NeuroTec Sitem-Insel Bern: Closing the Last Mile in Neurology
Abstract
1. Introduction
2. Motion and Emotion—Digital Biomarkers for Closed-Loop Deep Brain Stimulation
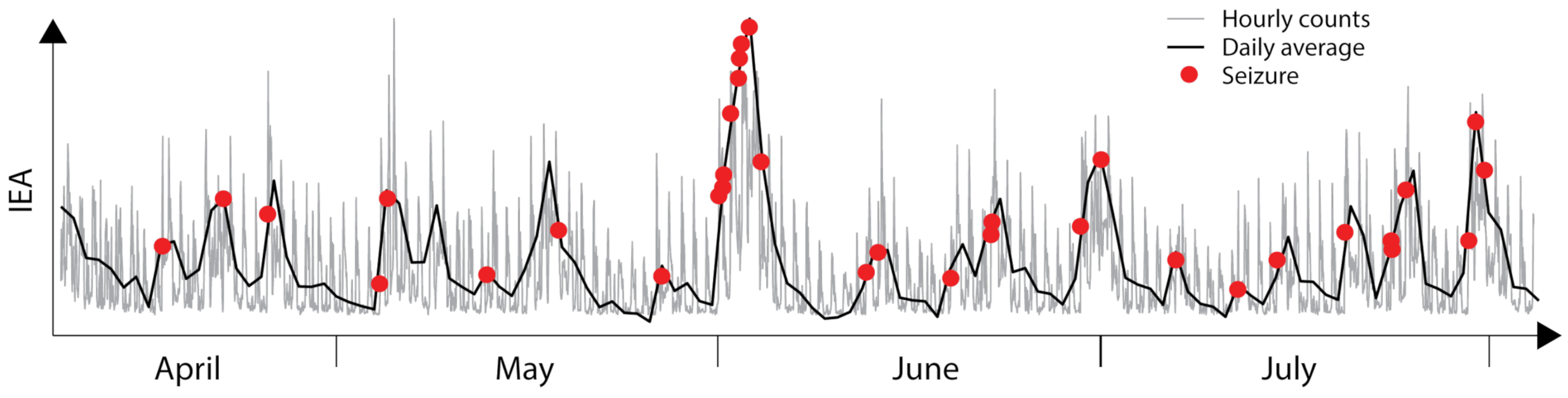
3. Personalized Chronotherapy Is Important for the Future of Epilepsy Care
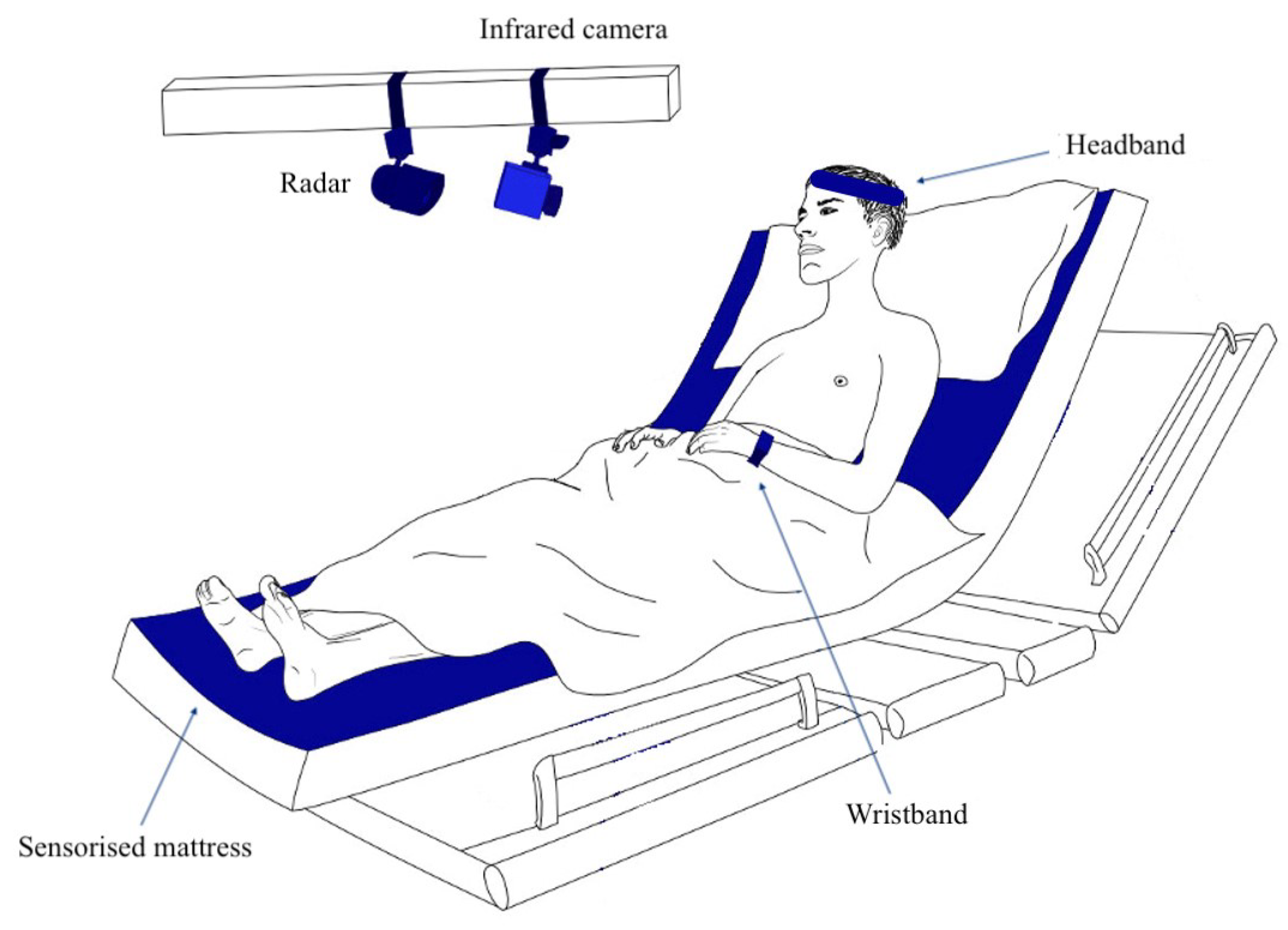
4. Detecting Primary Generalized Epileptic Seizures by Less Obtrusive Technology
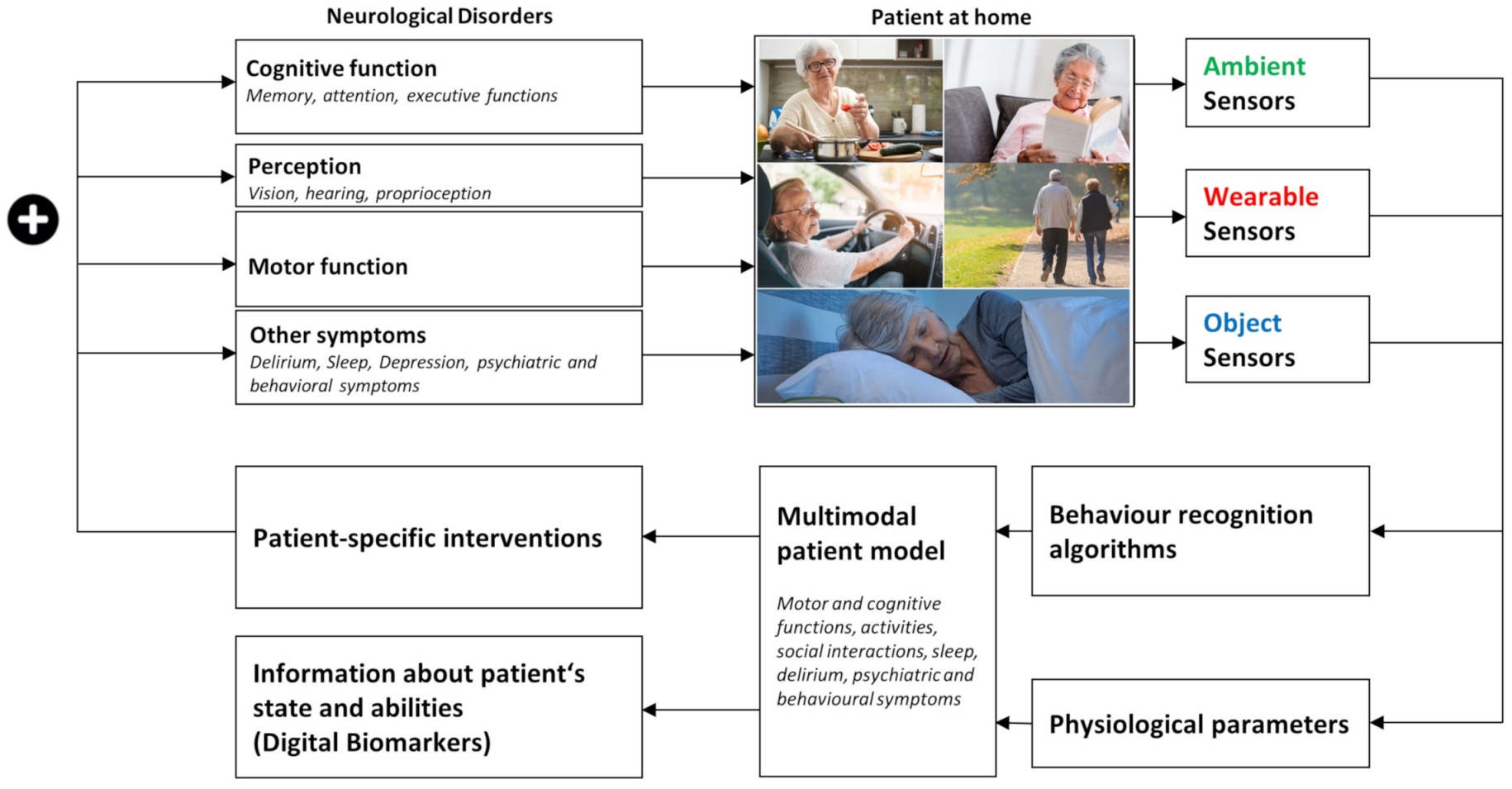
5. Monitoring Sleep at-Home
6. Computational Modeling and Machine Learning for Data Analysis
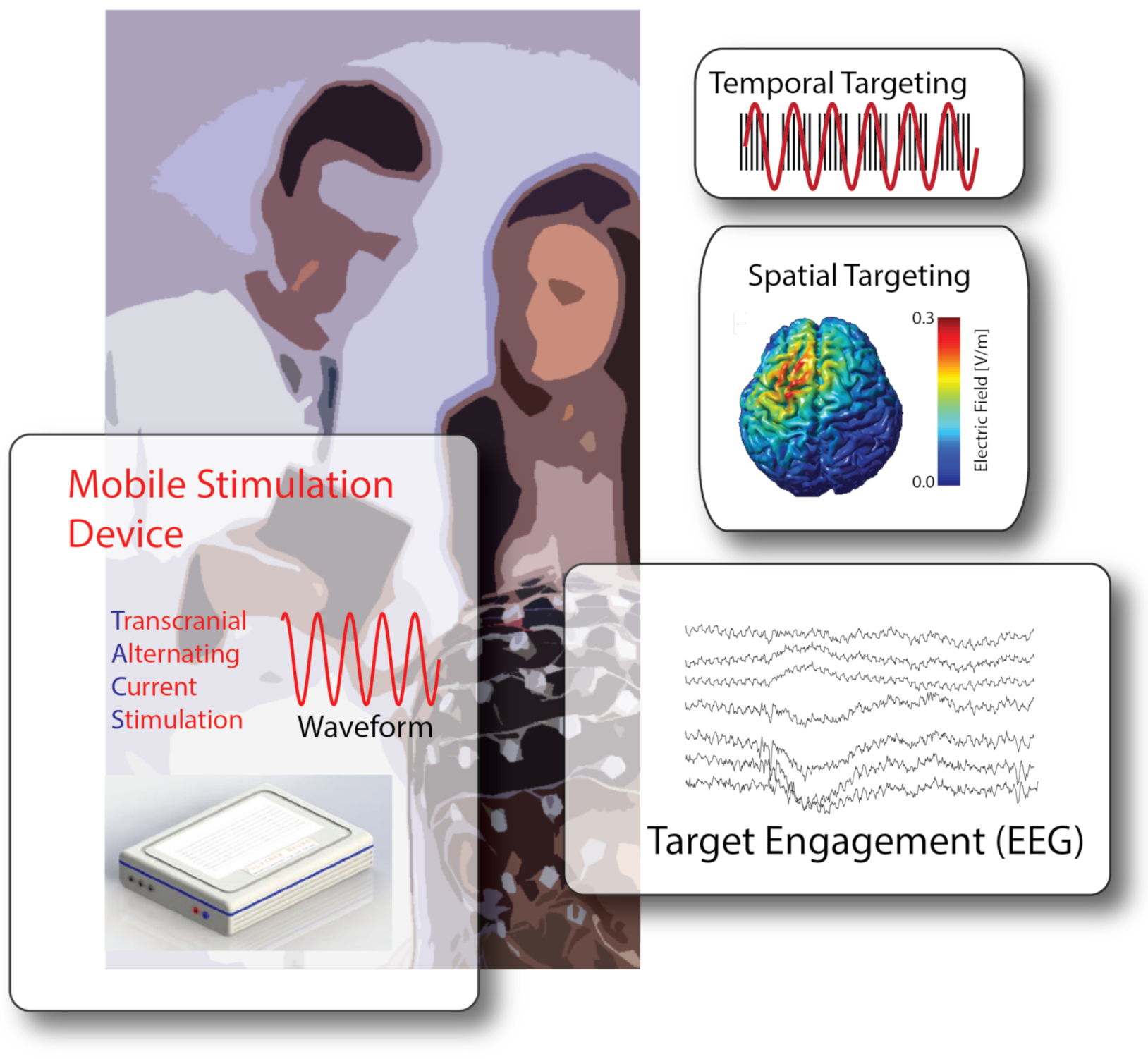
7. From Diagnostics to at-Home Therapies: The Case of Non-Invasive Brain Stimulation
8. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baud, M.O.; Proix, T.; Rao, V.R.; Schindler, K. Chance and risk in epilepsy. Curr. Opin. Neurol. 2020, 33, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Krack, P.; Volkmann, J.; Tinkhauser, G.; Deuschl, G. Deep brain stimulation in movement disorders: From experimental surgery to evidence-based therapy. Mov. Disord. 2019, 34, 1795–1810. [Google Scholar] [CrossRef]
- Torkildsen, Ø.; Linker, R.A.; Sesmero, J.M.; Fantaccini, S.; de la Rosa, R.S.; de Seze, J.; Duddy, M.; Chan, A. Living with secondary progressive multiple sclerosis in Europe: Perspectives of multiple stakeholders. Neurodegener. Dis. Manag. 