Two-Dimensional Materials for Selective Ion Transport Membrane: Synthesis and Application Advances
Abstract
1. Introduction
2. Materials and Preparation Methods
2.1. Chemical Vapor Deposition

2.2. Interface Synthesis

2.3. Solution Synthesis

2.4. Exfoliation

3. Application of 2D Materials
3.1. Desalination Seawater
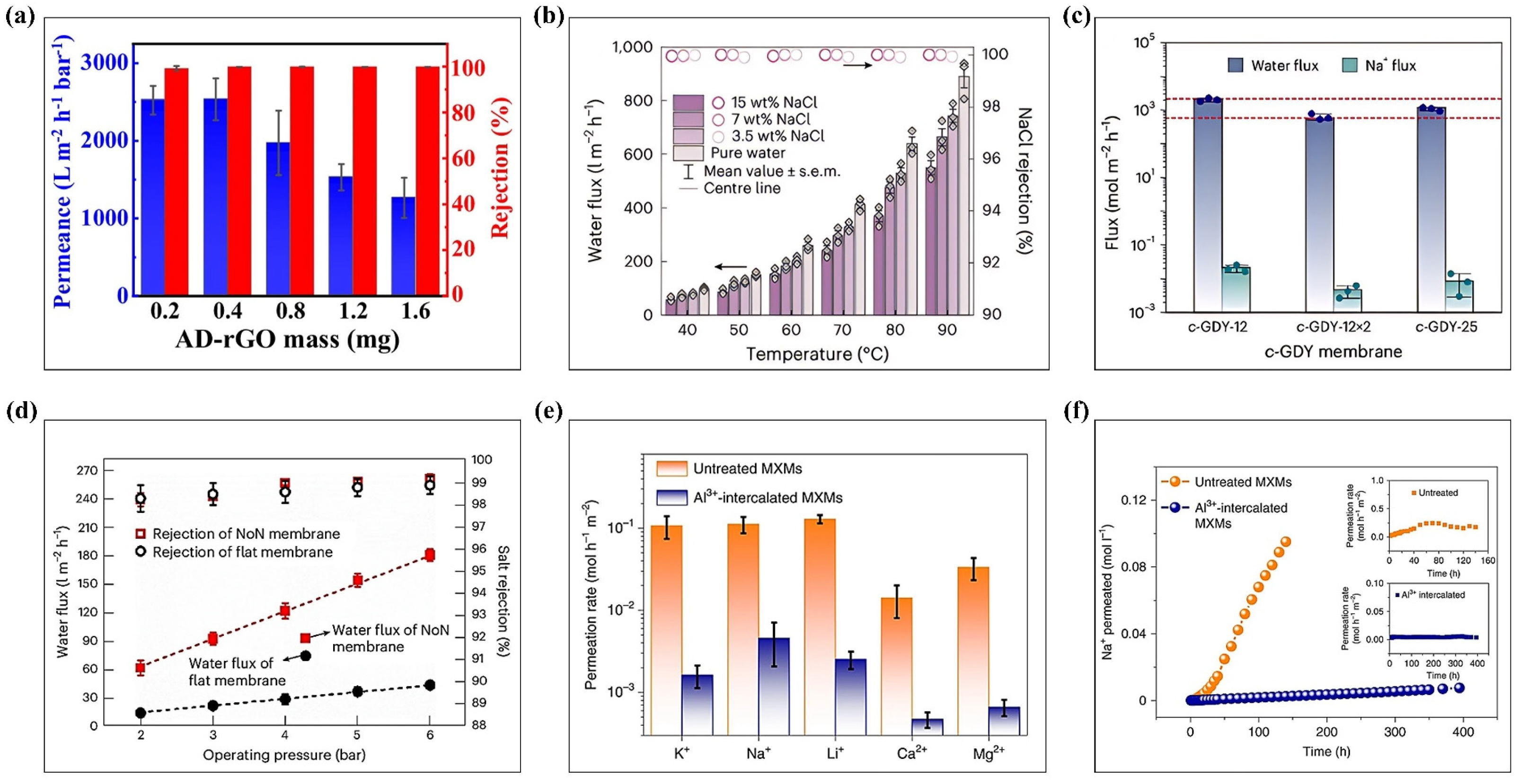
3.2. High-Value Ion Separation

3.3. Osmotic Energy Conversion
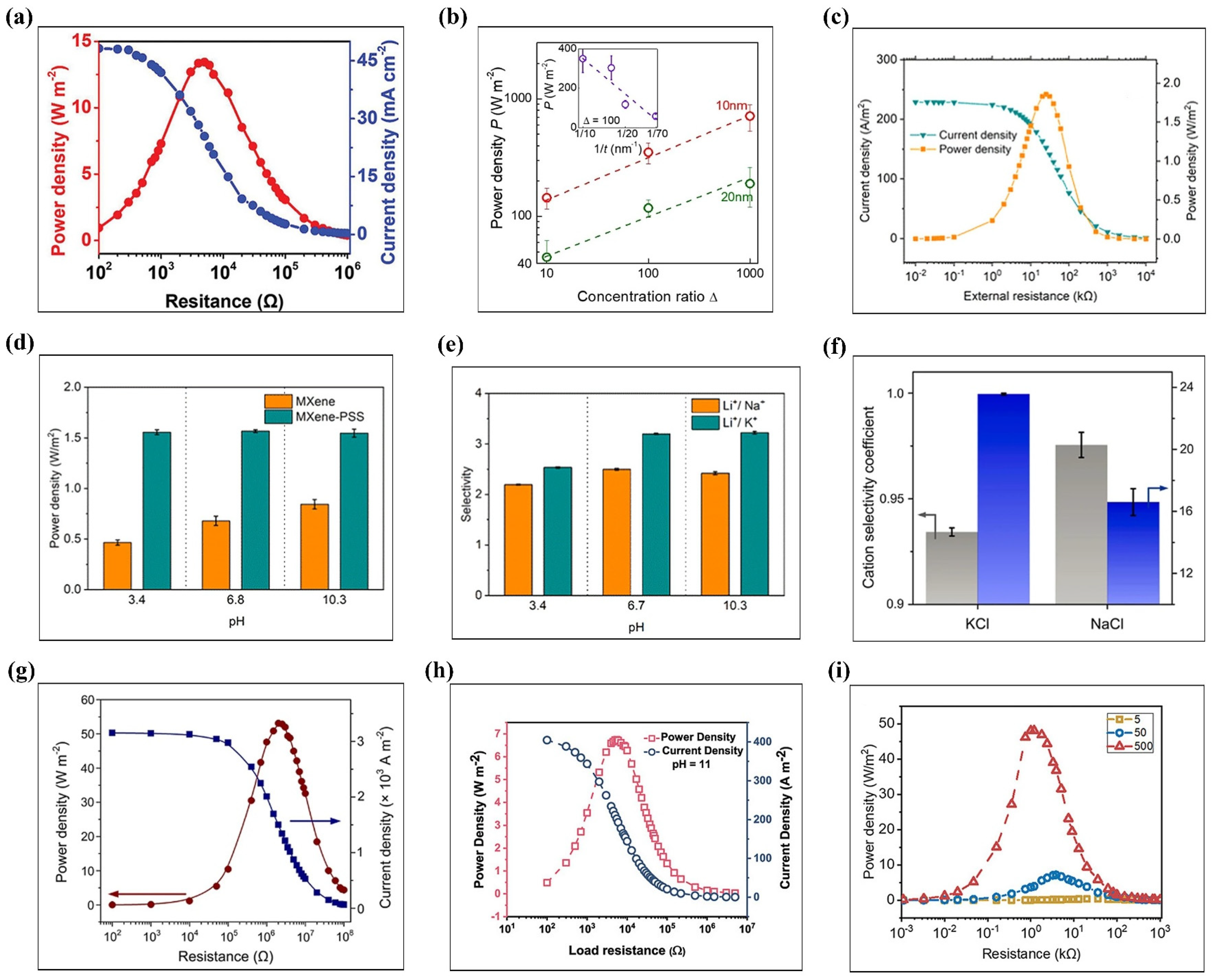
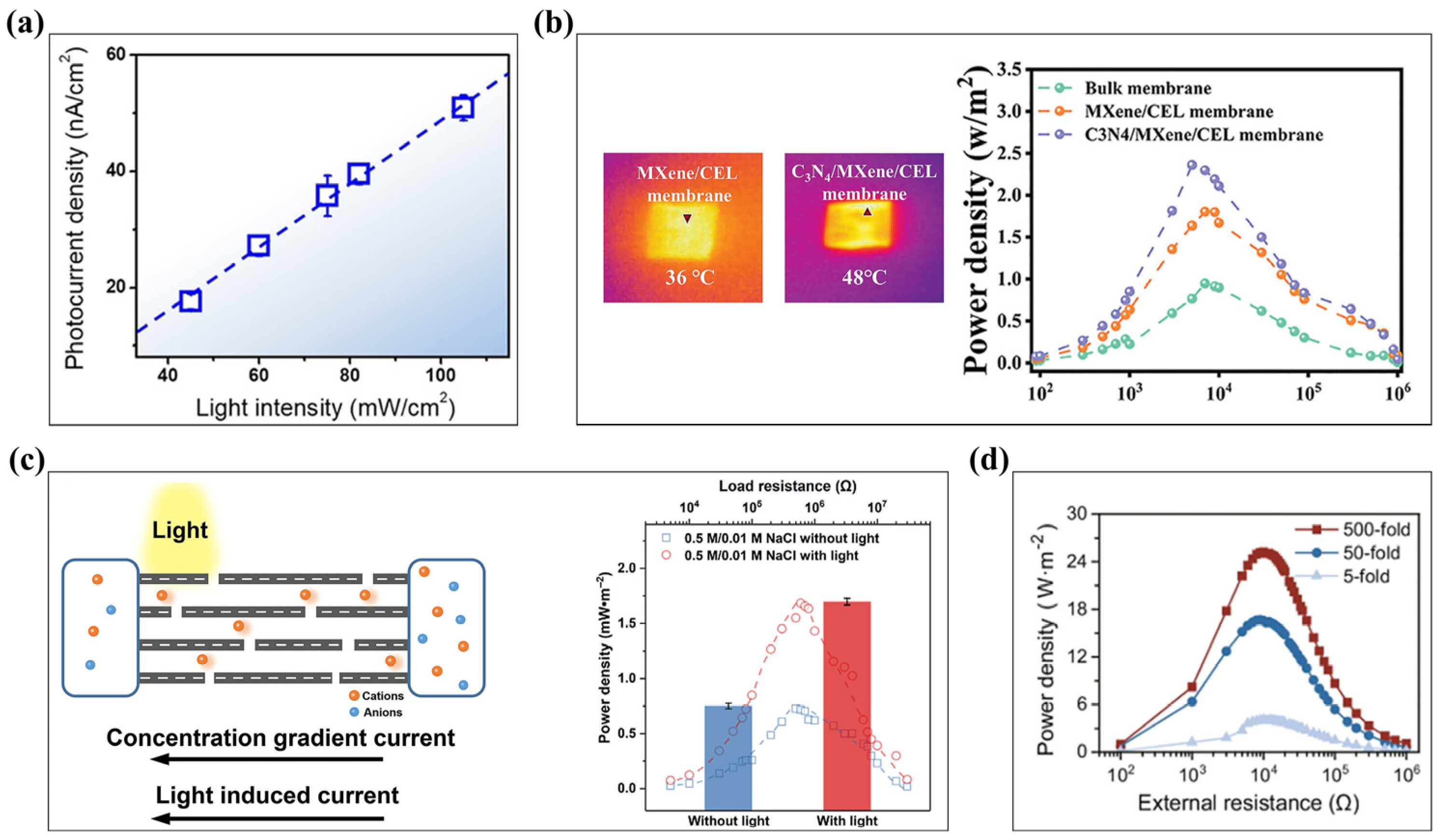
3.4. Proton Conduction in Energy Applications
3.4.1. Proton Conductivity
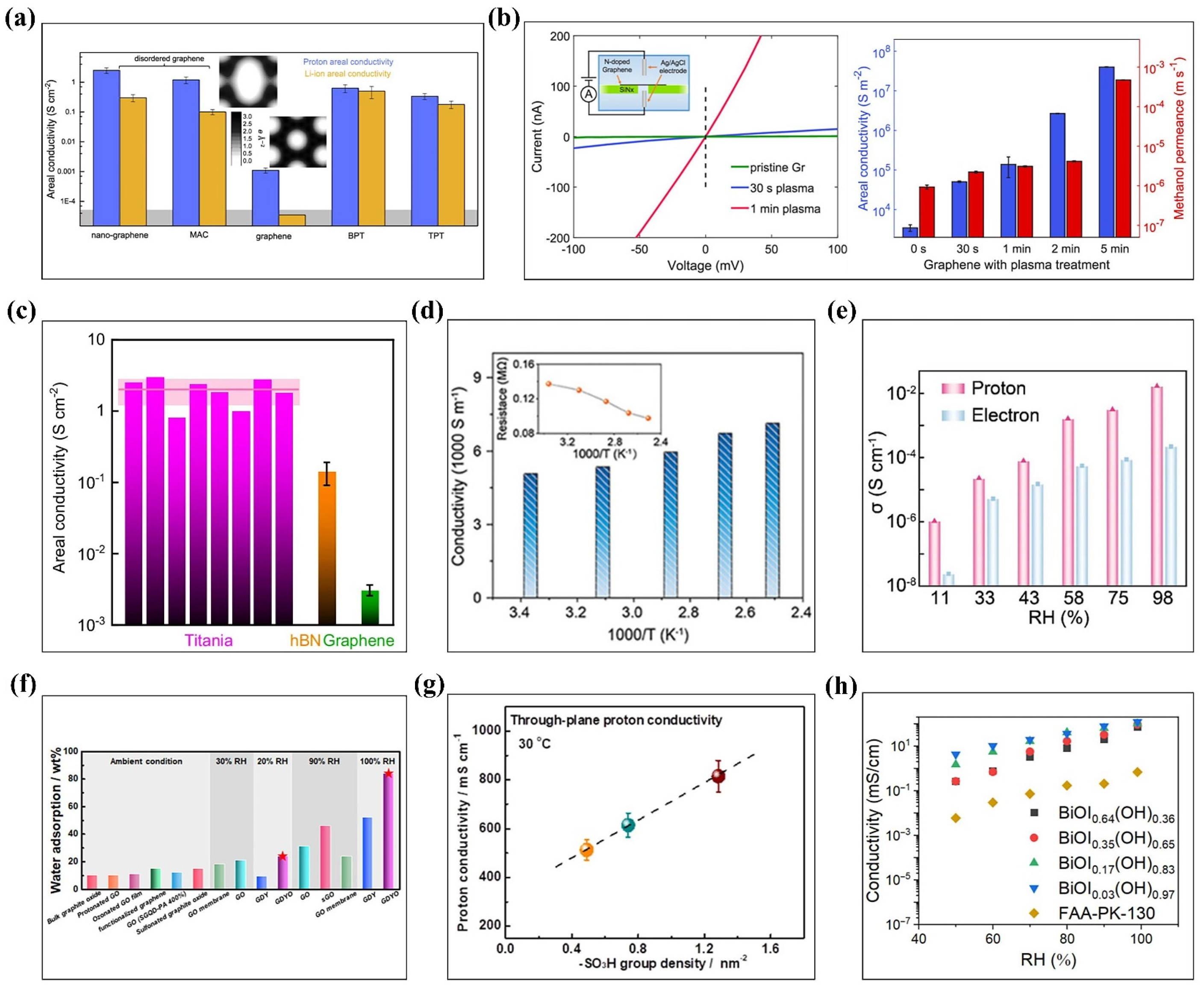
3.4.2. Direct Methanol Fuel Cells
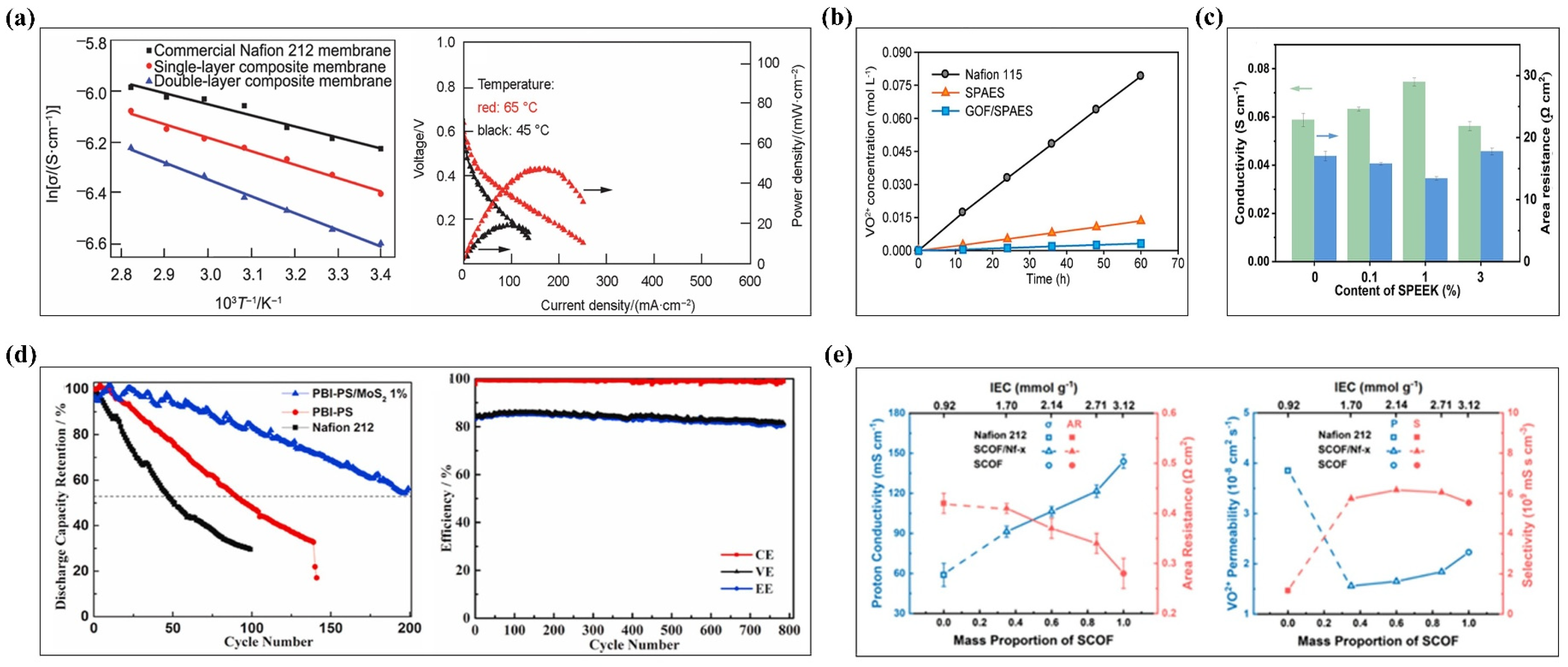
3.4.3. Vanadium Redox Flow Batteries
| Desalination Seawater | |||||
|---|---|---|---|---|---|
| Membranes | Technology | Mechanism | Test conditions | Performance | Ref. |
| AD-rGO | Nanofiltration | Size sieving | Water/NaCl | Water permeability: 2647 L m−2 h−1 bar−1; Salt rejection: 99.0% | [102] |
| ArGO-PSSNa | Forward Osmosis | Electrostatic repulsion | Water/NaCl | Water permeability: 47.0 L m−2 h−1; Salt rejection: 99.7% | [104] |
| Nanofiltration | Electrostatic repulsion | Water/NaCl | Water permeability: 48.6 L m−2 h−1 Salt rejection: 99.5% | ||
| GDY@PCHF | Vacuum Membrane Distillation | Size sieving | Water/NaCl | Water permeability: 700 L m−2 h−1; Salt rejection: 99.9% | [106] |
| c-GDY | Nanofiltration | Size sieving | Water/NaCl | Water permeability: ~32.9 mol m−2 h−1 bar−1; Salt rejection: 99.7% | [107] |
| NoN membranes | Nanofiltration | Size sieving | Water/Mg2SO4 | Water permeability: 30.0 L m−2 h−1 bar−1; Salt rejection: 99.0% | [109] |
| High-Value Ion Separation | |||||
| CMP-masked porous graphene | Electric field-driven | Size sieving | K+, Na+, Li+, Ca2+, Mg2+ | Selectivity: K+/Na+ = 20, K+/Mg2+ = 330; Li+/divalent ions > 900 | [115] |
| UHCD IEMs | Diffusion | Electrostatic repulsion | Na+, Cl− | Ionic conductivity enhance 4.5–13 times | [116] |
| M-SAT membranes | Diffusion | Size sieving Electrostatic repulsion | H+, Fe2+ | Selectivity: H+/Fe2+ > 800 | [117] |
| Nanofiltration | Water/Na2SO4 | Salt rejection: 100% | |||
| i-GO | Diffusion | Biological ion channel | K+, Ca2+, Mg2+, Fe3+, Cu2+ | Permeability: K+ 1.36 mol m−2 h−1 Selectivity: K+/Mg2+ = 9.11; K+/Ca2+ = 6.44; K+/Cu2+ = 8.93; K+/Fe3+ = 28.29 | [119] |
| Functionalized Graphene | Electric field-driven | Biological ion channel | H+, K+, Li+, Na+, Cs+, Cl− | K+/Li + =48.6;K+/Cl− ≈ 76; H+/Cl− ≈ 59.3; Li+/Cl− ≈ 36 | [120] |
| Nanopores COF-cys | Diffusion | pH response | K+, Na+ | Selectivity: K+/Na+ = 1.7 (pH 3.8); Na+/K+ = 2.9 (pH 8.9) | [122] |
| Osmotic Energy Conversion | |||||
| GPPS membranes | Reverse electrodialysis | Size sieving; Electrostatic repulsion | 0.5 M NaCl; 0.01 M NaCl | Max power density: 13.8 W m−2 | [125] |
| HGN membranes | Reverse electrodialysis | Size sieving Electrostatic repulsion | 1 M KCl; 0.001M KCl | Power density: >100 W m−2 | [126] |
| MoS2 membranes | Reverse electrodialysis | Size sieving | 0.5 M NaCl; 0.1 M NaCl; 0.0 1M NaCl | Max Power density: 6.7 W m−2 | [127] |
| MXene-PPS | Reverse electrodialysis | Size sieving Electrostatic repulsion | 0.5 M NaCl; 0.01 M NaCl | Power density: 1.57 W m−2 | [128] |
| MXene/PBONF | Reverse electrodialysis | Size sieving Electrostatic repulsion | 0.5 M NaCl; 0.01 M NaCl | Selectivity: Na+/Cl− 0.87 Power density: 15.7 W m−2 | [132] |
| MXene/ZIF-8 | Reverse electrodialysis | Size sieving Electrostatic repulsion Nano confined | 0.5 M NaCl; 0.01 M NaCl 0.5 M NaCl; 0.001 M NaCl | Selectivity: Na+/Cl− 0.906 Max Power density: 48.05 W m−2 | [133] |
| MXene/PS-b-P2VP | Reverse electrodialysis | Size sieving pH response | 0.1 M KCl; 0.5 M NaCl 0.01 M NaCl | Max Power density: 6.74 W m−2 | [134] |
| C3N4/MXene/CEL | Reverse electrodialysis | Photothermal drive | 0.5 M NaCl 0.01 M NaCl | Power density: 1.68 W m−2 | [137] |
| Cu-TCPP membranes | Reverse electrodialysis | Size sieving Electrostatic repulsion Photothermal drive | 0.5 M NaCl 0.01 M NaCl | Power density: 16.64 W m−2 | [138] |
| Proton Conductivity | |||||
| NGMs membranes | Transmembrane conductivity testing | Nitrogen-doped | 1M HCl | Proton conductivity: 1.4 × 105 S m−2 | [143] |
