Objective Sleep–Wake Findings in Patients with Post-COVID-19 Syndrome, Fatigue and Excessive Daytime Sleepiness
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Evaluation
2.2. Sleep Investigations
2.3. Data Availability
2.4. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AHI | Apnea–Hypopnea Index |
| BDI | Beck Depression Inventory |
| BMI | Body Mass Index |
| ESS | Epworth Sleepiness Scale |
| FSS | Fatigue Severity Scale |
| IQR | Interquartile Range |
| MSLT | Multiple Sleep Latency Test |
| NREM | Non-Rapid-Eye-Movement Sleep |
| OSA | Obstructive Sleep Apnea |
| PLMS | Periodic Limb Movement of Sleep |
| REM | Rapid-Eye-Movement Sleep |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| SOREMP | Sleep-Onset REM Period |
| SpO2 | Oxygen Desaturation |
References
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 24 November 2024).
- Carfi, A.; Bernabei, R.; Landi, F.; Gemelli Against, C.-P.-A.C.S.G. Persistent Symptoms in Patients After Acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Clinical Overview of Long COVID. Available online: https://www.cdc.gov/covid/hcp/clinical-overview/?CDC_AAref_Val=https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/post-covid-conditions.html (accessed on 24 November 2024).
- Ceravolo, M.G.; Arienti, C.; de Sire, A. Rehabilitation and COVID-19: The Cochrane Rehabilitation 2020 rapid living systematic review. Eur. J. Phys. Rehabil. Med. 2020, 56, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Marx, V. Scientists set out to connect the dots on long COVID. Nat. Methods 2021, 18, 449–453. [Google Scholar] [CrossRef]
- Reuken, P.A.; Scherag, A.; Stallmach, A. Postcoronavirus Disease Chronic Fatigue Is Frequent and Not Only Restricted to Hospitalized Patients. Crit. Care Med. 2021, 49, e1052–e1053. [Google Scholar] [CrossRef]
- Office of National Statistics. Prevalence of Ongoing Symptoms Following Coronavirus (COVID-19) Infection in the UK: 1 April 2021. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk/1april2021 (accessed on 24 November 2024).
- Sudre, C.H.; Murray, B.; Varsavsky, T. Attributes and predictors of long COVID. Nat. Med. 2021, 27, 626–631. [Google Scholar] [CrossRef]
- Frontera, J.A.; Sabadia, S.; Lalchan, R.; Fang, T.; Flusty, B.; Millar-Vernetti, P.; Snyder, T.; Berger, S.; Yang, D.; Granger, A.; et al. A Prospective Study of Neurologic Disorders in Hospitalized Patients With COVID-19 in New York City. Neurology 2021, 96, e575–e586. [Google Scholar] [CrossRef]
- Lund, L.C.; Hallas, J.; Nielsen, H. Post-acute effects of SARS-CoV-2 infection in individuals not requiring hospital admission: A Danish population-based cohort study. Lancet Infect. Dis. 2021, 21, 1373–1382. [Google Scholar] [CrossRef]
- Boldrini, M.; Canoll, P.D.; Klein, R.S. How COVID-19 Affects the Brain. JAMA Psychiatry 2021, 78, 682–683. [Google Scholar] [CrossRef]
- Meinhardt, J.; Radke, J.; Dittmayer, C.; Franz, J.; Thomas, C.; Mothes, R.; Laue, M.; Schneider, J.; Brunink, S.; Greuel, S.; et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat. Neurosci. 2021, 24, 168–175. [Google Scholar] [CrossRef]
- Gupta, A.; Madhavan, M.V.; Sehgal, K. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.H.; Wing, Y.K.; Yu, M.W.; Leung, C.M.; Ma, R.C.; Kong, A.P.; So, W.Y.; Fong, S.Y.; Lam, S.P. Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors: Long-term follow-up. Arch. Intern. Med. 2009, 169, 2142–2147. [Google Scholar] [CrossRef] [PubMed]
- Hickie, I.; Davenport, T.; Wakefield, D. Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: Prospective cohort study. BMJ 2006, 333, 575. [Google Scholar] [CrossRef] [PubMed]
- Kuratsune, H.; Kondo, K.; Ikuta, K. Chronic fatigue syndrome (CFS). Nihon Naika Gakkai Zasshi 2001, 90, 2431–2437. [Google Scholar] [CrossRef]