2021, 11, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Tarnanas, I.; Laskaris, N.; Tsolaki, M.; Muri, R.; Nef, T.; Mosimann, U.P. On the comparison of a novel serious game and electroencephalography biomarkers for early dementia screening. In Advances in Experimental Medicine and Biology; Springer International Publishing: Berlin/Heidelberg, Germany, 2014; pp. 63–77. [Google Scholar] [CrossRef]
- Klaffke, S.; Staedt, J. Sundowning and circadian rhythm disorders in dementia. Acta Neurol. Belg. 2006, 106, 168–175. [Google Scholar] [PubMed]
- Burish, M.J.; Chen, Z.; Yoo, S.H. Cluster Headache Is in Part a Disorder of the Circadian System. JAMA Neurol. 2018, 75, 783. [Google Scholar] [CrossRef]
- Wehr, T.A. Bipolar mood cycles and lunar tidal cycles. Mol. Psychiatry 2018, 23, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Facchin, L.; Schöne, C.; Mensen, A.; Bandarabadi, M.; Pilotto, F.; Saxena, S.; Libourel, P.A.; Bassetti, C.L.; Adamantidis, A.R. Slow waves promote sleep-dependent plasticity and functional recovery after stroke. J. Neurosci. 2020, 40, 8637–8651. [Google Scholar] [CrossRef] [PubMed]
- Baud, M.O.; Kleen, J.K.; Mirro, E.A.; Andrechak, J.C.; King-Stephens, D.; Chang, E.F.; Rao, V.R. Multi-day rhythms modulate seizure risk in epilepsy. Nat. Commun. 2018, 9, 88. [Google Scholar] [CrossRef]
- Ten Brinke, T.R.; Odekerken, V.J.J.; Dijk, J.M.; van den Munckhof, P.; Schuurman, P.R.; de Bie, R.M.A. Directional Deep Brain Stimulation: First experiences in centers across the globe. Brain Stimul. 2018, 11, 949–950. [Google Scholar] [CrossRef] [PubMed]