| Titania | Transmembrane conductivity testing | Titanium vacancy | 0.1M–1M HCl at 260 °C | Max proton conductivity: 200 S cm−1 | [144] |
| GDY | Transmembrane conductivity testing | Grotthuss | water vapor at rt. | Proton conductivity: 5.1 × 103 S cm−1 | [147] |
| GDYO | Au forked electrode testing | hydrogen-bond proton transport | 100% RH 348 K | Proton conductivity: 0.54 S cm−1 | [149] |
| TpBd-SO3H | Two electrode testing | hydrogen-bond proton transport | 1M HCl 100% RH, 90 °C | Proton conductivity: 1389 mS cm−1 | [151] |
| TpBd-(SO3H)2 iCOFMs | Two-probe testing | hydrogen-bond proton transport Nano confined | 100% RH, 90 °C | Proton conductivity: 0.66 S cm−1 | [152] |
| Direct Methanol Fuel Cell | |||||
| NH2-GDY@Nafion | Two electrode testing DMFC testing | Grotthuss Vehicle | 1 M Methanol solution at 80 °C | Power density: 42.5 mW cm−2 | [156] |
| 3NGDY-Nafion | Two electrode testing DMFC testing | Grotthuss Vehicle | 1 M Methanol solution at 65 °C | Power density: 80.48 mW cm−2 | [157] |
| Vanadium Redox Flow Batteries | |||||
| GOF/SPAES | VRFB testing | Size sieving Nano confined Grotthuss | 80 mA cm−2 in VRFB | VO2+ permeability reduced 4 times compared to Nafion 115 EE: 89% | [159] |
| TpPa SO3H/SPEEK | Two electrode testing VRFB testing | Size sieving Nano confined | 40 mA cm−2 in VRFB; 100% RH 20 °C in H2SO4 | Proton conductivity: 75 mS cm−1 EE: 81.0% | [66] |
| SCOF/Nf | Two electrode testing VRFB testing | Size sieving Electrostatic repulsion | 100 mA cm−2 in VRFB; 25 °C | Proton conductivity: 143.9 mS cm−1 H+/Vn+ selectivity:9.25 × 109 mS s cm−3 EE: 85.5% | [161] |
4. Conclusions and Perspectives
Author Contributions
Funding
Data availability Statement
Conflicts of Interest
References
- Zhou, Y. Worldwide carbon neutrality transition? Energy efficiency, renewable, carbon trading and advanced energy policies. Energy Rev. 2023, 2, 10026. [Google Scholar] [CrossRef]
- Yan, G.; Kenway, S.J.; Lam, K.L.; Lant, P.A. Water-energy trajectories for urban water and wastewater reveal the impact of city strategies. Appl. Energy 2024, 366, 123292. [Google Scholar] [CrossRef]
- Gao, W.; Wang, Z.; Duan, F.; Li, Y.; Shi, S.; Sun, Z.; Zhou, B.; Lv, L. Comprehensive assessment of membrane technology for typical water treatment processes: A critical review. Desalination 2025, 614, 119171. [Google Scholar] [CrossRef]
- Ji, T.; Zhang, C.; Xiao, X.; Wang, Y.; Cao, D.; Adomkevicius, A.; Zhao, Y.; Sun, X.; Fu, K.; Zhu, H. High Ion Conductive and Selective Membrane Achieved through Dual Ion Conducting Mechanisms. Small 2023, 19, e2206807. [Google Scholar] [CrossRef]
- Zhou, Z.; Shinde, D.B.; Guo, D.; Cao, L.; Al Nuaimi, R.; Zhang, Y.; Enakonda, L.R.; Lai, Z. Flexible Ionic Conjugated Microporous Polymer Membranes for Fast and Selective Ion Transport. Adv. Funct. Mater. 2021, 32, 2108672. [Google Scholar] [CrossRef]
- Borah, D.; Hazarika, G.; Gogoi, A.; Goswami, S.; Sawake, S.V.; Yadav, D.; Karki, S.; Gohain, M.B.; Sahu, L.R.; Ingole, P.G. Polymeric membranes for sustainable gas separation: A comprehensive review with challenges, innovations and future perspectives. Renew. Sustain. Energy Rev. 2025, 219, 115868. [Google Scholar] [CrossRef]
- Diao, X.; Zhang, X.; Li, Y.; Chen, X.; Zhao, Z.; Wang, P.; Liu, P.; Gao, H.; Wang, G. Heterogeneous network of 2D MOFs decorated 1D CNTs imparting multiple functionalities to composite phase change materials. Nano Res. Energy 2024, 3, e9120114. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, W.; Peng, J.; Zhang, W.; Liang, Z.; Wu, J.; Feng, J.; Li, H.; Huang, S. Metal–Organic Framework Derived Ultrafine Sb@Porous Carbon Octahedron via In Situ Substitution for High-Performance Sodium-Ion Batteries. ACS Nano 2021, 15, 15104–15113. [Google Scholar] [CrossRef]
- Li, J.; Yi, Y.; Zuo, X.; Hu, B.; Xiao, Z.; Lian, R.; Kong, Y.; Tong, L.; Shao, R.; Sun, J.; et al. Graphdiyne/Graphene/Graphdiyne Sandwiched Carbonaceous Anode for Potassium-Ion Batteries. ACS Nano 2022, 16, 3163–3172. [Google Scholar] [CrossRef]
- Ci, H.; Shi, Z.; Wang, M.; He, Y.; Sun, J. A review in rational design of graphene toward advanced Li–S batteries. Nano Res. Energy 2023, 2, e9120054. [Google Scholar] [CrossRef]