- Diem, L.; Fregolente-Gomes, L.; Warncke, J.D.; Hammer, H.; Friedli, C.; Kamber, N.; Jung, S.; Bigi, S.; Funke-Chambour, M.; Chan, A.; et al. Fatigue in Post-COVID-19 Syndrome: Clinical Phenomenology, Comorbidities and Association With Initial Course of COVID-19. J. Cent. Nerv. Syst. Dis. 2022, 14, 11795735221102727. [Google Scholar] [CrossRef]
- Diem, L.; Schwarzwald, A.; Friedli, C.; Hammer, H.; Gomes-Fregolente, L.; Warncke, J.; Weber, L.; Kamber, N.; Chan, A.; Bassetti, C.; et al. Multidimensional phenotyping of the post-COVID-19 syndrome: A Swiss survey study. CNS Neurosci. Ther. 2022, 28, 1953–1963. [Google Scholar] [CrossRef]
- Townsend, L.; Dyer, A.H.; Jones, K.; Dunne, J.; Mooney, A.; Gaffney, F.; O’Connor, L.; Leavy, D.; O’Brien, K.; Dowds, J.; et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS ONE 2020, 15, e0240784. [Google Scholar] [CrossRef]
- Sarkanen, T.; Partinen, M.; Bjorvatn, B.; Merikanto, I.; Benedict, C.; Nadorff, M.R.; Bolstad, C.J.; Espie, C.; Matsui, K.; Chung, F.; et al. Association between hypersomnolence and the COVID-19 pandemic: The International COVID-19 Sleep Study (ICOSS). Sleep. Med. 2023, 107, 108–115. [Google Scholar] [CrossRef]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Partinen, M.; Holzinger, B.; Morin, C.M.; Espie, C.; Chung, F.; Penzel, T.; Benedict, C.; Bolstad, C.J.; Cedernaes, J.; Chan, R.N.Y.; et al. Sleep and daytime problems during the COVID-19 pandemic and effects of coronavirus infection, confinement and financial suffering: A multinational survey using a harmonised questionnaire. BMJ Open 2021, 11, e050672. [Google Scholar] [CrossRef]
- Moura, A.E.F.; Oliveira, D.N.; Torres, D.M.; Tavares-Junior, J.W.L.; Nobrega, P.R.; Braga-Neto, P.; Sobreira-Neto, M.A. Central hypersomnia and chronic insomnia: Expanding the spectrum of sleep disorders in long COVID syndrome—A prospective cohort study. BMC Neurol. 2022, 22, 417. [Google Scholar] [CrossRef] [PubMed]
- Gulick, R.M.; Pau, A.K.; Daar, E.; Evans, L.; Gandhi, R.T.; Tebas, P.; Ridzon, R.; Masur, H.; Lane, H.C.; Adimora, A.A.; et al. National Institutes of Health COVID-19 Treatment Guidelines Panel: Perspectives and Lessons Learned. Ann. Intern. Med. 2024, 177, 1547–1557. [Google Scholar] [CrossRef] [PubMed]
- Valko, P.O.; Bassetti, C.L.; Bloch, K.E.; Held, U.; Baumann, C.R. Validation of the fatigue severity scale in a Swiss cohort. Sleep 2008, 31, 1601–1607. [Google Scholar] [CrossRef] [PubMed]
- Bloch, K.C.; Schoch, O.D.; Zhang, J.N.; Russi, E.W. German version fo th Epworth Sleepiness Scale. Respiration 1999, 65, 440–447. [Google Scholar] [CrossRef]
- Kühner, C.; Bürger, C.; Keller, F.; Hautzinger, M. Reliability and validity of the Revised Beck Depression Inventory (BDI-II). Results from German samples. Nervenarzt 2007, 78, 651–666. [Google Scholar] [CrossRef]
- Berry, R.B.; Brooks, R.; Gamaldo, C.; Harding, S.M.; Lloyd, R.M.; Quan, S.F.; Troester, M.T.; Vaughn, B.V. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications; American Academy of Sleep Medicine: Darien, IL, USA, 2020. [Google Scholar]
- Littner, M.R.; Kushida, C.; Wise, M.; Davila, D.G.; Morgenthaler, T.; Lee-Chiong, T.; Hirshkowitz, M.; Loube, D.L.; Bailey, D.; Berry, R.B. Practice parameters for clinical use of the multiple sleep latency test and the maintenance of wakefulness test. Sleep 2005, 28, 113–121. [Google Scholar] [CrossRef]
- Ameen, M.S.; Cheung, L.M.; Hauser, T.; Hahn, M.A.; Schabus, M. About the Accuracy and Problems of Consumer Devices in the Assessment of Sleep. Sensors 2019, 19, 4160. [Google Scholar] [CrossRef]
- Gonçalves, B.; Adamowicz, T.; Louzada, F.M.; Moreno, C.R.; Araujo, J.F. A fresh look at the use of nonparametric analysis in actimetry. Sleep Med. Rev. 2015, 20, 84–91. [Google Scholar] [CrossRef]
- R Core Team. R. A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 24 November 2024).
