A SERS Substrate for Ultrafast Photosynthetic Au Nanoparticle Growth on WO3 Nanowires
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Tungsten Oxide
2.2. Preparation of Au/WO3
2.3. SERS Measurement
3. Results and Discussion
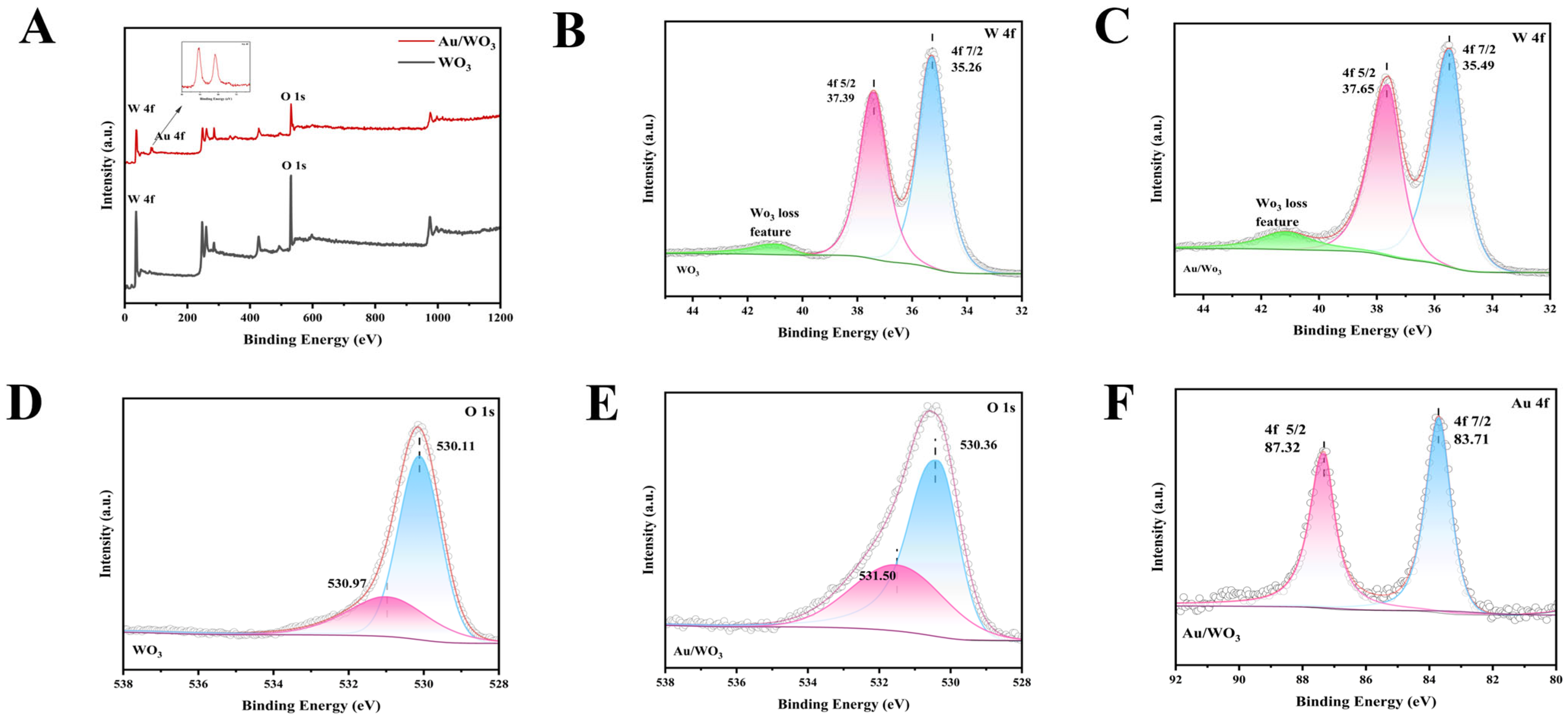
3.1. Morphology and Characterization of WO3 and Au/WO3
3.2. SERS Performance of WO3 and Au/WO3 SERS Substrates
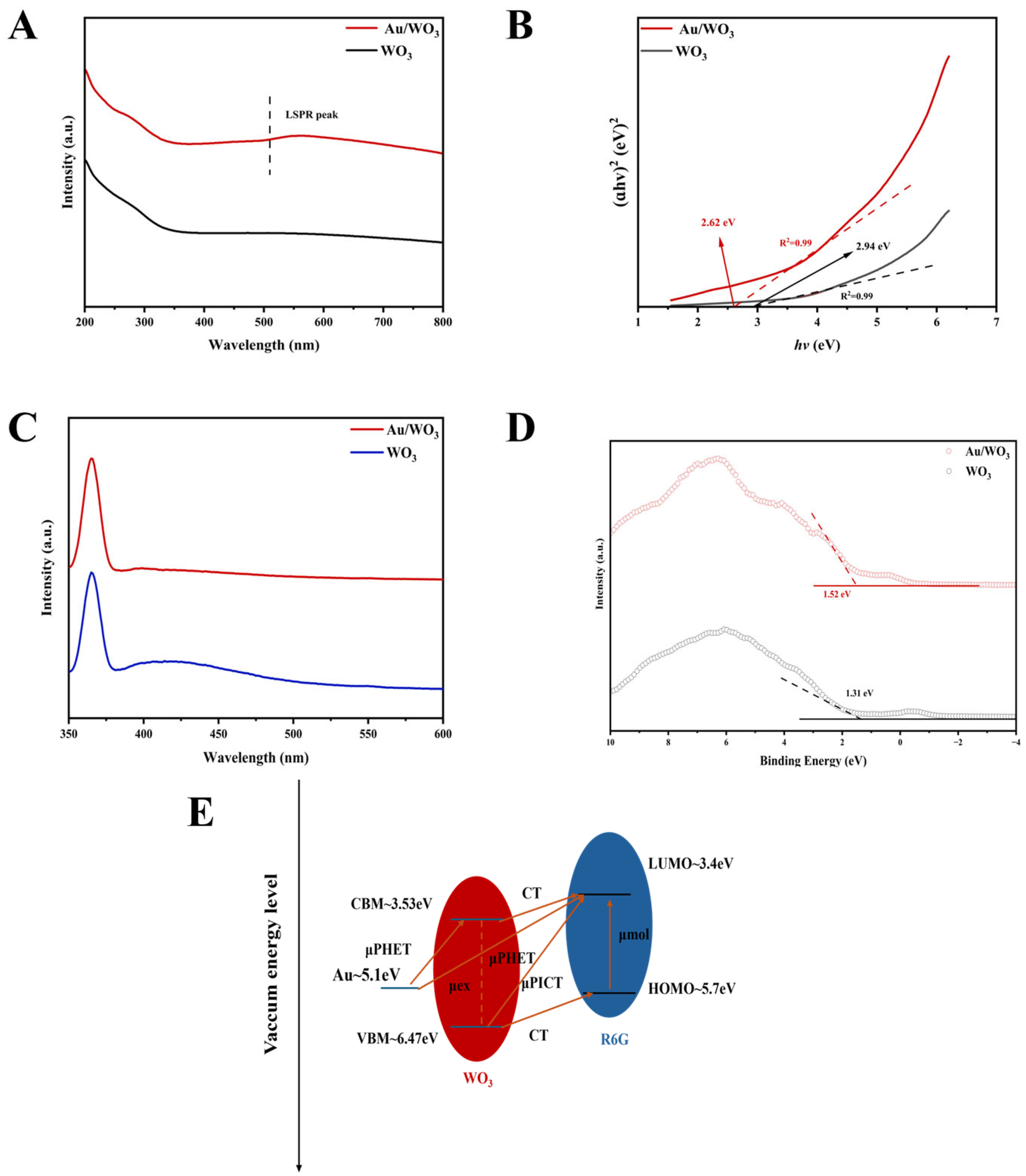
3.3. Mechanism Exploration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hassanain, W.A.; Izake, E.L. Toward Label-Free SERS Detection of Proteins through Their Disulfide Bond Structure. SLAS Discov. Adv. Sci. Drug Discov. 2020, 25, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Miao, Q.; Zhang, X.; Cui, P.; Wang, C.; Feng, Y.; Gan, L.; Fu, J.; Wang, S.; Dai, Z.; et al. Single-atom sites on perovskite chips for record-high sensitivity and quantification in SERS. Sci. China Mater. 2022, 65, 1601–1614. [Google Scholar] [CrossRef]
- Feng, R.; Fu, S.; Liu, H.; Wang, Y.; Liu, S.; Wang, K.; Chen, B.; Zhang, X.; Hu, L.; Chen, Q.; et al. Single-Atom Site SERS Chip for Rapid, Ultrasensitive, and Reproducible Direct-Monitoring of RNA Binding. Adv. Healthc. Mater. 2024, 13, e2301146. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Gao, Y.; Fang, Z.; Li, H.; Feng, R.; Wang, Y.; Feng, Y.; Li, W.; Zhang, S.; Hu, L.; et al. SERS detection for pesticide residue via a single-atom sites decoration strategy. Appl. Surf. Sci. 2023, 621, 156832. [Google Scholar] [CrossRef]
- Dai, X.; Fu, W.; Chi, H.; Mesias, V.S.D.; Zhu, H.; Leung, C.W.; Liu, W.; Huang, J. Optical tweezers-controlled hotspot for sensitive and reproducible surface-enhanced Raman spectroscopy characterization of native protein structures. Nat. Commun. 2021, 12, 1292. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Kim, T.; Cho, H.; Luo, J.; Lee, J.; Chueng, S.D.; Hou, Y.; Yin, P.T.; Han, J.; Kim, J.H.; et al. Hybrid Graphene-Gold Nanoparticle-Based Nucleic Acid Conjugates for Cancer-Specific Multimodal Imaging and Combined Therapeutics. Adv. Funct. Mater. 2020, 31, 2006918. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, J.; Xiao, L.; Zhao, X.; He, S.; Zhou, Y.; Liu, J.; Li, Y.; Peng, L.; Liu, W. Bio-inspired 3D Sub-Microstructures Coupling of Au Nanoparticles for SERS Detection of Trace Fentanyl. Adv. Funct. Mater. 2024, 34, 2411048. [Google Scholar] [CrossRef]