- Gao, X.; Zhu, Y.; Yi, D.; Zhou, J.; Zhang, S.; Yin, C.; Ding, F.; Zhang, S.; Yi, X.; Wang, J.; et al. Ultrathin graphdiyne film on graphene through solution-phase van der Waals epitaxy. Sci. Adv. 2018, 4, eaat6378. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.R.G.; Shekhirev, M.; Wyatt, B.C.; Anasori, B.; Gogotsi, Y.; Seh, Z.W. Fundamentals of MXene synthesis. Nat. Synth. 2022, 1, 601–614. [Google Scholar] [CrossRef]
- Li, J.; Zhao, N.; Liu, X.; Chang, X.; Zheng, W.; Zhang, J. Two-dimensional layered double hydroxides for advanced sensors. Co-ord. Chem. Rev. 2024, 523, 216262. [Google Scholar] [CrossRef]
- Tian, X.; Ye, C.; Zhang, L.; Sugumar, M.K.; Zhao, Y.; McKeown, N.B.; Margadonna, S.; Tan, R. Enhancing Membrane Materials for Efficient Li Recycling and Recovery. Adv. Mater. 2024, 37, e2402335. [Google Scholar] [CrossRef]
- Ali, A.; Tufa, R.A.; Macedonio, F.; Curcio, E.; Drioli, E. Membrane technology in renewable-energy-driven desalination. Renew. Sustain. Energy Rev. 2018, 81, 1–21. [Google Scholar] [CrossRef]
- Yang, F.; Yong, M.; Li, Z.; Yang, Z.; Zhang, X. Breaking the trade-off between lithium purity and lithium recovery: A comprehensive mathematical modeling based on membrane structure-property-performance relationships. Water Res. 2025, 281, 123678. [Google Scholar] [CrossRef]
- Shen, L.; Yi, M.; Japip, S.; Han, C.; Tian, L.; Lau, C.H.; Wang, Y. Breaking through permeability–selectivity trade-off of thin-film composite membranes assisted with crown ethers. AIChE J. 2021, 67, e17173. [Google Scholar] [CrossRef]
- Kidambi, P.R.; Chaturvedi, P.; Moehring, N.K. Subatomic species transport through atomically thin membranes: Present and future applications. Science 2021, 374, eabd7687. [Google Scholar] [CrossRef]
- Liu, G.; Jin, W.; Xu, N. Two-Dimensional-Material Membranes: A New Family of High-Performance Separation Membranes. Angew. Chem. Int. Ed. Engl. 2016, 55, 13384–13397. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, H.; Li, S.; Wang, L. Selective Ion Transport in Two-Dimensional Lamellar Nanochannel Membranes. Angew. Chem. Int. Ed. Engl. 2023, 62, e202218321. [Google Scholar] [CrossRef]
- Jiao, Y.; Yang, C.; Zhang, W.; Wang, Q.; Zhao, C. A review on direct osmotic power generation: Mechanism and membranes. Renew. Sustain. Energy Rev. 2023, 191, 114078. [Google Scholar] [CrossRef]
- Liu, L.; Cheng, Q. Mass transfer characteristic research on electrodialysis for desalination and regeneration of solution: A comprehensive review. Renew. Sustain. Energy Rev. 2020, 134, 110115. [Google Scholar] [CrossRef]
- León, T.; López, J.; Torres, R.; Grau, J.; Jofre, L.; Cortina, J.-L. Time-dependent 2-D model for transport of species analysis in electrodialysis: Concentration profiles and fluxes. Desalination 2023, 565, 116819. [Google Scholar] [CrossRef]
- Kang, Y.; Xia, Y.; Wang, H.; Zhang, X. 2D Laminar Membranes for Selective Water and Ion Transport. Adv. Funct. Mater. 2019, 29, 1902014. [Google Scholar] [CrossRef]
- Luo, J.; Qiao, R.; Ding, B. Enhancement of ion selectivity and permeability in two-dimensional material membranes. Matter 2024, 7, 3351–3389. [Google Scholar] [CrossRef]
- Yu, X.; Ren, W. Ion and Water Transport in 2D Nanofluidic Channels. Adv. Funct. Mater. 2024, 34, 2313968. [Google Scholar] [CrossRef]
- Wang, S.; Yang, L.; He, G.; Shi, B.; Li, Y.; Wu, H.; Zhang, R.; Nunes, S.; Jiang, Z. Two-dimensional nanochannel membranes for molecular and ionic separations. Chem. Soc. Rev. 2020, 49, 1071–1089. [Google Scholar] [CrossRef]
- Kang, Y.; Wang, Y.; Zhang, H.; Wang, Z.; Zhang, X.; Wang, H. Functionalized 2D membranes for separations at the 1-nm scale. Chem. Soc. Rev. 2024, 53, 7939–7959. [Google Scholar] [CrossRef]
- Cui, Y.; Gao, L.; Ying, C.; Tian, J.; Liu, Z. Two-Dimensional Material-Based Nanofluidic Devices and Their Applications. ACS Nano 2025, 19, 1911–1943. [Google Scholar] [CrossRef]
- Tiwary, S.K.; Singh, M.; Chavan, S.V.; Karim, A. Graphene oxide-based membranes for water desalination and purification. npj 2D Mater. Appl. 2024, 8, 27. [Google Scholar] [CrossRef]