- Sjoberg, D.W.K.; Curry, M.; Lavery, J.; Larmarange, J. Reproducible Summary Tables with the gtsummary Package. R. J. 2021, 13, 570–580. [Google Scholar] [CrossRef]
- American Academy of Sleep Medicine. International Classification of Sleep Disorders—Third Edition (ICSD-3); American Academy of Sleep Medicine: Darien, IL, USA, 2014. [Google Scholar]
- Bassetti, C.L.A.; Adamantidis, A.R.; Burdakov, D.; Han, F.; Gay, S.; Kallweit, U.; Khatami, R.; Koning, F.; Kornum, B.R.; Lammers, G.J.; et al. Narcolepsy—Clinical features, etio-pathophysiology, diagnosis and management of a hypothalamic, immune-mediated disease. Nat. Rev. Neurol. 2019, 15, 519–539. [Google Scholar] [CrossRef]
- Lopez, R.; Doukkali, A.; Barateau, L.; Evangelista, E.; Chenini, S.; Jaussent, I.; Dauvilliers, Y. Test-Retest Reliability of the Multiple Sleep Latency Test in Central Disorders of Hypersomnolence. Sleep 2017, 40, zsx164. [Google Scholar] [CrossRef] [PubMed]
- Trotti, L.M.; Staab, B.A.; Rye, D.B. Test-retest reliability of the multiple sleep latency test in narcolepsy without cataplexy and idiopathic hypersomnia. J. Clin. Sleep Med. 2013, 9, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Kazaglis, L.; Cho, Y.; Howell, M.J.; Mahowald, M.W. Test–retest reliability of two consecutive mean sleep latency tests in patients with hypersomnia. Sleep Biol. Rhythm. 2017, 15, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Mignot, E.; Lin, L.; Finn, L.; Lopes, C.; Pluff, K.; Sundstrom, M.L.; Young, T. Correlates of sleep-onset REM periods during the Multiple Sleep Latency Test in community adults. Brain 2006, 129, 1609–1623. [Google Scholar] [CrossRef]
- Sparasci, D.; Gobbi, C.; Castelnovo, A.; Riccitelli, G.C.; Disanto, G.; Zecca, C.; Manconi, M. Fatigue, sleepiness and depression in multiple sclerosis: Defining the overlaps for a better phenotyping. J. Neurol. 2022, 269, 4961–4971. [Google Scholar] [CrossRef]
- Bei, B.; Manber, R.; Allen, N.B.; Trinder, J.; Wiley, J.F. Too Long, Too Short, or Too Variable? Sleep Intraindividual Variability and Its Associations With Perceived Sleep Quality and Mood in Adolescents During Naturalistically Unconstrained Sleep. Sleep 2016, 40, zsw067. [Google Scholar] [CrossRef]