- Wu, L.; Tang, X.; Wu, T.; Zeng, W.; Zhu, X.; Hu, B.; Zhang, S. A review on current progress of Raman-based techniques in food safety: From normal Raman spectroscopy to SESORS. Food Res. Int. 2023, 169, 112944. [Google Scholar] [CrossRef]
- Afroozeh, A. A Review of Developed Surface-Enhanced Raman Spectroscopy (SERS)-Based Sensors for the Detection of Common Hazardous Substances in the Agricultural Industry. Plasmonics 2024, 20, 63–81. [Google Scholar] [CrossRef]
- Li, X.; Xu, C.; Yan, L.; Feng, Y.; Li, H.; Ye, C.; Zhang, M.; Jiang, C.; Li, J.; Wu, Y. A plasmonic AgNP decorated heterostructure substrate for synergetic surface-enhanced Raman scattering identification and quantification of pesticide residues in real samples. Anal. Methods 2022, 14, 3250–3259. [Google Scholar] [CrossRef]
- Ustun, O.; Yilmaz, A.; Yilmaz, M. Catalytic and SERS activities of WO3-based nanowires: The effect of oxygen vacancies, silver nanoparticle doping, and the type of organic dye. Phys. Chem. Chem. Phys. 2022, 24, 18615–18626. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Meng, S.; Liu, D.; Deng, Q.; Wang, C. In Situ SERS Monitoring of Schiff Base Reactions via Nanoparticles on a Mirror Platform. Catalysts 2024, 14, 803. [Google Scholar] [CrossRef]
- Han, X.X.; Rodriguez, R.S.; Haynes, C.L.; Ozaki, Y.; Zhao, B. Surface-enhanced Raman spectroscopy. Nat. Rev. Methods Prim. 2021, 1, 87. [Google Scholar] [CrossRef]
- Zhan, C.; Chen, X.-J.; Yi, J.; Li, J.-F.; Wu, D.-Y.; Tian, Z.-Q. From plasmon-enhanced molecular spectroscopy to plasmon-mediated chemical reactions. Nat. Rev. Chem. 2018, 2, 216–230. [Google Scholar] [CrossRef]
- Ding, S.-Y.; You, E.-M.; Tian, Z.-Q.; Moskovits, M. Electromagnetic theories of surface-enhanced Raman spectroscopy. Chem. Soc. Rev. 2017, 46, 4042–4076. [Google Scholar] [CrossRef] [PubMed]