- Gu, P.; Liu, S.; Cheng, X.; Zhang, S.; Wu, C.; Wen, T.; Wang, X. Recent strategies, progress, and prospects of two-dimensional metal carbides (MXenes) materials in wastewater purification: A review. Sci. Total. Environ. 2023, 912, 169533. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Fal′Ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Asif, M.B.; Iftekhar, S.; Maqbool, T.; Pramanik, B.K.; Tabraiz, S.; Sillanpää, M.; Zhang, Z. Two-dimensional nanoporous and lamellar membranes for water purification: Reality or a myth? Chem. Eng. J. 2022, 432, 134335. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Hou, J.; Jiang, L.; Wang, H. Angstrom-scale ion channels towards single-ion selectivity. Chem. Soc. Rev. 2022, 51, 2224–2254. [Google Scholar] [CrossRef]
- Xin, W.; Jiang, L.; Wen, L. Two-Dimensional Nanofluidic Membranes toward Harvesting Salinity Gradient Power. Acc. Chem. Res. 2021, 54, 4154–4165. [Google Scholar] [CrossRef]
- Zhu, L.; Cao, Y.; Xu, T.; Yang, H.; Wang, L.; Dai, L.; Pan, F.; Chen, C.; Si, C. Covalent organic framework membranes for energy storage and conversion. Energy Environ. Sci. 2025, 18, 5675–5739. [Google Scholar] [CrossRef]
- Senila, L.; Kovacs, E.; Senila, M. A Review of Polylactic Acid (PLA) and Poly(3-hydroxybutyrate) (PHB) as Bio-Sourced Polymers for Membrane Production Applications. Membranes 2025, 15, 210. [Google Scholar] [CrossRef]
- Cai, Z.; Liu, B.; Zou, X.; Cheng, H.-M. Chemical Vapor Deposition Growth and Applications of Two-Dimensional Materials and Their Heterostructures. Chem. Rev. 2018, 118, 6091–6133. [Google Scholar] [CrossRef]
- Luo, L.; Hou, L.; Cui, X.; Zhan, P.; He, P.; Dai, C.; Li, R.; Dong, J.; Zou, Y.; Liu, G.; et al. Self-condensation-assisted chemical vapour deposition growth of atomically two-dimensional MOF single-crystals. Nat. Commun. 2024, 15, 3618. [Google Scholar] [CrossRef]
- Baek, K.; Yun, G.; Kim, Y.; Kim, D.; Hota, R.; Hwang, I.; Xu, D.; Ko, Y.H.; Gu, G.H.; Suh, J.H.; et al. Free-Standing, Single-Monomer-Thick Two-Dimensional Polymers through Covalent Self-Assembly in Solution. J. Am. Chem. Soc. 2013, 135, 6523–6528. [Google Scholar] [CrossRef]
- Huo, C.; Yan, Z.; Song, X.; Zeng, H. 2D materials via liquid exfoliation: A review on fabrication and applications. Sci. Bull. 2015, 60, 1994–2008. [Google Scholar] [CrossRef]
- Cun, H.; Miao, Z.; Hemmi, A.; Al-Hamdani, Y.; Iannuzzi, M.; Osterwalder, J.; Altman, M.S.; Greber, T. High-Quality Hexagonal Boron Nitride from 2D Distillation. ACS Nano 2020, 15, 1351–1357. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, T.; Wu, P.; Lee, H.W.; Liu, Z.; Tang, T.W.; Tang, S.-Y.; Kang, T.; Park, J.-H.; Wang, J.; et al. Boosting Monolayer Transition Metal Dichalcogenides Growth by Hydrogen-Free Ramping during Chemical Vapor Deposition. Nano Lett. 2024, 24, 8277–8286. [Google Scholar] [CrossRef]
- Rubio-Giménez, V.; Arnauts, G.; Wang, M.; Mata, E.S.O.; Huang, X.; Lan, T.; Tietze, M.L.; Kravchenko, D.E.; Smets, J.; Wauteraerts, N.; et al. Chemical Vapor Deposition and High-Resolution Patterning of a Highly Conductive Two-Dimensional Coordination Polymer Film. J. Am. Chem. Soc. 2022, 145, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Fu, S.; Fu, Y.; Chen, Y.; Tadayon, K.; Hambsch, M.; Pohl, D.; Yang, Y.; Müller, A.; Zhao, F.; et al. Ammonia-Assisted Chemical Vapor Deposition Growth of Two-Dimensional Conjugated Coordination Polymer Thin Films. J. Am. Chem. Soc. 2025, 147, 18190–18196. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Giusto, P.; Wen, L.; Jiang, L.; Antonietti, M. Nanofluidic Ion Transport and Energy Conversion through Ultrathin Free-Standing Polymeric Carbon Nitride Membranes. Angew. Chem. Int. Ed. Engl. 2018, 57, 10123–10126. [Google Scholar] [CrossRef]
- Wetzl, C.; Silvestri, A.; Garrido, M.; Hou, H.; Criado, A.; Prato, M. The Covalent Functionalization of Surface-Supported Graphene: An Update. Angew. Chem. Int. Ed. Engl. 2022, 62, e202212857. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Bhoyate, S.; Kim, Y.-H.; Lee, Y.H.; Conlin, P.; Cho, K.; Choi, W. Unusually High Ion Conductivity in Large-Scale Patternable Two-Dimensional MoS2 Film. ACS Nano 2021, 15, 12267–12275. [Google Scholar] [CrossRef]
- Kutagulla, S.; Carmichael, P.; Coupin, M.; Mutyala, D.; Ignacio, N.; Le, N.H.; Bohn, I.T.C.; Kim, J.-W.; Mason, K.S.; Warner, J.; et al. Ozonated Monolayer Graphene for Extended Performance and Durability in Hydrogen Fuel Cell Electric Vehicles. ACS Nano 2025, 19, 9422–9431. [Google Scholar] [CrossRef]