- Lopez, R.; Barateau, L.; Evangelista, E.; Dauvilliers, Y. Depression and Hypersomnia: A Complex Association. Sleep Med. Clin. 2017, 12, 395–405. [Google Scholar] [CrossRef]
- Nevsimalova, S.; Skibova, J.; Galuskova, K.; Prihodova, I.; Dostalova, S.; Maurovich-Horvat, E.; Šonka, K. Central Disorders of Hypersomnolence: Association with Fatigue, Depression and Sleep Inertia Prevailing in Women. Brain Sci. 2022, 12, 1491. [Google Scholar] [CrossRef]
- Heidbreder, A.; Sonnweber, T.; Stefani, A.; Ibrahim, A.; Cesari, M.; Bergmann, M.; Brandauer, E.; Tancevski, I.; Loffler-Ragg, J.; Hogl, B. Video-polysomnographic findings after acute COVID-19: REM sleep without atonia as sign of CNS pathology? Sleep Med. 2021, 80, 92–95. [Google Scholar] [CrossRef]
- Senaratna, C.V.; Perret, J.L.; Lodge, C.J.; Lowe, A.J.; Campbell, B.E.; Matheson, M.C.; Hamilton, G.S.; Dharmage, S.C. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Med. Rev. 2017, 34, 70–81. [Google Scholar] [CrossRef]
- Goyal, A.; Saxena, K.; Kar, A.; Khurana, A.; Bhagtana, P.K.; Sridevi, C.; Pakhare, A. Obstructive sleep apnea is highly prevalent in COVID19 related moderate to severe ARDS survivors: Findings of level I polysomnography in a tertiary care hospital. Sleep Med. 2022, 91, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Cade, B.E.; Dashti, H.S.; Hassan, S.M.; Redline, S.; Karlson, E.W. Sleep Apnea and COVID-19 Mortality and Hospitalization. Am. J. Respir. Crit. Care Med. 2020, 202, 1462–1464. [Google Scholar] [CrossRef] [PubMed]
- Voncken, S.F.J.; Feron, T.M.H.; Laven, S.A.J.S.; Karaca, U.; Beerhorst, K.; Klarenbeek, P.; Straetmans, J.M.J.A.A.; de Vries, G.J.; Kolfoort-Otte, A.A.B.; de Kruif, M.D. Impact of obstructive sleep apnea on clinical outcomes in patients hospitalized with COVID-19. Sleep Breath. 2022, 26, 1399–1407. [Google Scholar] [CrossRef] [PubMed]
- Labarca, G.; Henríquez-Beltrán, M.; Lamperti, L.; Nova-Lamperti, E.; Sanhueza, S.; Cabrera, C.; Quiroga, R.; Antilef, B.; Ormazábal, V.; Zúñiga, F. Impact of obstructive sleep apnea (OSA) in COVID-19 survivors, symptoms changes between 4-months and 1 year after the COVID-19 infection. Front. Med. 2022, 9, 884218. [Google Scholar] [CrossRef]
