Management of Acute Demyelinating Attacks in the Pediatric Population: A Swiss Consensus Statement
Abstract
:1. Introduction
2. Methods
3. Results
3.1. First Survey
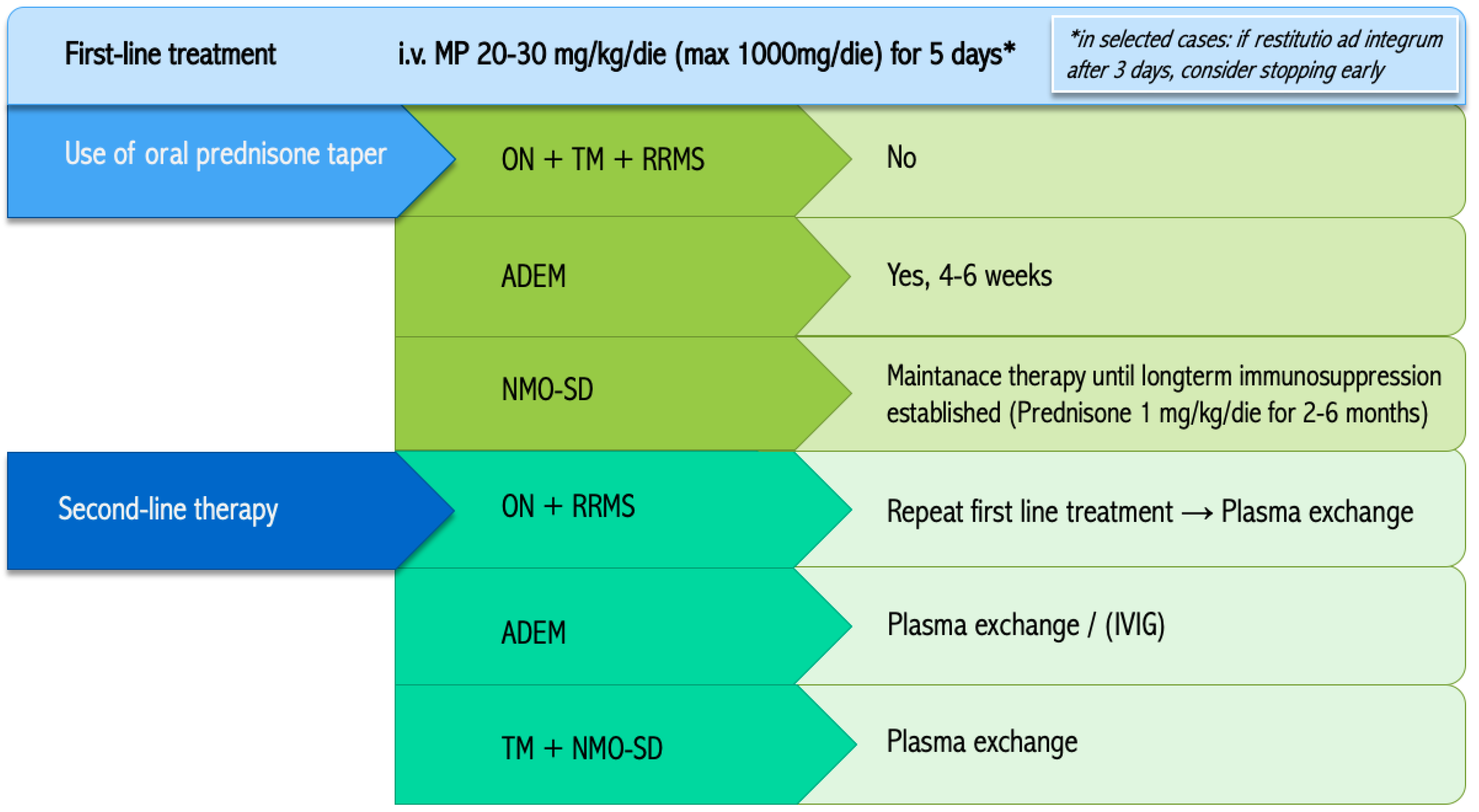
3.1.1. First-Line Therapy
3.1.2. Taper Regimen
3.1.3. Second-Line Treatment
3.2. Second Survey
4. Discussion
4.1. Epidemiology: First Swiss Data on Incidence
4.2. The Oral Prednisone Taper: A Challenge
4.3. Applicability of the Consensus Statement: Benefits and Points of Caution
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bar-Or, A.; Hintzen, R.Q.; Dale, R.C.; Rostasy, K.; Brück, W.; Chitnis, T. Immunopathophysiology of pediatric CNS inflammatory demyelinating diseases. Neurology 2016, 87, S12–S19. [Google Scholar] [CrossRef] [Green Version]
- Metz, I.; Ms, S.D.W.; Popescu, B.F.G.; Frischer, J.; Parisi, J.E.; Guo, Y.; Lassmann, H.; Brück, W.; Lucchinetti, C.F. Pathologic heterogeneity persists in early active multiple sclerosis lesions. Ann. Neurol. 2014, 75, 728–738. [Google Scholar] [CrossRef] [Green Version]
- Lucchinetti, C.F.; Popescu, B.F.; Bunyan, R.F.; Moll, N.M.; Roemer, S.F.; Lassmann, H.; Brück, W.; Parisi, J.E.; Scheithauer, B.W.; Giannini, C.; et al. Inflammatory Cortical Demyelination in Early Multiple Sclerosis. N. Engl. J. Med. 2011, 365, 2188–2197. [Google Scholar] [CrossRef] [Green Version]
- Young, N.P.; Weinshenker, B.G.; Parisi, J.E.; Scheithauer, B.; Giannini, C.; Roemer, S.F.; Thomsen, K.M.; Mandrekar, J.N.; Erickson, B.J.; Lucchinetti, C.F. Perivenous demyelination: Association with clinically defined acute disseminated encephalomyelitis and comparison with pathologically confirmed multiple sclerosis. Brain 2010, 133, 333–348. [Google Scholar] [CrossRef] [Green Version]
- Banwell, B.; Kennedy, J.; Sadovnick, D.; Arnold, D.L.; Magalhaes, S.; Wambera, K.; Connolly, M.B.; Yager, J.; Mah, J.K.; Shah, N.; et al. Incidence of acquired demyelination of the CNS in Canadian children. Neurology 2009, 72, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Pohl, D.; Hennemuth, I.; Von Kries, R.; Hanefeld, F. Paediatric multiple sclerosis and acute disseminated encephalomyelitis in Germany: Results of a nationwide survey. Eur. J. Nucl. Med. Mol. Imaging 2007, 166, 405–412. [Google Scholar] [CrossRef]