- Meidahl, A.C.; Tinkhauser, G.; Herz, D.M.; Cagnan, H.; Debarros, J.; Brown, P. Adaptive Deep Brain Stimulation for Movement Disorders: The Long Road to Clinical Therapy. Mov. Disord. 2017, 32, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Tinkhauser, G.; Pogosyan, A.; Tan, H.; Herz, D.M.; Kühn, A.A.; Brown, P. Beta burst dynamics in Parkinson’s disease OFF and ON dopaminergic medication. Brain 2017, 140, 2968–2981. [Google Scholar] [CrossRef]
- Bouthour, W.; Mégevand, P.; Donoghue, J.; Lüscher, C.; Birbaumer, N.; Krack, P. Biomarkers for closed-loop deep brain stimulation in Parkinson disease and beyond. Nat. Rev. Neurol. 2019, 15, 343–352. [Google Scholar] [CrossRef]
- Little, S.; Brown, P. Debugging Adaptive Deep Brain Stimulation for Parkinson’s Disease. Mov. Disord. 2020, 35, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Ossig, C.; Antonini, A.; Buhmann, C.; Classen, J.; Csoti, I.; Falkenburger, B.; Schwarz, M.; Winkler, J.; Storch, A. Wearable sensor-based objective assessment of motor symptoms in Parkinson’s disease. J. Neural Transm. 2015, 123, 57–64. [Google Scholar] [CrossRef]
- Rovini, E.; Maremmani, C.; Cavallo, F. How wearable sensors can support Parkinson’s disease diagnosis and treatment: A systematic review. Front. Neurosci. 2017, 11, 555. [Google Scholar] [CrossRef] [PubMed]
- Schütz, N.; Saner, H.; Rudin, B.; Botros, A.; Pais, B.; Santschi, V.; Buluschek, P.; Gatica-Perez, D.; Urwyler, P.; Marchal-Crespo, L.; et al. Validity of pervasive computing based continuous physical activity assessment in community-dwelling old and oldest-old. Sci. Rep. 2019, 9, 9662. [Google Scholar] [CrossRef]