- Mandel, H.L.; Colleen, G.; Abedian, S.; Ammar, N.; Charles Bailey, L.; Bennett, T.D.; Daniel Brannock, M.; Brosnahan, S.B.; Chen, Y.; Chute, C.G.; et al. Risk of post-acute sequelae of SARS-CoV-2 infection associated with pre-coronavirus disease obstructive sleep apnea diagnoses: An electronic health record-based analysis from the RECOVER initiative. Sleep 2023, 46, zsad126. [Google Scholar] [CrossRef]
- Lévy, P.; Kohler, M.; McNicholas, W.T.; Barbé, F.; McEvoy, R.D.; Somers, V.K.; Lavie, L.; Pépin, J.-L. Obstructive sleep apnoea syndrome. Nat. Rev. Dis. Primers 2015, 1, 1–21. [Google Scholar] [CrossRef]
- Breville, G.; Adler, D.; Uginet, M.; Assal, F.; Tamisier, R.; Lalive, P.H.; Pepin, J.-L.; Allali, G. Does Endothelial Vulnerability in OSA Syndrome Promote COVID-19 Encephalopathy? Chest 2021, 160, e161–e164. [Google Scholar] [CrossRef]
- Dauvilliers, Y.; Lopez, R.; Ohayon, M.; Bayard, S. Hypersomnia and depressive symptoms: Methodological and clinical aspects. BMC Med. 2013, 11, 78. [Google Scholar] [CrossRef]
- Benítez, I.D.; Moncusí-Moix, A.; Vaca, R.; Gort-Paniello, C.; Minguez, O.; Santisteve, S.; Carmona, P.; Torres, G.; Fagotti, J.; Labarca, G. Sleep and circadian health of critical COVID-19 survivors 3 months after hospital discharge. Crit. Care Med. 2022, 50, 945. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Reviews. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef]
- Wong, A.C.; Devason, A.S.; Umana, I.C.; Cox, T.O.; Dohnalova, L.; Litichevskiy, L.; Perla, J.; Lundgren, P.; Etwebi, Z.; Izzo, L.T.; et al. Serotonin reduction in post-acute sequelae of viral infection. Cell 2023, 186, 4851–4867.e4820. [Google Scholar] [CrossRef] [PubMed]
| Demographics | N = 31 1 |
|---|---|
| Age (years) | 47 (32, 53) |
| Gender | |
| Male | 23% (7/31) |
| Female | 77% (24/31) |
| BMI (kg/m2) | 25.4 (23.5, 28.4) |
| Time since COVID-19 infection (weeks) | 31 (24, 45) |
| Duration of acute infection (days) | 14 (10, 21) |
| Severe Symptoms | 7.1% (2/28) |
| Questionnaires | N = 31 |
| Epworth sleepiness scale (ESS) | 15.00 (13.00, 17.00) |
| Fatigue severity scale (FSS) | 6.33 (5.65, 6.44) |
| Beck depression inventory (BDI-II) | 20 (14, 28) |
| Polysomnography | N = 31 |
|---|---|
| Apnea–hypopnea index (/h) | 8 (4, 22) |