- Yeh, E.A.; Graves, J.S.; Benson, L.A.; Wassmer, E.; Waldman, A. Pediatric optic neuritis. Neurology 2016, 87, S53–S58. [Google Scholar] [CrossRef] [Green Version]
- Absoud, M.; Greenberg, B.; Lim, M.; Lotze, T.; Thomas, T.; Deiva, K. Pediatric transverse myelitis. Neurology 2016, 87, S46–S52. [Google Scholar] [CrossRef] [PubMed]
- Bigi, S.; Banwell, B. Pediatric Multiple Sclerosis. J. Child Neurol. 2012, 27, 1378–1383. [Google Scholar] [CrossRef] [PubMed]
- Waldman, A.T.; Gorman, M.P.; Rensel, M.R.; Austin, T.E.; Hertz, D.P.; Kuntz, N.L.; Network of Pediatric Multiple Sclerosis Centers of Excellence of the National Multiple Sclerosis Society. Management of pediatric central nervous system demyelinating disorders: Consensus of United States neurologists. J. Child Neurol. 2011, 26, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Huppke, P.; Gärtner, J. A Practical Guide to Pediatric Multiple Sclerosis. Neuropediatrics 2010, 41, 157–162. [Google Scholar] [CrossRef] [Green Version]
- Dale, R.C.; Brilot, F.; Banwell, B. Pediatric central nervous system inflammatory demyelination: Acute disseminated encephalomyelitis, clinically isolated syndromes, neuromyelitis optica, and multiple sclerosis. Curr. Opin. Neurol. 2009, 22, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Perumal, J.S.; Caon, C.; Hreha, S.; Zabad, R.; Tselis, A.; Lisak, R.; Khan, O. Oral prednisone taper following intravenous steroids fails to improve disability or recovery from relapses in multiple sclerosis. Eur. J. Neurol. 2008, 15, 677–680. [Google Scholar] [CrossRef] [PubMed]
- Okoli, C.; Pawlowski, S.D. The Delphi method as a research tool: An example, design considerations and applications. Inf. Manag.-Amster. 2004, 42, 15–29. [Google Scholar] [CrossRef] [Green Version]
- Hasson, F.; Keeney, S.; McKenna, H. Research guidelines for the Delphi survey technique. J. Adv. Nurs. 2000, 32, 1008–1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bevölkerungsbestand per 31.12.2018. 2018. Available online: https://www.bfs.admin.ch/bfs/de/home/statistiken/bevoelkerung/stand-entwicklung/bevoelkerung.assetdetail.14087554.html (accessed on 1 August 2020).