- Li, G.; Li, Y.; Liu, H.; Guo, Y.; Li, Y.; Zhu, D. Architecture of graphdiyne nanoscale films. Chem. Commun. 2010, 46, 3256–3258. [Google Scholar] [CrossRef]
- Zhou, J.; Gao, X.; Liu, R.; Xie, Z.; Yang, J.; Zhang, S.; Zhang, G.; Liu, H.; Li, Y.; Zhang, J.; et al. Synthesis of Graphdiyne Nanowalls Using Acetylenic Coupling Reaction. J. Am. Chem. Soc. 2015, 137, 7596–7599. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Tan, Y.; Yang, Q.; Bera, A.; Xiong, Z.; Yagmurcukardes, M.; Kim, M.; Zou, Y.; Wang, G.; Mishchenko, A.; et al. Gas permeation through graphdiyne-based nanoporous membranes. Nat. Commun. 2022, 13, 4031. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhou, J.; Du, R.; Xie, Z.; Deng, S.; Liu, R.; Liu, Z.; Zhang, J. Robust Superhydrophobic Foam: A Graphdiyne-Based Hierarchical Architecture for Oil/Water Separation. Adv. Mater. 2015, 28, 168–173. [Google Scholar] [CrossRef]
- Matsuoka, R.; Sakamoto, R.; Hoshiko, K.; Sasaki, S.; Masunaga, H.; Nagashio, K.; Nishihara, H. Crystalline Graphdiyne Nanosheets Produced at a Gas/Liquid or Liquid/Liquid Interface. J. Am. Chem. Soc. 2017, 139, 3145–3152. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Yang, Y.; Wang, J.; Fu, R.; Zhao, X.; Zhao, L.; Ming, Y.; Hu, Y.; Lin, H.; Tao, X.; et al. High-performance graphdiyne-based electrochemical actuators. Nat. Commun. 2018, 9, 752. [Google Scholar] [CrossRef]
- Wang, L.; Han, Y.; Xie, M.; Li, X.; Chen, Q.; Tang, Y.; Liu, Y.; Ge, H.; Li, H.; Cai, L.; et al. Synthesis of Hexabenzocoronene-Cored Graphdiyne Nanosheets through Dehydrogenative Coupling on Au(111) Surface. Angew. Chem. Int. Ed. Engl. 2024, 63, e202411722. [Google Scholar] [CrossRef]
- Kaliya, K.; Bhardwaj, N.; Ruchika; Saneja, A. An Imine-Based Two-Dimensional Covalent Organic Framework for Gemcitabine Delivery. Colloids Interfaces 2025, 9, 8. [Google Scholar] [CrossRef]
- Liu, K.; Qi, H.; Dong, R.; Shivhare, R.; Addicoat, M.; Zhang, T.; Sahabudeen, H.; Heine, T.; Mannsfeld, S.; Kaiser, U.; et al. On-water surface synthesis of crystalline, few-layer two-dimensional polymers assisted by surfactant monolayers. Nat. Chem. 2019, 11, 994–1000. [Google Scholar] [CrossRef]
- Ying, Y.; Tong, M.; Ning, S.; Ravi, S.K.; Peh, S.B.; Tan, S.C.; Pennycook, S.J.; Zhao, D. Ultrathin Two-Dimensional Membranes Assembled by Ionic Covalent Organic Nanosheets with Reduced Apertures for Gas Separation. J. Am. Chem. Soc. 2020, 142, 4472–4480. [Google Scholar] [CrossRef]
- Liu, X.; Lin, W.; Al Mohawes, K.B.; Khashab, N.M. Ultrahigh Proton Selectivity by Assembled Cationic Covalent Organic Framework Nanosheets. Angew. Chem. Int. Ed. Engl. 2024, 64, e202419034. [Google Scholar] [CrossRef]
- Khan, N.A.; Zhang, R.; Wu, H.; Shen, J.; Yuan, J.; Fan, C.; Cao, L.; Olson, M.A.; Jiang, Z. Solid–Vapor Interface Engineered Covalent Organic Framework Membranes for Molecular Separation. J. Am. Chem. Soc. 2020, 142, 13450–13458. [Google Scholar] [CrossRef]
- Qian, Y.; Wu, Y.; Qiu, S.; He, X.; Liu, Y.; Kong, X.; Tian, W.; Jiang, L.; Wen, L. A Bioinspired Free-Standing 2D Crown-Ether-Based Polyimine Membrane for Selective Proton Transport. Angew. Chem. Int. Ed. Engl. 2023, 62, e202300167. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, T.; Wu, X.; Jiang, C.; Zhang, T.; Jin, B.; Ji, H.; Bai, W.; Bai, R. From 1D Polymers to 2D Polymers: Preparation of Free-Standing Single-Monomer-Thick Two-Dimensional Conjugated Polymers in Water. ACS Nano 2017, 11, 7223–7229. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Zhang, F.; Zhang, W.; Qiang, P.; Yu, K.; Fu, X.; Wu, D.; Bi, S. Semiconducting 2D Triazine-Cored Covalent Organic Frameworks with Unsubstituted Olefin Linkages. J. Am. Chem. Soc. 2019, 141, 14272–14279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, J.; Han, G.; Bai, Y.; Ge, Q.; Ma, J.; Lau, C.H.; Shao, L. Molecularly soldered covalent organic frameworks for ultrafast precision sieving. Sci. Adv. 2021, 7, eabe8706. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Y.; Zhang, D.; Xu, F.; Dai, L.; Qu, K.; Cao, H.; Xia, Y.; Li, S.; Huang, K.; et al. Crystallizing Self-Standing Covalent Organic Framework Membranes for Ultrafast Proton Transport in Flow Batteries. Angew. Chem. Int. Ed. Engl. 2023, 62, e202313571. [Google Scholar] [CrossRef]