- Magiorkinis, E.; Sidiropoulou, K.; Diamantis, A. Hallmarks in the history of epilepsy: From antiquity till the twentieth century. In Novel Aspects on Epilepsy; InTech: London, UK, 2011. [Google Scholar] [CrossRef][Green Version]
- Elger, C.E.; Hoppe, C. Diagnostic challenges in epilepsy: Seizure under-reporting and seizure detection. Lancet Neurol. 2018, 17, 279–288. [Google Scholar] [CrossRef]
- Leguia, M.G.; Andrzejak, R.G.; Rummel, C.; Fan, J.M.; Mirro, E.A.; Tcheng, T.K.; Rao, V.R.; Baud, M.O. Seizure cycles in focal epilepsy. JAMA Neurol. 2021, 78, 454–463. [Google Scholar] [CrossRef]
- Proix, T.; Truccolo, W.; Leguia, M.G.; Tcheng, T.K.; King-Stephens, D.; Rao, V.R.; Baud, M.O. Forecasting seizure risk in adults with focal epilepsy: A development and validation study. Lancet Neurol. 2021, 20, 127–135. [Google Scholar] [CrossRef]
- Duun-Henriksen, J.; Baud, M.; Richardson, M.P.; Cook, M.; Kouvas, G.; Heasman, J.M.; Friedman, D.; Peltola, J.; Zibrandtsen, I.C.; Kjaer, T.W. A new era in electroencephalographic monitoring? Subscalp devices for ultra-long-term recordings. Epilepsia 2020, 61, 1805–1817. [Google Scholar] [CrossRef] [PubMed]
- Eichenwald, K. A Mind Unraveled: A Memoir; Ballantine Books: New York, NY, USA, 2018. [Google Scholar]
- Bassetti, C.L.; Ferini-Strambi, L.; Brown, S.; Adamantidis, A.; Benedetti, F.; Bruni, O.; Cajochen, C.; Dolenc-Groselj, L.; Ferri, R.; Gais, S.; et al. Neurology and psychiatry: Waking up to opportunities of sleep: State of the art and clinical/research priorities for the next decade. Eur. J. Neurol. 2015, 22, 1337–1354. [Google Scholar] [CrossRef]