| Apnea–hypopnea index NREM (/h) | 6 (3, 22) |
| Apnea–hypopnea index REM (/h) | 13 (5, 24) |
| Apnea index (/h) | 0.4 (0.1, 1.2) |
| Oxygen desaturation index (/h) | 3 (2, 10) |
| Mean SpO2 (%) | 94.70 (93.75, 95.45) |
| Time SpO2 < 90% (min) | 0.0 (0.0, 0.4) |
| Sleep latency (min) | 12 (5, 32) |
| Total sleep time (min) | 366 (321, 452) |
| Sleep efficiency (%) | 86 (73, 93) |
| REM latency (min) | 144 (79, 218) |
| Arousal index (/h) | 29 (16, 48) |
| Mean heart rate (bpm) | 64 (57, 71) |
| PLMS (/h) | 2 (0, 7) |
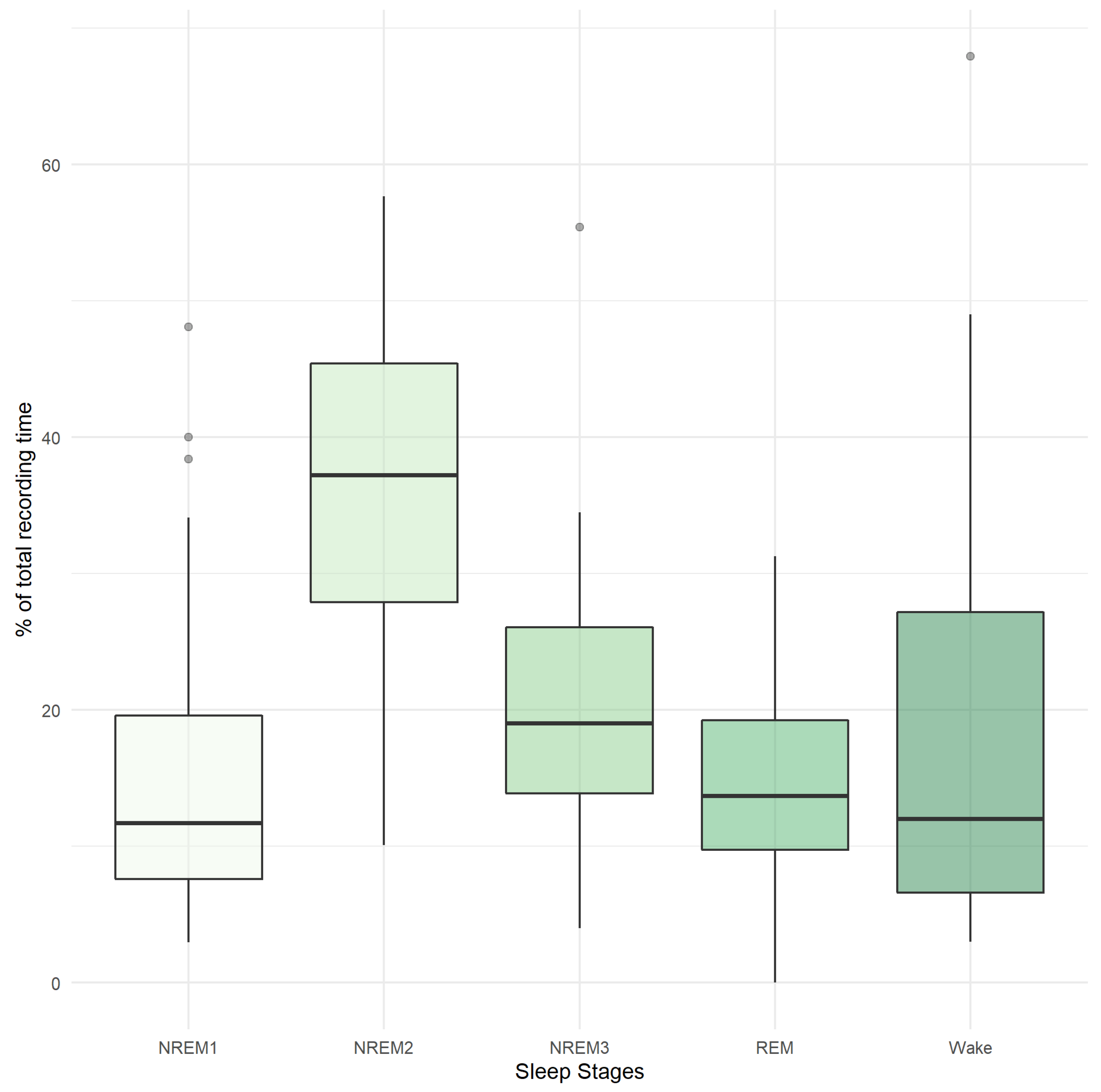
| NREM1 (% TRT) | 12 (6, 17) |
| NREM2 (% TRT) | 37 (28, 45) |
| NREM3 (% TRT) | 19 (16, 26) |
| REM (% TRT) | 15.8 (10.1, 19.8) |
| Wake (% TRT) | 12 (6, 27) |
| MSLT | N = 15 |
| Mean sleep latency in MSLT (min) | 12.2 (9.4, 15.6) |
| Maximal NREM stage in MSLT | |
| NREM1 | 13% (2/15) |
| NREM2 | 67% (10/15) |
| NREM3 | 20% (3/15) |
| SOREMP in MSLT | 0% (0/15) |
| Variables | AHI > 15/h, N = 11 1 | AHI < 15/h, N = 20 1 | p-Value 2 |
|---|---|---|---|
| Age (years) | 51 (47, 55) | 44 (30, 52) | 0.060 |
| Gender | 0.7 | ||
| Male | 27% (3/11) | 20% (4/20) | |
| Female | 73% (8/11) | 80% (16/20) | |
| Severe symptoms | 12% (1/8) | 5.0% (1/20) | 0.5 |
| BMI (kg/m2) | 28.1 (26.1, 31.4) | 25.2 (22.9, 26.2) | 0.026 |
| FSS | 6.11 (5.55, 6.38) | 6.42 (5.77, 6.47) | 0.3 |
| ESS | 15.00 (14.00, 16.50) | 14.00 (12.00, 17.00) | 0.3 |
| BDI-II | 16 (10, 20) | 22 (16, 31) | 0.056 |
| Apnea–hypopnea index (/h) | 24 (22, 40) | 5 (4, 8) | <0.001 |
| Apnea–hypopnea index REM (/h) | 39 (14, 46) | 9 (4, 18) | 0.010 |
| Oxygen desaturation index (/h) | 15 (10, 32) | 2 (1, 3) | <0.001 |
| Sleep latency (min) | 14 (3, 19) | 12 (8, 38) | 0.14 |
| Total sleep time (min) | 366 (328, 447) | 364 (321, 444) | >0.9 |
| Sleep efficiency (%) | 88 (75, 93) | 85 (71, 93) | 0.5 |
| NREM1 (% TRT) | 24 (12, 33) | 10 (6, 13) | 0.006 |
| NREM2 (% TRT) | 34 (20, 43) | 38 (30, 46) | 0.3 |
| NREM3 (% TRT) | 19 (15, 21) | 20 (16, 27) | 0.5 |
| REM (% TRT) | 10.9 (6.7, 17.2) | 15.9 (11.1, 20.5) | 0.094 |
| Wake (% TRT) | 12 (7, 26) | 12 (6, 29) | 0.8 |
| Arousal index (/h) | 46 (34, 55) | 17 (14, 27) | 0.001 |
| PLMS (/h) | 3 (0, 7) | 2 (0, 7) | 0.9 |
| Mean sleep latency in MSLT (min) | 10.2 (8.3, 12.1) | 13.1 (10.8, 16.0) | 0.2 |