- Alessandri, I.; Lombardi, J.R. Enhanced Raman Scattering with Dielectrics. Chem. Rev. 2016, 116, 14921–14981. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Shao, X.; Gu, X.; Wang, K.; Ning, X.; Xia, J.; Xie, M.; Tang, Y.; Li, Q.; Tian, S. CuO@AgNPs nanozyme cavity arrays on screen-printed electrodes for ultrasensitive and on-site SERS detection. Chem. Eng. J. 2023, 471, 144522. [Google Scholar] [CrossRef]
- Wang, X.; Shi, W.; Jin, Z.; Huang, W.; Lin, J.; Ma, G.; Li, S.; Guo, L. Remarkable SERS Activity Observed from Amorphous ZnO Nanocages. Angew. Chem. Int. Ed. Engl. 2017, 56, 9851–9855. [Google Scholar] [CrossRef]
- Estevez, M.; Alvarez, M.; Lechuga, L. Integrated optical devices for lab-on-a-chip biosensing applications. Laser Photon-Rev. 2011, 6, 463–487. [Google Scholar] [CrossRef]
- Simas, M.V.; Davis, G.A.; Hati, S.; Pu, J.; Goodpaster, J.V.; Sardar, R. Anisotropically Shaped Plasmonic WO3−x Nanostructure-Driven Ultrasensitive SERS Detection and Machine Learning-Based Differentiation of Nitro-Explosives. ACS Appl. Mater. Interfaces 2025, 17, 11309–11324. [Google Scholar] [CrossRef]
- Lombardi, J.R.; Birke, R.L. Theory of Surface-Enhanced Raman Scattering in Semiconductors. J. Phys. Chem. C 2014, 118, 11120–11130. [Google Scholar] [CrossRef]
- Park, W.-H.; Kim, Z.H. Charge Transfer Enhancement in the SERS of a Single Molecule. Nano Lett. 2010, 10, 4040–4048. [Google Scholar] [CrossRef]
- Wang, X.; Guo, L. SERS Activity of Semiconductors: Crystalline and Amorphous Nanomaterials. Angew. Chem. Int. Ed. Engl. 2019, 59, 4231–4239. [Google Scholar] [CrossRef]
- Li, Q.; Wang, J.; Ding, Q.; Chen, M.; Ma, F. Coupling effect on charge-transfer mechanism of surface-enhanced resonance Raman scattering. J. Raman Spectrosc. 2017, 48, 560–569. [Google Scholar] [CrossRef]
- Langer, J.; Jimenez de Aberasturi, D.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and Future of Surface-Enhanced Raman Scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef]
- Yang, B.; Jin, S.; Guo, S.; Park, Y.; Chen, L.; Zhao, B.; Jung, Y.M. Recent Development of SERS Technology: Semiconductor-Based Study. ACS Omega 2019, 4, 20101–20108. [Google Scholar] [CrossRef] [PubMed]