- Shi, B.; Pang, X.; Lyu, B.; Wu, H.; Shen, J.; Guan, J.; Wang, X.; Fan, C.; Cao, L.; Zhu, T.; et al. Spacer-Engineered Ionic Channels in Covalent Organic Framework Membranes toward Ultrafast Proton Transport. Adv. Mater. 2023, 35, e2211004. [Google Scholar] [CrossRef]
- Pang, X.; Shi, B.; Liu, Y.; Li, Y.; Zhang, Y.; Wang, T.; Xu, S.; Wang, X.; Liu, Z.; Xing, N.; et al. Phosphorylated Covalent Organic Framework Membranes Toward Ultrafast Single Lithium-Ion Transport. Adv. Mater. 2024, 36, e2413022. [Google Scholar] [CrossRef]
- You, X.; Wu, H.; Zhang, R.; Su, Y.; Cao, L.; Yu, Q.; Yuan, J.; Xiao, K.; He, M.; Jiang, Z. Metal-coordinated sub-10 nm membranes for water purification. Nat. Commun. 2019, 10, 4160. [Google Scholar] [CrossRef]
- Li, R.; Yao, Z.; Li, Z.; Liao, L.; Sun, H.; Cong, C.; Huang, X.; Wu, K.; Wang, T.; Tian, H.; et al. Mechanical exfoliation of non-layered metal oxides into ultrathin flakes. Nat. Synth. 2024, 4, 106–115. [Google Scholar] [CrossRef]
- Tian, W.; Kang, M.-A.; Shakya, J.; Li, Q.; Sui, X.; Liu, M.; Wang, H.; Hamedi, M.M. Liquid-phase exfoliation of 2D transition metal dichalcogenide nanosheets in water. Chem. Eng. J. 2025, 513, 162587. [Google Scholar] [CrossRef]
- Yang, R.; Mei, L.; Zhang, Q.; Fan, Y.; Shin, H.S.; Voiry, D.; Zeng, Z. High-yield production of mono- or few-layer transition metal dichalcogenide nanosheets by an electrochemical lithium ion intercalation-based exfoliation method. Nat. Protoc. 2022, 17, 358–377. [Google Scholar] [CrossRef]
- Zou, Y.-C.; Mogg, L.; Clark, N.; Bacaksiz, C.; Milovanovic, S.; Sreepal, V.; Hao, G.-P.; Wang, Y.-C.; Hopkinson, D.G.; Gorbachev, R.; et al. Ion exchange in atomically thin clays and micas. Nat. Mater. 2021, 20, 1677–1682. [Google Scholar] [CrossRef] [PubMed]
- Alhabeb, M.; Maleski, K.; Mathis, T.S.; Sarycheva, A.; Hatter, C.B.; Uzun, S.; Levitt, A.; Gogotsi, Y. Selective Etching of Silicon from Ti3SiC2 (MAX) To Obtain 2D Titanium Carbide (MXene). Angew. Chem. Int. Ed. Engl. 2018, 57, 5444–5448. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lin, H.; Moss, D.J.; Loh, K.P.; Jia, B. Graphene oxide for photonics, electronics and optoelectronics. Nat. Rev. Chem. 2023, 7, 162–183. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Velarde, S.; Euán-Díaz, E.C.; Castañeda-Priego, R. Ordering and Dynamics of Interacting Colloidal Particles under Soft Confinement. Colloids Interfaces 2021, 5, 29. [Google Scholar] [CrossRef]
- Kruk, T.; Warszyński, P. Conductive Nanofilms with Oppositely Charged Reduced Graphene Oxides as a Base for Electroactive Coatings and Sensors. Colloids Interfaces 2021, 5, 20. [Google Scholar] [CrossRef]
- Kiran, P.S.; Prasad, S.; Satish, C.; Indupuri, S.; Kumar, K.V.; Keshri, S.; Keshri, A.K. Breaking Strong Lip to Lip Interactions in Hexagonal Boron Nitride: A Facile, Scalable Exfoliation Protocol. Small 2025, 21, e2504831. [Google Scholar] [CrossRef]
- Lu, X.; Gabinet, U.R.; Ritt, C.L.; Feng, X.; Deshmukh, A.; Kawabata, K.; Kaneda, M.; Hashmi, S.M.; Osuji, C.O.; Elimelech, M. Relating Selectivity and Separation Performance of Lamellar Two-Dimensional Molybdenum Disulfide (MoS2) Membranes to Nanosheet Stacking Behavior. Environ. Sci. Technol. 2020, 54, 9640–9651. [Google Scholar] [CrossRef]
- Ventura-Martinez, K.; Zhu, Y.; Booth, A.; Hatzell, K.B. Impact of Asymmetric Microstructure on Ion Transport in Ti3C2Tx Membranes. Nano Lett. 2024, 24, 13551–13557. [Google Scholar] [CrossRef]
- Huang, Y.; Pan, Y.-H.; Yang, R.; Bao, L.-H.; Meng, L.; Luo, H.-L.; Cai, Y.-Q.; Liu, G.-D.; Zhao, W.-J.; Zhou, Z.; et al. Universal mechanical exfoliation of large-area 2D crystals. Nat. Commun. 2020, 11, 2453. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.-Y.; Kim, M.; Kim, S.-I.; Xu, S.; Choi, J.-H.; Whang, D.; Watanabe, K.; Taniguchi, T.; Park, D.S.; Seo, J.; et al. Layer-engineered large-area exfoliation of graphene. Sci. Adv. 2020, 6, eabc6601. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Xu, Y.; Sun, L.; Ward, Z.; Wang, H.; Ratnayake, G.; Wang, C.; Zhao, M.; He, H.; Gao, J.; et al. Two-dimensional Nanosheets by Liquid Metal Exfoliation. Adv. Mater. 2024, 37, e2416375. [Google Scholar] [CrossRef] [PubMed]