- Leng, Y.; Musiek, E.S.; Hu, K.; Cappuccio, F.P.; Yaffe, K. Association between circadian rhythms and neurodegenerative diseases. Lancet Neurol. 2019, 18, 307–318. [Google Scholar] [CrossRef]
- Rakusa, M.; Sieminski, M.; Rakusa, S.; Falup-Pecurariu, C.; Fronzcek, R.; Hidalgo, H.; Muntean, M.L.; Pijpers, A.; Cochen De Cock, V.; Pizza, F.; et al. Awakening to sleep disorders in Europe: Survey on education, knowledge, and treatment competence of European residents and neurologists. Eur. J. Neurol. 2021. [Google Scholar] [CrossRef]
- Postuma, R.B.; Gagnon, J.F.; Vendette, M.; Montplaisir, J.Y. Markers of neurodegeneration in idiopathic rapid eye movement sleep behaviour disorder and Parkinson’s disease. Brain 2009, 132, 3298–3307. [Google Scholar] [CrossRef]
- Winer, J.R.; Mander, B.A.; Kumar, S.; Reed, M.; Baker, S.L.; Jagust, W.J.; Walker, M.P. Sleep disturbance forecasts β-amyloid accumulation across subsequent years. Curr. Biol. 2020, 30, 4291–4298. [Google Scholar] [CrossRef]
- Rundo, J.V.; Downey, R. Polysomnography. In Clinical Neurophysiology: Basis and Technical Aspects; Elsevier: Amsterdam, The Netherlands, 2019; pp. 381–392. [Google Scholar] [CrossRef]
