Self-Reported Sensations During tACS Linked to Cortical Excitability Measured by Transcranial Magnetic Stimulation? A Pilot Study in an Older Adult Sample. Clin. Transl. Neurosci. 2025, 9, 20. https://doi.org/10.3390/ctn9020020
Seiler A, Park J, Frohlich F. Are Self-Reported Sensations During tACS Linked to Cortical Excitability Measured by Transcranial Magnetic Stimulation? A Pilot Study in an Older Adult Sample. Clinical and Translational Neuroscience. 2025; 9(2):20. https://doi.org/10.3390/ctn9020020
Chicago/Turabian StyleSeiler, Andrea, Jimin Park, and Flavio Frohlich. 2025. "Are Self-Reported Sensations During tACS Linked to Cortical Excitability Measured by Transcranial Magnetic Stimulation? A Pilot Study in an Older Adult Sample" Clinical and Translational Neuroscience 9, no. 2: 20. https://doi.org/10.3390/ctn9020020
APA StyleSeiler, A., Park, J., & Frohlich, F. (2025). Are Self-Reported Sensations During tACS Linked to Cortical Excitability Measured by Transcranial Magnetic Stimulation? A Pilot Study in an Older Adult Sample. Clinical and Translational Neuroscience, 9(2), 20. https://doi.org/10.3390/ctn9020020