- Perala, R.S.; Chandrasekar, N.; Balaji, R.; Alexander, P.S.; Humaidi, N.Z.N.; Hwang, M.T. A comprehensive review on graphene-based materials: From synthesis to contemporary sensor applications. Mater. Sci. Eng. R Rep. 2024, 159, 100805. [Google Scholar] [CrossRef]
- Khyzhun, O.; Solonin, Y.; Dobrovolsky, V. Electronic structure of hexagonal tungsten trioxide: XPS, XES, and XAS studies. J. Alloys Compd. 2001, 320, 1–6. [Google Scholar] [CrossRef]
- Zou, J.-W.; Li, Z.-D.; Kang, H.-S.; Zhao, W.-Q.; Liu, J.-C.; Chen, Y.-L.; Ma, L.; Hou, H.-Y.; Ding, S.-J. Strong Visible Light Absorption and Abundant Hotspots in Au-Decorated WO3 Nanobricks for Efficient SERS and Photocatalysis. ACS Omega 2021, 6, 28347–28355. [Google Scholar] [CrossRef]
- Yu, J.; Chen, C.; Zhang, Q.; Lin, J.; Yang, X.; Gu, L.; Zhang, H.; Liu, Z.; Wang, Y.; Zhang, S.; et al. Au Atoms Anchored on Amorphous C3N4 for Single-Site Raman Enhancement. J. Am. Chem. Soc. 2022, 144, 21908–21915. [Google Scholar] [CrossRef]
- Chen, B.; Fan, L.; Li, C.; Xia, L.; Wang, K.; Wang, J.; Pang, D.; Zhu, Z.; Ma, P. Au nanoparticles decorated β-Bi2O3 as highly-sensitive SERS substrate for detection of methylene blue and methyl orange. Analyst 2024, 149, 4283–4294. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, H.; Tian, Y.; Jiao, A.; Chen, F.; Chen, M. Photochemical synthesis of ZnO@Au nanorods as an advanced reusable SERS substrate for ultrasensitive detection of light-resistant organic pollutant in wastewater. Talanta 2019, 194, 680–688. [Google Scholar] [CrossRef]
- Huang, J.; Ma, D.; Chen, F.; Chen, D.; Bai, M.; Xu, K.; Zhao, Y. Green in Situ Synthesis of Clean 3D Chestnutlike Ag/WO3−x Nanostructures for Highly Efficient, Recyclable and Sensitive SERS Sensing. ACS Appl. Mater. Interfaces 2017, 9, 7436–7446. [Google Scholar] [CrossRef]
- Zhai, Y.; DuChene, J.S.; Wang, Y.-C.; Qiu, J.; Johnston-Peck, A.C.; You, B.; Guo, W.; DiCiaccio, B.; Qian, K.; Zhao, E.W.; et al. Polyvinylpyrrolidone-induced anisotropic growth of gold nanoprisms in plasmon-driven synthesis. Nat. Mater. 2016, 15, 889–895. [Google Scholar] [CrossRef]
- Uboldi, C.; Bonacchi, D.; Lorenzi, G.; Hermanns, M.I.; Pohl, C.; Baldi, G.; E Unger, R.; Kirkpatrick, C.J. Gold nanoparticles induce cytotoxicity in the alveolar type-II cell lines A549 and NCIH441. Part. Fibre Toxicol. 2009, 6, 18. [Google Scholar] [CrossRef]