- Mantua, J.; Gravel, N.; Spencer, R. Reliability of sleep measures from four personal health monitoring devices compared to research-based actigraphy and polysomnography. Sensors 2016, 16, 646. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.M.; Schade, M.M.; Mathew, G.M.; Gartenberg, D.; Buxton, O.M. Detecting sleep using heart rate and motion data from multisensor consumer-grade wearables, relative to wrist actigraphy and polysomnography. Sleep 2020, 43, zsaa045. [Google Scholar] [CrossRef]
- Massaroni, C.; Nicolò, A.; Presti, D.L.; Sacchetti, M.; Silvestri, S.; Schena, E. Contact-based methods for measuring respiratory rate. Sensors 2019, 19, 908. [Google Scholar] [CrossRef] [PubMed]
- Dietmann, A.; Wenz, E.; van der Meer, J.; Ringli, M.; Warncke, J.D.; Edwards, E.; Schmidt, M.H.; Bernasconi, C.A.; Nirkko, A.; Strub, M.; et al. The Swiss Primary Hypersomnolence and Narcolepsy Cohort study (SPHYNCS): Study protocol for a prospective, multicentre cohort observational study. J. Sleep Res. 2021, e13296. [Google Scholar] [CrossRef]
- Fereshtehnejad, S.M.; Yao, C.; Pelletier, A.; Montplaisir, J.Y.; Gagnon, J.F.; Postuma, R.B. Evolution of prodromal Parkinson’s disease and dementia with Lewy bodies: A prospective study. Brain 2019, 142, 2051–2067. [Google Scholar] [CrossRef] [PubMed]
- Musiek, E.S.; Bhimasani, M.; Zangrilli, M.A.; Morris, J.C.; Holtzman, D.M.; Ju, Y.S. Circadian Rest-Activity Pattern Changes in Aging and Preclinical Alzheimer Disease. JAMA Neurol. 2018, 75, 582–590. [Google Scholar] [CrossRef]
- Goodfellow, I.; Bengio, Y.; Courville, A. Deep Learning; MIT Press: Cambridge, MA, USA, 2016; Available online: http://www.deeplearningbook.org (accessed on 1 June 2021).