- Ritt, C.L.; Werber, J.R.; Deshmukh, A.; Elimelech, M. Monte Carlo Simulations of Framework Defects in Layered Two-Dimensional Nanomaterial Desalination Membranes: Implications for Permeability and Selectivity. Environ. Sci. Technol. 2019, 53, 6214–6224. [Google Scholar] [CrossRef]
- Nicklin, C. Capturing Surface Processes. Science 2014, 343, 739–740. [Google Scholar] [CrossRef]
- Chen, L.; Shi, G.; Shen, J.; Peng, B.; Zhang, B.; Wang, Y.; Bian, F.; Wang, J.; Li, D.; Qian, Z.; et al. Ion sieving in graphene oxide membranes via cationic control of interlayer spacing. Nature 2017, 550, 380–383. [Google Scholar] [CrossRef]
- Paton, K.R.; Varrla, E.; Backes, C.; Smith, R.J.; Khan, U.; O’Neill, A.; Boland, C.S.; Lotya, M.; Istrate, O.M.; King, P.; et al. Scalable production of large quantities of defect-free few-layer graphene by shear exfoliation in liquids. Nat. Mater. 2014, 13, 624–630. [Google Scholar] [CrossRef]
- Islam, A.; Mukherjee, B.; Pandey, K.K.; Keshri, A.K. Ultra-Fast, Chemical-Free, Mass Production of High Quality Exfoliated Graphene. ACS Nano 2021, 15, 1775–1784. [Google Scholar] [CrossRef]
- Wang, N.; Yang, G.; Wang, H.; Yan, C.; Sun, R.; Wong, C.-P. A universal method for large-yield and high-concentration exfoliation of two-dimensional hexagonal boron nitride nanosheets. Mater. Today 2019, 27, 33–42. [Google Scholar] [CrossRef]
- Pendse, A.; Cetindag, S.; Wang, K.; Li, D.; Castellano, R.J.; Yang, D.-C.; Wang, T.; Shan, J.W.; Kim, S. Intrinsic ion transport of highly charged sub-3-nm boron nitride nanotubes. Mater. Today 2022, 60, 79–90. [Google Scholar] [CrossRef]
- Hu, C.Y.; Achari, A.; Rowe, P.; Xiao, H.; Suran, S.; Li, Z.; Huang, K.; Chi, C.; Cherian, C.T.; Sreepal, V.; et al. pH-dependent water permeability switching and its memory in MoS2 membranes. Nature 2023, 616, 719–723. [Google Scholar] [CrossRef]
- Ries, L.; Petit, E.; Michel, T.; Diogo, C.C.; Gervais, C.; Salameh, C.; Bechelany, M.; Balme, S.; Miele, P.; Onofrio, N.; et al. Enhanced sieving from exfoliated MoS2 membranes via covalent functionalization. Nat. Mater. 2019, 18, 1112–1117. [Google Scholar] [CrossRef]
- Kim, C.; Koh, D.-Y.; Lee, Y.; Choi, J.; Cho, H.S.; Choi, M. Bottom-up synthesis of two-dimensional carbon with vertically aligned ordered micropores for ultrafast nanofiltration. Sci. Adv. 2023, 9, eade7871. [Google Scholar] [CrossRef]
- Yi, M.; Wang, M.; Wang, Y.; Wang, Y.; Chang, J.; Kheirabad, A.K.; He, H.; Yuan, J.; Zhang, M. Poly(ionic liquid)-Armored MXene Membrane: Interlayer Engineering for Facilitated Water Transport. Angew. Chem. Int. Ed. Engl. 2022, 61, e202202515. [Google Scholar] [CrossRef]
- Liu, L.; Orbay, M.; Luo, S.; Duluard, S.; Shao, H.; Harmel, J.; Rozier, P.; Taberna, P.-L.; Simon, P. Exfoliation and Delamination of Ti3C2Tx MXene Prepared via Molten Salt Etching Route. ACS Nano 2021, 16, 111–118. [Google Scholar] [CrossRef]
- Xia, Z.; Chen, W.; Shevate, R.; Wang, Y.; Gao, F.; Wang, D.; Kazi, O.A.; Mane, A.U.; Lee, S.S.; Elam, J.W.; et al. Tunable Ion Transport with Freestanding Vermiculite Membranes. ACS Nano 2022, 16, 18266–18273. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, J.; Yong, M.; Zeng, X.; Tebyetekerwa, M.; Sun, K.; Bie, C.; Xing, C.; Wang, H.; Andreeva, D.V.; et al. From Layered Crystals to Permselective Membranes: History, Fundamentals, and Opportunities. Chem. Rev. 2025, 125, 6753–6818. [Google Scholar] [CrossRef] [PubMed]
- DuChanois, R.M.; Porter, C.J.; Violet, C.; Verduzco, R.; Elimelech, M. Membrane Materials for Selective Ion Separations at the Water–Energy Nexus. Adv. Mater. 2021, 33, 2101312. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, H.; Zhang, X. Facile Fabrication of Freestanding Ultrathin Reduced Graphene Oxide Membranes for Water Purification. Adv. Mater. 2014, 27, 249–254. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.; Yu, Z.; Gao, Y.; Lu, Q.; Ma, C.; Liu, K.; Yuan, Q.; Yang, Y. Fast water transport and ionic sieving in ultrathin stacked nanoporous 2D membranes. Natl. Sci. Rev. 2025, 12, nwae482. [Google Scholar] [CrossRef]
- Xu, W.L.; Fang, C.; Zhou, F.; Song, Z.; Liu, Q.; Qiao, R.; Yu, M. Self-Assembly: A Facile Way of Forming Ultrathin, High-Performance Graphene Oxide Membranes for Water Purification. Nano Lett. 2017, 17, 2928–2933. [Google Scholar] [CrossRef]