- Lopez-Sanchez, J.A.; Dimitratos, N.; Hammond, C.; Brett, G.L.; Kesavan, L.; White, S.; Miedziak, P.; Tiruvalam, R.; Jenkins, R.L.; Carley, A.F.; et al. Facile removal of stabilizer-ligands from supported gold nanoparticles. Nat. Chem. 2011, 3, 551–556. [Google Scholar] [CrossRef]
- Amendola, V.; Litti, L.; Meneghetti, M. LDI-MS Assisted by Chemical-Free Gold Nanoparticles: Enhanced Sensitivity and Reduced Background in the Low-Mass Region. Anal. Chem. 2013, 85, 11747–11754. [Google Scholar] [CrossRef]
- Ma, S.; Xue, J.; Zhou, Y.; Zhang, Z. Photochemical synthesis of ZnO/Ag2O heterostructures with enhanced ultraviolet and visible photocatalytic activity. J. Mater. Chem. A 2014, 2, 7272–7280. [Google Scholar] [CrossRef]
- Ameer, F.S.; Pittman, C.U.; Zhang, D. Quantification of Resonance Raman Enhancement Factors for Rhodamine 6G (R6G) in Water and on Gold and Silver Nanoparticles: Implications for Single-Molecule R6G SERS. J. Phys. Chem. C 2013, 117, 27096–27104. [Google Scholar] [CrossRef]
- Zheng, Q.; Fu, Y.-C.; Xu, J.-Q. Advances in the chemical sensors for the detection of DMMP—A simulant for nerve agent sarin. Procedia Eng. 2010, 7, 179–184. [Google Scholar] [CrossRef]
- Bo, X.; Tang, A.; Dou, M.; Li, Z.; Wang, F. Controllable electrodeposition and mechanism research of nanostructured Bi2Te3 thin films with high thermoelectric properties. Appl. Surf. Sci. 2019, 486, 65–71. [Google Scholar] [CrossRef]
- Lin, N.; Lin, Y.; Qian, X.; Wang, X.; Su, W. Construction of a 2D/2D WO3/LaTiO2N Direct Z-Scheme Photocatalyst for Enhanced CO2 Reduction Performance Under Visible Light. ACS Sustain. Chem. Eng. 2021, 9, 13686–13694. [Google Scholar] [CrossRef]
- Dokou, E.; Stangland, E.E.; Andres, R.P.; Delgass, W.N.; Barteau, M.A. Comparison of AFM and HRTEM to determine the metal particle morphology and loading of an Au/TiO2 catalyst. Catal. Lett. 2000, 70, 1–7. [Google Scholar] [CrossRef]
- Sahu, B.K.; Dwivedi, A.; Pal, K.K.; Pandian, R.; Dhara, S.; Das, A. Optimized Au NRs for efficient SERS and SERRS performances with molecular and longitudinal surface plasmon resonance. Appl. Surf. Sci. 2021, 537, 147615. [Google Scholar] [CrossRef]
