The Past and Future of Psychiatric Sleep Research
Abstract
:1. Introduction
2. Psychiatric Sleep Research until the Turn of the Millennium
3. REM Sleep Pressure in Depression
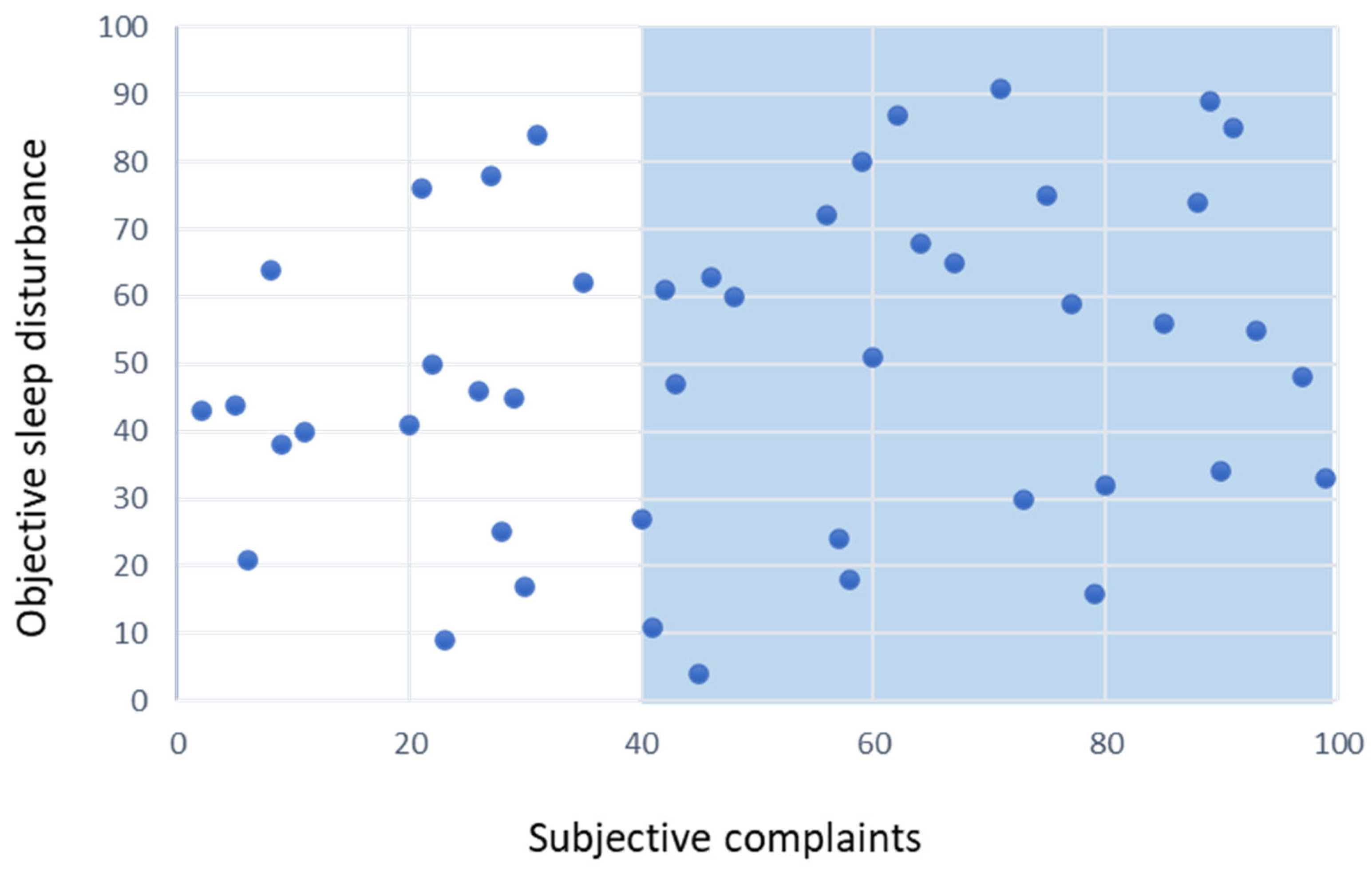
4. The Case of Insomnia Disorder
- -
- Another sleep–wake disorder better explains the insomnia or it occurs exclusively during the course of another sleep–wake disorder (criterion F);
- -
- The insomnia is attributable to the physiological effects of a substance (criterion G);
- -
- Coexisting mental disorders and medical conditions adequately explain the predominant complaint of insomnia (criterion H).