- Williams, J.M.; Samal, A.; Rao, P.K.; Johnson, M.R. Paired trial classification: A novel deep learning technique for MVPA. Front. Neurosci. 2020, 14, 417. [Google Scholar] [CrossRef]
- Mehrer, J.; Spoerer, C.J.; Kriegeskorte, N.; Kietzmann, T.C. Individual differences among deep neural network models. Nat. Commun. 2020, 11, 5725. [Google Scholar] [CrossRef]
- Tzovaras, B.G.; Tzovara, A. The Personal Data Is Political. In Philosophical Studies Series; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 133–140. [Google Scholar] [CrossRef]
- Tzovara, A.; Simonin, A.; Oddo, M.; Rossetti, A.O.; Lucia, M.D. Neural detection of complex sound sequences in the absence of consciousness. Brain 2015, 138, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Dietmann, A.; Gallino, C.; Wenz, E.; Mathis, J.; Bassetti, C.L.A. Multiple sleep latency test and polysomnography in patients with central disorders of hypersomnolence. Sleep Med. 2021, 79, 6–10. [Google Scholar] [CrossRef]
- Cossy, N.; Tzovara, A.; Simonin, A.; Rossetti, A.O.; Lucia, M.D. Robust discrimination between EEG responses to categories of environmental sounds in early coma. Front. Psychol. 2014, 5, 155. [Google Scholar] [CrossRef] [PubMed]
- Tzovara, A.; Rossetti, A.O.; Juan, E.; Suys, T.; Viceic, D.; Rusca, M.; Oddo, M.; Lucia, M.D. Prediction of awakening from hypothermic postanoxic coma based on auditory discrimination. Ann. Neurol. 2016, 79, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.L.; Kam, J.W.Y.; Tzovara, A.; Knight, R.T. Insights into human cognition from intracranial EEG: A review of audition, memory, internal cognition, and causality. J. Neural Eng. 2020, 17, 051001. [Google Scholar] [CrossRef]
- Schnakers, C.; Vanhaudenhuyse, A.; Giacino, J.; Ventura, M.; Boly, M.; Majerus, S.; Moonen, G.; Laureys, S. Diagnostic accuracy of the vegetative and minimally conscious state: Clinical consensus versus standardized neurobehavioral assessment. BMC Neurol. 2009, 9, 35. [Google Scholar] [CrossRef]
- Kempny, A.M.; James, L.; Yelden, K.; Duport, S.; Farmer, S.F.; Playford, E.D.; Leff, A.P. Patients with a severe prolonged disorder of consciousness can show classical EEG responses to their own name compared with others’ names. Neuroimage Clin. 2018, 19, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Bekinschtein, T.A.; Dehaene, S.; Rohaut, B.; Tadel, F.; Cohen, L.; Naccache, L. Neural signature of the conscious processing of auditory regularities. Proc. Natl. Acad. Sci. USA 2009, 106, 1672–1677. [Google Scholar] [CrossRef]
- Grootswagers, T.; Wardle, S.G.; Carlson, T.A. Decoding dynamic brain patterns from evoked responses: A tutorial on multivariate pattern analysis applied to time series neuroimaging data. J. Cogn. Neurosci. 2017, 29, 677–697. [Google Scholar] [CrossRef]
- Lucia, M.D.; Tzovara, A. Decoding auditory EEG responses in healthy and clinical populations: A comparative study. J. Neurosci. Methods 2015, 250, 106–113. [Google Scholar] [CrossRef]
- Antal, A.; Alekseichuk, I.; Bikson, M.; Brockmöller, J.; Brunoni, A.; Chen, R.; Cohen, L.; Dowthwaite, G.; Ellrich, J.; Flöel, A.; et al. Low intensity transcranial electric stimulation: Safety, ethical, legal regulatory and application guidelines. Clin. Neurophysiol. 2017, 128, 1774–1809. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Ugawa, Y. Adverse events of tDCS and tACS: A review. Clin. Neurophysiol. Pract. 2017, 2, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Kurmann, R.; Gast, H.; Schindler, K.; Fröhlich, F. Rational design of transcranial alternating current stimulation: Identification, engagement, and validation of network oscillations as treatment targets. Clin. Transl. Neurosci. 2018, 2, 2514183X18793515. [Google Scholar] [CrossRef]
- Thut, G.; Veniero, D.; Romei, V.; Miniussi, C.; Schyns, P.; Gross, J. Rhythmic TMS causes local entrainment of natural oscillatory signatures. Curr. Biol. 2011, 21, 1176–1185. [Google Scholar] [CrossRef]
- Antal, A.; Boros, K.; Poreisz, C.; Chaieb, L.; Terney, D.; Paulus, W. Comparatively weak after-effects of transcranial alternating current stimulation (tACS) on cortical excitability in humans. Brain Stimul. 2008, 1, 97–105. [Google Scholar] [CrossRef]