- Kim, N.H.; Kim, K. Surface-enhanced resonance Raman scattering of rhodamine 6G on Pt nanoaggregates. J. Raman Spectrosc. 2005, 36, 623–628. [Google Scholar] [CrossRef]
- Taranenko, N.; Alarie, J.-P.; Stokes, D.L.; Vo-Dinh, T. Surface-Enhanced Raman Detection of Nerve Agent Simulant (DMMP and DIMP) Vapor on Electrochemically Prepared Silver Oxide Substrates. J. Raman Spectrosc. 1996, 27, 379–384. [Google Scholar] [CrossRef]
- Kim, S.; Park, S.; Park, S.; Lee, C. Acetone sensing of Au and Pd-decorated WO3 nanorod sensors. Sens. Actuators B Chem. 2015, 209, 180–185. [Google Scholar] [CrossRef]
- Foster, A.S.; Gejo, F.L.; Shluger, A.L.; Nieminen, R.M. Vacancy and interstitial defects in hafnia. Phys. Rev. B 2002, 65, 174117. [Google Scholar] [CrossRef]
- Cong, S.; Yuan, Y.; Chen, Z.; Hou, J.; Yang, M.; Su, Y.; Zhang, Y.; Li, L.; Li, Q.; Geng, F.; et al. Noble metal-comparable SERS enhancement from semiconducting metal oxides by making oxygen vacancies. Nat. Commun. 2015, 6, 7800. [Google Scholar] [CrossRef]
- Liu, H.; Chen, L.; Li, B.; Song, H.; Tan, C.L.; Shi, Y.; Yan, S. Semiconducting Tungsten Trioxide Thin Films for High-Performance SERS Biosensors. Nanomaterials 2025, 15, 1393. [Google Scholar] [CrossRef]
- McMillan, K.S.; McCluskey, A.G.; Sorensen, A.; Boyd, M.; Zagnoni, M. Emulsion technologies for multicellular tumour spheroid radiation assays. Analyst 2015, 141, 100–110. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, L.; Wang, S.; Yu, Y.; Lei, D. Oxygen Vacancies Enhance SERS Performance of Tungsten-Doped Vanadium Dioxide Nanoparticles. Adv. Mater. Technol. 2024, 10, 2401304. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, R.; Yadav, R.M. Polyacrylonitrile as a versatile matrix for gold nanoparticle-based SERS substrates. Nanoscale Adv. 2024, 6, 1065–1073. [Google Scholar] [CrossRef]
- Yu, J.; Chen, C.; Lin, J.; Meng, X.; Qiu, L.; Wang, X. Amorphous Co(OH)2 nanocages achieving efficient photo-induced charge transfer for significant SERS activity. J. Mater. Chem. C 2022, 10, 1632–1637. [Google Scholar] [CrossRef]