5. Summary: The Future of Psychiatric Sleep Research
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kraepelin, E. Psychiatrie. Ein Lehrbuch für Studierende und Ärzte; Verlag Johann Ambrosius Barth: Leipzig, Germany, 1913. [Google Scholar]
- Schulz, H.; Salzarulo, P. The development of sleep medicine: A historical sketch. J. Clin. Sleep Med. 2016, 12, 1041–1052. [Google Scholar] [CrossRef]
- Berger, H. Über das Elektrenkephalogram des Menschen. Arch. Für Psychiatr. Nervenkrankh. 1929, 87, 527–570. [Google Scholar] [CrossRef]
- Aserinsky, E.; Kleitman, N. Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science 1953, 118, 273–274. [Google Scholar] [CrossRef] [PubMed]
- Krueger, J.M.; Frank, M.G.; Wisor, J.P.; Roy, S. Sleep function: Toward elucidating an enigma. Sleep Med. Rev. 2016, 28, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Daan, S.; Beersma, D.G.; Borbély, A. Timing of human sleep: Recovery process gated by a circadian pacemaker. Am. J. Physiol. 1984, 246, R161–R183. [Google Scholar] [CrossRef] [PubMed]
- Kupfer, D.; Foster, F.G. Interval between onset of sleep and rapid-eye-movement sleep as an indicator of depression. Lancet 1972, 2, 684–686. [Google Scholar] [CrossRef] [PubMed]
- Baglioni, C.; Nanovska, S.; Regen, W.; Spiegelhalder, K.; Feige, B.; Nissen, C.; Reynolds, C.F.; Riemann, D. Sleep and mental disorders: A meta-analysis of polysomnographic research. Psychol. Bull. 2016, 142, 969–990. [Google Scholar] [CrossRef] [PubMed]
- Benca, R.M.; Obermeyer, W.H.; Thisted, R.A.; Gillin, J.C. Sleep and psychiatric disorders. A meta-analysis. Arch. Gen. Psychiatry 1992, 49, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Riemann, D.; Berger, M. Sleep, age, depression and the cholinergic REM induction test with RS 86. Prog. Neuropsychopharmacol. Biol. Psychiatry 1992, 16, 311–316. [Google Scholar] [CrossRef]
- Pollmächer, T.; Mullington, J.; Lauer, C.J. REM sleep disinhibition at sleep onset: A comparison between narcolepsy and depression. Biol. Psychiatry 1997, 42, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Giles, D.E.; Jarrett, R.B.; Roffwarg, H.P.; Rush, A.J. Reduced rapid eye movement latency. A predictor of recurrence in depression. Neuropsychopharmacology 1987, 1, 33–39. [Google Scholar] [CrossRef]
- Modell, S.; Lauer, C.J. Rapid eye movement (REM) sleep: An endophenotype for depression. Curr. Psychiatry Rep. 2007, 9, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Modell, S.; Ising, M.; Holsboer, F.; Lauer, C.J. The Munich vulnerability study on affective disorders: Premorbid polysomnographic profile of affected high-risk probands. Biol. Psychiatry 2005, 58, 694–699. [Google Scholar] [CrossRef]
- Pillai, V.; Kalmbach, D.A.; Ciesla, J.A. A meta-analysis of electroencephalographic sleep in depression: Evidence for genetic biomarkers. Biol. Psychiatry 2011, 70, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Solelhac, G.; Berger, M.; Strippoli, M.P.F.; Marchi, N.A.; Stephan, A.; Petit, J.M.; Bayon, V.; Imler, T.; Haba-Rubio, J.; Raffray, T.; et al. Objective polysomnography-based sleep features and major depressive disorder subtypes in the general population. Psychiatry Res. 2023, 324, 115213. [Google Scholar] [CrossRef]
- World Health Organization (WHO). The ICD-10 Classification of Mental and Behavioural Disorders; World Health Organization: Geneva, Switzerland, 1993. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; American Psychiatric Association: Washington, DC, USA, 2000. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- World Health Organization. ICD-11. International Classification of Diseases; 11th Revision; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Riemann, D.; Benz, F.; Dressle, R.J.; Espie, C.A.; Johann, A.F.; Blanken, T.F.; Leerssen, J.; Wassing, R.; Henry, A.L.; Kyle, S.D.; et al. Insomnia disorder: State of the science and challenges for the future. J. Sleep Res. 2022, 31, e13604. [Google Scholar] [CrossRef]