- Fröhlich, F. Endogenous and exogenous electric fields as modifiers of brain activity: Rational design of noninvasive brain stimulation with transcranial alternating current stimulation. Dialogues Clin. Neurosci. 2014, 16, 93. [Google Scholar]
- Ahn, S.; Mellin, J.M.; Alagapan, S.; Alexander, M.L.; Gilmore, J.H.; Jarskog, L.F.; Fröhlich, F. Targeting reduced neural oscillations in patients with schizophrenia by transcranial alternating current stimulation. Neuroimage 2019, 186, 126–136. [Google Scholar] [CrossRef]
- Alexander, M.L.; Alagapan, S.; Lugo, C.E.; Mellin, J.M.; Lustenberger, C.; Rubinow, D.R.; Fröhlich, F. Double-blind, randomized pilot clinical trial targeting alpha oscillations with transcranial alternating current stimulation (tACS) for the treatment of major depressive disorder (MDD). Transl. Psychiatry 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.M.; Sellers, K.K.; Fröhlich, F. Transcranial alternating current stimulation modulates large-scale cortical network activity by network resonance. J. Neurosci. 2013, 33, 11262–11275. [Google Scholar] [CrossRef] [PubMed]
- Force, R.B.; Riddle, J.; Jarskog, L.F.; Fröhlich, F. A case study of the feasibility of weekly tACS for the treatment of auditory hallucinations in schizophrenia. Brain Stimul. Basic Transl. Clin. Res. Neuromodul. 2021, 14, 361–363. [Google Scholar]
- Lustenberger, C.; Boyle, M.R.; Alagapan, S.; Mellin, J.M.; Vaughn, B.V.; Fröhlich, F. Feedback-controlled transcranial alternating current stimulation reveals a functional role of sleep spindles in motor memory consolidation. Curr. Biol. 2016, 26, 2127–2136. [Google Scholar] [CrossRef] [PubMed]
- Lam, A.D.; Noebels, J. Night watch on the titanic: Detecting early signs of epileptogenesis in Alzheimer Disease. Epilepsy Curr. 2020, 20, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Price, W.N.; Cohen, I.G. Privacy in the age of medical big data. Nat. Med. 2019, 25, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Leeming, G.; Cunningham, J.; Ainsworth, J. A Ledger of Me: Personalizing Healthcare Using Blockchain Technology. Front. Med. 2019, 6. [Google Scholar] [CrossRef]
- Wang, T.; Lu, Y.; Cao, Z.; Shu, L.; Zheng, X.; Liu, A.; Xie, M. When Sensor-Cloud Meets Mobile Edge Computing. Sensors 2019, 19, 5324. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Leng, Y.; Cheng, Y.; Wang, J. Secure data storage based on blockchain and coding in edge computing. Math. Biosci. Eng. 2019, 16, 1874–1892. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Warnecke, J.M.; Haghi, M.; Deserno, T.M. Unobtrusive Health Monitoring in Private Spaces: The Smart Vehicle. Sensors 2020, 20, 2442. [Google Scholar] [CrossRef] [PubMed]
- Strain, T.; Wijndaele, K.; Dempsey, P.C.; Sharp, S.J.; Pearce, M.; Jeon, J.; Lindsay, T.; Wareham, N.; Brage, S. Wearable-device-measured physical activity and future health risk. Nat. Med. 2020, 26, 1385–1391. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schindler, K.A.; Nef, T.; Baud, M.O.; Tzovara, A.; Yilmaz, G.; Tinkhauser, G.; Gerber, S.M.; Gnarra, O.; Warncke, J.D.; Schütz, N.; et al. NeuroTec Sitem-Insel Bern: Closing the Last Mile in Neurology. Clin. Transl. Neurosci. 2021, 5, 13. https://doi.org/10.3390/ctn5020013
Schindler KA, Nef T, Baud MO, Tzovara A, Yilmaz G, Tinkhauser G, Gerber SM, Gnarra O, Warncke JD, Schütz N, et al. NeuroTec Sitem-Insel Bern: Closing the Last Mile in Neurology. Clinical and Translational Neuroscience. 2021; 5(2):13. https://doi.org/10.3390/ctn5020013
Chicago/Turabian StyleSchindler, Kaspar A., Tobias Nef, Maxime O. Baud, Athina Tzovara, Gürkan Yilmaz, Gerd Tinkhauser, Stephan M. Gerber, Oriella Gnarra, Jan D. Warncke, Narayan Schütz, and et al. 2021. "NeuroTec Sitem-Insel Bern: Closing the Last Mile in Neurology" Clinical and Translational Neuroscience 5, no. 2: 13. https://doi.org/10.3390/ctn5020013
APA StyleSchindler, K. A., Nef, T., Baud, M. O., Tzovara, A., Yilmaz, G., Tinkhauser, G., Gerber, S. M., Gnarra, O., Warncke, J. D., Schütz, N., Knobel, S. E. J., Schmidt, M. H., Krack, P., Fröhlich, F., Sznitman, R., Rothen, S., & Bassetti, C. L. A. (2021). NeuroTec Sitem-Insel Bern: Closing the Last Mile in Neurology. Clinical and Translational Neuroscience, 5(2), 13. https://doi.org/10.3390/ctn5020013