- Wei, W.; Yao, Y.; Zhao, Q.; Xu, Z.; Wang, Q.; Zhang, Z.; Gao, Y. Oxygen defect-induced localized surface plasmon resonance at the WO3−x quantum dot/silver nanowire interface: SERS and photocatalysis. Nanoscale 2019, 11, 5535–5547. [Google Scholar] [CrossRef]
- Sneed, B.T.; Young, A.P.; Tsung, C.-K. Building up strain in colloidal metal nanoparticle catalysts. Nanoscale 2015, 7, 12248–12265. [Google Scholar] [CrossRef]
- Ji, X.; Song, X.; Li, J.; Bai, Y.; Yang, W.; Peng, X. Size Control of Gold Nanocrystals in Citrate Reduction: The Third Role of Citrate. J. Am. Chem. Soc. 2007, 129, 13939–13948. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Bai, H.; Li, X.; Li, W.; Zhai, J.; Li, J.; Xi, G. Improved Surface-Enhanced Raman Spectroscopy Sensitivity on Metallic Tungsten Oxide by the Synergistic Effect of Surface Plasmon Resonance Coupling and Charge Transfer. J. Phys. Chem. Lett. 2018, 9, 4096–4100. [Google Scholar] [CrossRef]
- Sun, J.; Hu, H.; Zheng, D.; Zhang, D.; Deng, Q.; Zhang, S.; Xu, H. Light-Emitting Plexciton: Exploiting Plasmon–Exciton Interaction in the Intermediate Coupling Regime. ACS Nano 2018, 12, 10393–10402. [Google Scholar] [CrossRef]
- Yang, S.; Yao, J.; Quan, Y.; Hu, M.; Su, R.; Gao, M.; Han, D.; Yang, J. Monitoring the charge-transfer process in a Nd-doped semiconductor based on photoluminescence and SERS technology. Light Sci. Appl. 2020, 9, 117. [Google Scholar] [CrossRef]
- Wang, X.-J.; Tian, X.; Sun, Y.-J.; Zhu, J.-Y.; Li, F.-T.; Mu, H.-Y.; Zhao, J. Enhanced Schottky effect of a 2D–2D CoP/g-C3N4 interface for boosting photocatalytic H2 evolution. Nanoscale 2018, 10, 12315–12321. [Google Scholar] [CrossRef] [PubMed]
- Handoko, A.D.; Steinmann, S.N.; Seh, Z.W. Theory-guided materials design: Two-dimensional MXenes in electro- and photocatalysis. Nanoscale Horiz. 2019, 4, 809–827. [Google Scholar] [CrossRef]
- Hung, S.-F.; Xu, A.; Wang, X.; Li, F.; Hsu, S.-H.; Li, Y.; Wicks, J.; Cervantes, E.G.; Rasouli, A.S.; Li, Y.C.; et al. A Metal-Supported Single-Atom Catalytic Site Enables Carbon Dioxide Hydrogenation. Nat. Commun. 2022, 13, 819. [Google Scholar] [CrossRef]