| Variables | N = 29 1 |
|---|---|
| Bedtime (HH:mm) | 21:51:00 to 00:54:00 |
| Get up time (HH:mm) | 04:22:00 to 09:51:00 |
| Time in bed (hours) | 8.23 (7.92, 9.27) |
| Variability of time in bed (hours) | 5.20 (4.00, 6.60) |
| Sleep duration (hours) | 8.15 (7.85, 9.07) |
| Sleep efficiency (%) | 82.8 (79.3, 86.6) |
| Mean wake bout (minutes) | 00:01:45 to 00:03:27 |
| Inactivity index (%) | 40 (37, 44) |
| Relative amplitude | 0.90 (0.86, 0.94) |
| Inter-daily stability | 0.47 (0.39, 0.54) |
| L5 | 23:00:00 to 03:00:00 |
| M10 | 04:00:00 to 13:00:00 |
| Duration (days) | |
| 7–13 | 12 (41%) |
| >13 | 17 (59%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Swiss Federation of Clinical Neuro-Societies. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fregolente, L.G.; Diem, L.; Warncke, J.D.; van der Meer, J.; Schwarzwald, A.; Schäfer, C.; Hammer, H.; Chan, A.; Hoepner, R.; Bassetti, C.L.A. Objective Sleep–Wake Findings in Patients with Post-COVID-19 Syndrome, Fatigue and Excessive Daytime Sleepiness. Clin. Transl. Neurosci. 2025, 9, 15. https://doi.org/10.3390/ctn9010015
Fregolente LG, Diem L, Warncke JD, van der Meer J, Schwarzwald A, Schäfer C, Hammer H, Chan A, Hoepner R, Bassetti CLA. Objective Sleep–Wake Findings in Patients with Post-COVID-19 Syndrome, Fatigue and Excessive Daytime Sleepiness. Clinical and Translational Neuroscience. 2025; 9(1):15. https://doi.org/10.3390/ctn9010015
Chicago/Turabian StyleFregolente, Livia G., Lara Diem, Jan D. Warncke, Julia van der Meer, Anina Schwarzwald, Carolin Schäfer, Helly Hammer, Andrew Chan, Robert Hoepner, and Claudio L. A. Bassetti. 2025. "Objective Sleep–Wake Findings in Patients with Post-COVID-19 Syndrome, Fatigue and Excessive Daytime Sleepiness" Clinical and Translational Neuroscience 9, no. 1: 15. https://doi.org/10.3390/ctn9010015
APA StyleFregolente, L. G., Diem, L., Warncke, J. D., van der Meer, J., Schwarzwald, A., Schäfer, C., Hammer, H., Chan, A., Hoepner, R., & Bassetti, C. L. A. (2025). Objective Sleep–Wake Findings in Patients with Post-COVID-19 Syndrome, Fatigue and Excessive Daytime Sleepiness. Clinical and Translational Neuroscience, 9(1), 15. https://doi.org/10.3390/ctn9010015