- Levic, Z.; Micic, D.; Nikolić, J.; Stojisavljević, N.; Sokić, D.; Jankovic, S.; Kendereški, A.; Mavra, M. Short-term high dose steroid therapy does not affect the hypothalamic-pituitary-adrenal axis in relapsing multiple sclerosis patients. Clinical assessment by the insulin tolerance test. J. Endocrinol. Investig. 1996, 19, 30–34. [Google Scholar] [CrossRef]
- Beck, R.W.; Cleary, P.A.; Anderson, M.M.; Keltner, J.L.; Shults, W.T.; Kaufman, D.I.; Buckley, E.G.; Corbett, J.J.; Kupersmith, M.J.; Miller, N.R.; et al. A Randomized, Controlled Trial of Corticosteroids in the Treatment of Acute Optic Neuritis. N. Engl. J. Med. 1992, 326, 581–588. [Google Scholar] [CrossRef]
- Treatment of Acute Exacerbations of Multiple Sclerosis in Adults. 2019. Available online: https://www.uptodate.com (accessed on 19 April 2020).
- Kimbrough, D.; Fujihara, K.; Jacob, A.; Lana-Peixoto, M.A.; Leite, M.I.; Levy, M.; Marignier, R.; Nakashima, I.; Palace, J.; de Seze, J.; et al. Treatment of neuromyelitis optica: Review and recommendations. Mult. Scler. Relat. Disord. 2012, 1, 180–187. [Google Scholar] [CrossRef] [Green Version]
- Sellner, J.; Boggild, M.; Clanet, M.; Hintzen, R.Q.; Illes, Z.; Montalban, X.; Du Pasquier, R.; Polman, C.H.; Sorensen, P.S.; Hemmer, B. EFNS guidelines on diagnosis and management of neuromyelitis optica. Eur. J. Neurol. 2010, 17, 1019–1032. [Google Scholar] [CrossRef]
- Pohl, D.; Alper, G.; Van Haren, K.; Kornberg, A.J.; Lucchinetti, C.F.; Tenembaum, S.; Belman, A.L. Acute disseminated encephalomyelitis: Updates on an inflammatory CNS syndrome. Neurology 2016, 87, S38–S45. [Google Scholar] [CrossRef]
- Cole, J.; Evans, E.; Mwangi, M.; Mar, S. Acute Disseminated Encephalomyelitis in Children: An Updated Review Based on Current Diagnostic Criteria. Pediatr. Neurol. 2019, 100, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Anlar, B.; Guven, A.; Haspolat, S.; Yakut, A.; Serdaroglu, A.; Senbil, N.; Karaagaoglu, E.; Oguz, K.K.; Basaran, C.; Köse, G.; et al. Acute Disseminated Encephalomyelitis in Children: Outcome and Prognosis. Neuropediatrics 2003, 34, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Sloka, J.S.; Stefanelli, M. The mechanism of action of methylprednisolone in the treatment of multiple sclerosis. Mult. Scler. J. 2005, 11, 425–432. [Google Scholar] [CrossRef]
- Rimsza, M.E. Complications of Corticosteroid Therapy. Arch. Pediatr. Adolesc. Med. 1978, 132, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Patil, C.G.; Lad, S.P.; Katznelson, L.; Laws, E.R., Jr. Brain atrophy and cognitive deficits in Cushing’s disease. Neurosurg. Focus 2007, 23, E11. [Google Scholar] [CrossRef] [PubMed]
- Zivadinov, R. Steroids and brain atrophy in multiple sclerosis. J. Neurol. Sci. 2005, 233, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Hacohen, Y.; Banwell, B. Treatment Approaches for MOG-Ab-Associated Demyelination in Children. Curr. Treat. Options Neurol. 2019, 21, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armangue, T.; Capobianco, M.; de Chalus, A.; Laetitia, G.; Deiva, K.; Bruijstens, A.L.; Wendel, E.-M.; Lechner, C.; Bartels, F.; Finke, C.; et al. E.U. paediatric MOG consortium consensus: Part 3—Biomarkers of paediatric myelin oligodendrocyte glycoprotein antibody-associated disorders. Eur. J. Paediatr. Neurol. 2020, 29, 22–31. [Google Scholar] [CrossRef]