- Lei, Z.; Zhang, X.; Zhao, Y.; Wei, A.; Tao, L.; Yang, Y.; Zheng, Z.; Tao, L.; Yu, P.; Li, J. Enhanced Raman Scattering on Two-Dimensional Palladium Diselenide. Nanoscale 2022, 14, 4181–4187. [Google Scholar] [CrossRef]
- Yang, L.; Peng, Y.; Yang, Y.; Liu, J.; Li, Z.; Ma, Y.; Zhang, Z.; Wei, Y.; Li, S.; Huang, Z.; et al. Green and Sensitive Flexible Semiconductor SERS Substrates: Hydrogenated Black TiO2 Nanowires. ACS Appl. Nano Mater. 2018, 1, 4516–4527. [Google Scholar] [CrossRef]
- Lin, J.; Shang, Y.; Li, X.; Yu, J.; Wang, X.; Guo, L. Ultrasensitive SERS Detection by Defect Engineering on Single Cu2O Superstructure Particle. Adv. Mater. 2017, 29, 1604797. [Google Scholar] [CrossRef]
- Zheng, X.; Zhong, H.; Wang, Z.; Li, J.; Hu, Y.; Li, H.; Jia, J.; Zhang, S.; Ren, F. Fabrication of Stable Substoichiometric WOx Films with High SERS Sensitivity by Thermal Treatment. Vacuum 2022, 198, 110884. [Google Scholar] [CrossRef]
- Sun, Z.; Gao, Y.; Ban, C.; Meng, J.; Wang, J.; Wang, K.; Gan, L. 3 Nm-Wide WO3−x Nanorods with Abundant Oxygen Vacancies as Substrates for High-Sensitivity SERS Detection. ACS Appl. Nano Mater. 2023, 6, 8635–8642. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, S.; Deng, Q.; Zhang, L.; Feng, Y.; Fan, L.; Liu, Y.; Liu, D.; Wang, C. A SERS Substrate for Ultrafast Photosynthetic Au Nanoparticle Growth on WO3 Nanowires. Colloids Interfaces 2025, 9, 70. https://doi.org/10.3390/colloids9050070
Meng S, Deng Q, Zhang L, Feng Y, Fan L, Liu Y, Liu D, Wang C. A SERS Substrate for Ultrafast Photosynthetic Au Nanoparticle Growth on WO3 Nanowires. Colloids and Interfaces. 2025; 9(5):70. https://doi.org/10.3390/colloids9050070
Chicago/Turabian StyleMeng, Shiyong, Qingsong Deng, Lin Zhang, Yibo Feng, Lei Fan, Yuxin Liu, Danmin Liu, and Cong Wang. 2025. "A SERS Substrate for Ultrafast Photosynthetic Au Nanoparticle Growth on WO3 Nanowires" Colloids and Interfaces 9, no. 5: 70. https://doi.org/10.3390/colloids9050070
APA StyleMeng, S., Deng, Q., Zhang, L., Feng, Y., Fan, L., Liu, Y., Liu, D., & Wang, C. (2025). A SERS Substrate for Ultrafast Photosynthetic Au Nanoparticle Growth on WO3 Nanowires. Colloids and Interfaces, 9(5), 70. https://doi.org/10.3390/colloids9050070