- Ferini-Strambi, L.; Auer, R.; Bjorvatn, B.; Castronovo, V.; Franco, O.; Gabutti, L.; Galbiati, A.; Hajak, G.; Khatami, R.; Kitajima, T.; et al. European Sleep Foundation. Insomnia disorder: Clinical and research challenges for the 21st century. Eur. J. Neurol. 2021, 28, 2156–2167. [Google Scholar] [CrossRef]
- Spielman, A.J.; Caruso, L.S.; Glovinsky, P.B. A behavioral perspective on insomnia treatment. Psychiatr. Clin. North. Am. 1987, 10, 541–553. [Google Scholar] [CrossRef]
- Van Someren, E.J.W. Brain mechanisms of insomnia: New perspectives on causes and consequences. Physiol. Rev. 2021, 101, 995–1046. [Google Scholar] [CrossRef]
- Dikeos, D.; Wichniak, A.; Ktonas, P.Y.; Mikoteit, T.; Crönlein, T.; Eckert, A.; Kopřivová, J.; Ntafouli, M.; Spiegelhalder, K.; Hatzinger, M.; et al. The potential of biomarkers for diagnosing insomnia: Consensus statement of the WFSBP Task Force on Sleep Disorders. World J. Biol. Psychiatry 2023, 24, 614–642. [Google Scholar] [CrossRef] [PubMed]
- Åkerstedt, T.; Schwarz, J.; Theorell-Haglöw, J.; Lindberg, E. What do women mean by poor sleep? A large population-based sample with polysomnographical indicators, inflammation, fatigue, depression, and anxiety. Sleep Med. 2023, 109, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Benz, F.; Riemann, D.; Domschke, K.; Spiegelhalder, K.; Johann, A.F.; Marshall, N.S.; Feige, B. How many hours do you sleep? A comparison of subjective and objective sleep duration measures in a sample of insomnia patients and good sleepers. J. Sleep Res. 2023, 32, e13802. [Google Scholar] [CrossRef] [PubMed]
- Baglioni, C.; Regen, W.; Teghen, A.; Spiegelhalder, K.; Feige, B.; Nissen, C.; Riemann, D. Sleep changes in the disorder of insomnia: A meta-analysis of polysomnographic studies. Sleep Med. Rev. 2014, 18, 195–213. [Google Scholar] [CrossRef]
- Frase, L.; Nissen, C.; Spiegelhalder, K.; Feige, B. The importance and limitations of polysomnography in insomnia disorder-a critical appraisal. J. Sleep Res. 2023, e14036. [Google Scholar] [CrossRef]
- Behr, M.; Acker, J.; Cohrs, S.; Deuschle, M.; Danker-Hopfe, H.; Göder, R.; Norra, C.; Richter, K.; Riemann, D.; Schilling, C.; et al. Prävalenz schlafbezogener Atmungsstörungen bei stationären Patienten mit psychischen Erkrankungen. Nervenarzt 2018, 89, 807–813. [Google Scholar] [CrossRef]
- Ragnoli, B.; Pochetti, P.; Raie, A.; Malerba, M. Comorbid Insomnia and Obstructive Sleep Apnea (COMISA): Current Concepts of Patient Management. Int. J. Environ. Res. Public. Health 2021, 18, 9248. [Google Scholar] [CrossRef] [PubMed]
- Keckeis, M.; Lattova, Z.; Maurovich-Horvat, E.; Beitinger, P.A.; Birkmann, S.; Lauer, C.J.; Wetter, T.C.; Wilde-Frenz, J.; Pollmächer, T. Impaired glucose tolerance in sleep disorders. PLoS ONE 2010, 15, e9444. [Google Scholar] [CrossRef]
- Tschepp, J.; Lauer, C.J.; Wilde-Frenz, J.; Pollmächer, T. No Impaired Glucose Tolerance in Primary Insomnia Patients with Normal Results of Polysomnography. Front. Neurol. 2017, 27, 303. [Google Scholar] [CrossRef]
- Johnson, K.A.; Gordon, C.J.; Chapman, J.L.; Hoyos, C.M.; Marshall, N.S.; Miller, C.B.; Grunstein, R.R. The association of insomnia disorder characterised by objective short sleep duration with hypertension, diabetes and body mass index: A systematic review and meta-analysis. Sleep Med. Rev. 2021, 59, 101456. [Google Scholar] [CrossRef]