- Bruijstens, A.L.; Wendel, E.-M.; Lechner, C.; Bartels, F.; Finke, C.; Breu, M.; Flet-Berliac, L.; de Chalus, A.; Adamsbaum, C.; Capobianco, M.; et al. E.U. paediatric MOG consortium consensus: Part 5—Treatment of paediatric myelin oligodendrocyte glycoprotein antibody-associated disorders. Eur. J. Paediatr. Neurol. 2020, 29, 41–53. [Google Scholar] [CrossRef]
| ADS | Patient Currently in Participants Care n (%) | New Diagnosis Per Year n (%) |
|---|---|---|
| Optic Neuritis | 18 (25.4) | 11 (27.5) |
| Transverse Myelitis | 8 (11.3) | 6 (15) |
| Acute demyelinating encephalomyelitis | 20 (28.9) | 12 (30) |
| RRMS | 22 (30.3) | 9 (22.5) |
| NMO-SD | 3 (4.2) | 2 (5) |
| Total | 71 (100) | 40 (100) |
| ADS | Yes, Always n (%) | It Depends * n (%) | No n (%) |
|---|---|---|---|
| ON | 4 (36.4) | 2 (18.2) | 5 (45.5) |
| TM | 5 (50) | 3 (30) | 2 (20) |
| ADEM | 4 (40) | 1 (10) | 5 (50) |
| RRMS | 4 (40) | 2 (20) | 4 (40) |
| NMO-SD | 4 (40) | 3 (30) | 2 (20) |
| ADS | Repeat First Line Treatment n (%) | Plasma Exchange n (%) | IVIG n (%) | Others, Please Specify n (%) * |
|---|---|---|---|---|
| ON | 5 (50) | 1 (10) | 3 (30) | 1 (10) |
| TM | 4 (40) | 3 (30) | 2 (20) | 1 (10) |
| ADEM | 5 (50) | 1 (10) | 2 (20) | 1 (10) |
| RRMS | 5 (50) | 2 (20) | - | 3 (30) |
| NMO-SD | 4 (40) | 3 (30) | - | 3 (30) |
| ADS | Statement |
|---|---|
| ON + RRMS | In case of insufficient response in an acute optic neuritis or RRMS, repeat the first-line treatment with i.v. MP. If further treatment is required, we suggest the use of plasma exchange. |
| ADEM | In case of poor response to i.v. MP, we suggest using plasma exchange in fulminant forms of ADEM and IVIG in the remainder. |
| TM + NMO-SD | In case of poor response to i.v. MP for TM or ADS in NMO-SD, we suggest to use plasma exchange. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hofer, S.; Bauder, F.; Capone Mori, A.; Chan, A.; Dill, P.; Garcia-Tarodo, S.; Goeggel Simonetti, B.; Hackenberg, A.; Kalser, J.; Maier, O.; et al. Management of Acute Demyelinating Attacks in the Pediatric Population: A Swiss Consensus Statement. Clin. Transl. Neurosci. 2021, 5, 17. https://doi.org/10.3390/ctn5020017
Hofer S, Bauder F, Capone Mori A, Chan A, Dill P, Garcia-Tarodo S, Goeggel Simonetti B, Hackenberg A, Kalser J, Maier O, et al. Management of Acute Demyelinating Attacks in the Pediatric Population: A Swiss Consensus Statement. Clinical and Translational Neuroscience. 2021; 5(2):17. https://doi.org/10.3390/ctn5020017
Chicago/Turabian StyleHofer, Seline, Florian Bauder, Andrea Capone Mori, Andrew Chan, Patricia Dill, Stéphanie Garcia-Tarodo, Barbara Goeggel Simonetti, Annette Hackenberg, Judith Kalser, Oliver Maier, and et al. 2021. "Management of Acute Demyelinating Attacks in the Pediatric Population: A Swiss Consensus Statement" Clinical and Translational Neuroscience 5, no. 2: 17. https://doi.org/10.3390/ctn5020017
APA StyleHofer, S., Bauder, F., Capone Mori, A., Chan, A., Dill, P., Garcia-Tarodo, S., Goeggel Simonetti, B., Hackenberg, A., Kalser, J., Maier, O., Schmid, R., Strozzi, S., Bigi, S., & on behalf of the “Medico Scientific Advisory Board” of the Swiss Multiple Sclerosis Society. (2021). Management of Acute Demyelinating Attacks in the Pediatric Population: A Swiss Consensus Statement. Clinical and Translational Neuroscience, 5(2), 17. https://doi.org/10.3390/ctn5020017