- Markham, A. Daridorexant: First Approval. Drugs 2022, 82, 601–607. [Google Scholar] [CrossRef]
- Riemann, D.; Baglioni, C.; Bassetti, C.; Bjorvatn, B.; Dolenc Groselj, L.; Ellis, J.G.; Espie, C.A.; Garcia-Borreguero, D.; Gjerstad, M.; Gonçalves, M.; et al. European guideline for the diagnosis and treatment of insomnia. J. Sleep Res. 2017, 26, 675–700. [Google Scholar] [CrossRef]
- Ferrarelli, F. Sleep Abnormalities in Schizophrenia: State of the Art and Next Steps. Am. J. Psychiatry 2021, 178, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Plante, D.T. The Evolving Nexus of Sleep and Depression. Am. J. Psychiatry 2021, 178, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Insel, T.; Cuthbert, B.; Garvey, M.; Heinssen, R.; Pine, D.S.; Quinn, K.; Sanislow, C.; Wang, P. Research domain criteria (RDoC): Toward a new classification framework for research on mental disorders. Am. J. Psychiatry 2010, 167, 748–751. [Google Scholar] [CrossRef]
- Penzel, T. Sleep scoring moving from visual scoring towards automated scoring. Sleep 2022, 10, 45. [Google Scholar] [CrossRef]
- Yoon, H.; Choi, S.H. Technologies for sleep monitoring at home: Wearables and nearables. Biomed. Eng. Lett. 2023, 13, 313–327. [Google Scholar] [PubMed]
| Mental Disorder | Sleep Continuity | Sleep Stages | REM-Sleep | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Sleep Efficiency | Sleep Latency | N of Awakenings | N1 | N2 | N3 | REM % | Latency | REM Density | |
| Major depression | ↓ | ↓ | ↑ | n.s. | ↑ | n.s. | ↑ | ↓ | ↑ |
| Post traumatic stress disorder | ↓ | n.s. | ↑ | n.s. | n.s. | ↓ | n.s. | ↓ | ↑ |
| Panic disorder | ↓ | n.s. | n.s. | - | n.s. | n.s. | n.s. | n.s. | - |
| Schizophrenia | ↓ | ↑ | ↑ | n.s. | ↓ | ↓ | n.s. | ↓ | n.s. |
| Borderline personality disorder | ↓ | n.s. | ↑ | n.s. | n.s. | n.s. | n.s. | ↓ | n.s. |
| Anorexia nervosa | ↓ | n.s. | - | ↑ | n.s. | n.s. | n.s. | n.s. | - |
| Attention deficit disorder | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | - |
A. A predominant complaint of dissatisfaction with sleep quantity or quality, associated with one (or more) of the following symptoms:
|
| B. The sleep disturbance causes clinically significant distress or impairment in social, occupational, educational, academic, behavioral, or other important areas of functioning. |
| C. The sleep difficulty occurs at least 3 nights per week. |
| E. The sleep difficulty occurs despite adequate opportunity for sleep. |
| F. The insomnia is not better explained by and does not occur exclusively during the course of another sleep-wake disorder (e.g., narcolepsy, a breathing-related sleep disorder, a circadian rhythm sleep-wake disorder, a parasomnia). |
| H. Coexisting mental disorders and medical conditions do not adequately explain the predominant complaint of insomnia. |
| G. The insomnia is not attributable to the physiological effects of a substance (e.g., a drug of abuse, a medication). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pollmächer, T. The Past and Future of Psychiatric Sleep Research. Clin. Transl. Neurosci. 2023, 7, 37. https://doi.org/10.3390/ctn7040037
Pollmächer T. The Past and Future of Psychiatric Sleep Research. Clinical and Translational Neuroscience. 2023; 7(4):37. https://doi.org/10.3390/ctn7040037
Chicago/Turabian StylePollmächer, Thomas. 2023. "The Past and Future of Psychiatric Sleep Research" Clinical and Translational Neuroscience 7, no. 4: 37. https://doi.org/10.3390/ctn7040037
APA StylePollmächer, T. (2023). The Past and Future of Psychiatric Sleep Research. Clinical and Translational Neuroscience, 7(4), 37. https://doi.org/10.3390/ctn7040037