- Han, C.; Jiang, J.; Mu, L.; Zhao, W.; Liu, J.; Lan, J.; Hu, S.; Yang, H.; Gao, S.; Zhou, F.; et al. Quasi-vertically asymmetric channels of graphene oxide membrane for ultrafast ion sieving. Nat. Commun. 2025, 16, 1020. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, J.; Zhang, J.; Gong, X.; Zhou, J.; Zhang, N.; Su, Y. Smart and solvent-switchable graphene-based membrane for graded molecular sieving. Nat. Commun. 2025, 16, 5363. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xing, J.; Wei, G.; Wang, X.; Chen, S.; Quan, X. Electrostatic-induced ion-confined partitioning in graphene nanolaminate membrane for breaking anion–cation co-transport to enhance desalination. Nat. Commun. 2024, 15, 4324. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Sengupta, B.; Kazi, O.A.; Martinson, A.B.F.; Elam, J.W.; Darling, S.B. Pillared Laminar Vermiculite Membranes with Tunable Monovalent and Multivalent Ion Selectivity. Adv. Mater. 2025, 37, e2417994. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, X.; Gong, D.; Zhu, C.; Liu, G.; Fan, J.; Wu, P.; Li, Z.; Pan, Y.; Shi, G.; et al. Ultrahigh-water-flux desalination on graphdiyne membranes. Nat. Water 2023, 1, 800–807. [Google Scholar] [CrossRef]
- Li, J.; Zhou, K.; Liu, Q.; Tian, B.; Liu, X.; Cao, L.; Cao, H.; Li, G.; Zhang, X.; Han, Y.; et al. Synthesis of two-dimensional ordered graphdiyne membranes for highly efficient and selective water transport. Nat. Water 2025, 3, 307–318. [Google Scholar] [CrossRef]
- Shen, J.; Cai, Y.; Zhang, C.; Wei, W.; Chen, C.; Liu, L.; Yang, K.; Ma, Y.; Wang, Y.; Tseng, C.-C.; et al. Fast water transport and molecular sieving through ultrathin ordered conjugated-polymer-framework membranes. Nat. Mater. 2022, 21, 1183–1190. [Google Scholar] [CrossRef]
- Liu, S.-H.; Shi, W.; Hung, W.-S.; Shi, L.; Xue, B.; She, J.; Song, Z.; Lu, X.; Gray, S.; Lee, K.-R.; et al. Interfacial self-organization of large-area mixed-dimensional polyamide membranes for rapid aqueous nanofiltration. Nat. Water 2024, 2, 1238–1248. [Google Scholar] [CrossRef]
- Yang, H.; Yang, L.; Wang, H.; Xu, Z.; Zhao, Y.; Luo, Y.; Nasir, N.; Song, Y.; Wu, H.; Pan, F.; et al. Covalent organic framework membranes through a mixed-dimensional assembly for molecular separations. Nat. Commun. 2019, 10, 2101. [Google Scholar] [CrossRef]
- Ding, L.; Li, L.; Liu, Y.; Wu, Y.; Lu, Z.; Deng, J.; Wei, Y.; Caro, J.; Wang, H. Effective ion sieving with Ti3C2Tx MXene membranes for production of drinking water from seawater. Nat. Sustain. 2020, 3, 296–302. [Google Scholar] [CrossRef]
- Dang, C.; Helal, A.S.; Zhu, L.; Xu, G.; Zhu, M. Industrial pathways to lithium extraction from seawater: Challenges and perspectives. Nano Res. Energy 2023, 2, e9120059. [Google Scholar] [CrossRef]
- Juve, J.-M.A.; Christensen, F.M.S.; Wang, Y.; Wei, Z. Electrodialysis for metal removal and recovery: A review. Chem. Eng. J. 2022, 435, 134857. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Z.; Yang, K.; Wei, J.; Li, Z.; Ma, C.; Yang, X.; Wang, T.; Zeng, G.; Yu, G.; et al. Removal of chloride from water and wastewater: Removal mechanisms and recent trends. Sci. Total. Environ. 2022, 821, 153174. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhao, K.; Chi, H.-Y.; Shen, Y.; Song, S.; Hsu, K.-J.; Chevalier, M.; Shi, W.; Agrawal, K.V. Electrochemical-repaired porous graphene membranes for precise ion-ion separation. Nat. Commun. 2024, 15, 4006. [Google Scholar] [CrossRef]
- Kitto, D.; Espinoza, C.; Díaz, J.C.; Zamora, J.; Kamcev, J. Fast and selective ion transport in ultrahigh-charge-density membranes. Nat. Chem. Eng. 2025, 2, 252–260. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Z.; Zhu, J.; Tian, M.; Zheng, S.; Wang, F.; Wang, X.; Wang, L. Ion sieving by a two-dimensional Ti3C2Tx alginate lamellar membrane with stable interlayer spacing. Nat. Commun. 2020, 11, 3540. [Google Scholar] [CrossRef]
- Xu, R.; Yu, H.; Ren, J.; Zhang, W.; Kang, Y.; Wang, Z.; Feng, F.; Xia, X.; Liu, J.Z.; Peng, L.; et al. Regulate Ion Transport in Subnanochannel Membranes by Ion-Pairing. J. Am. Chem. Soc. 2025, 147, 17144–17151. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, P.; Li, P.; Ji, Y.; Liu, G.; Jin, W. Designing Biomimic Two-Dimensional Ionic Transport Channels for Efficient Ion Sieving. ACS Nano 2021, 15, 5209–5220. [Google Scholar] [CrossRef]
- Guo, L.; Liu, Y.; Zeng, H.; Zhang, S.; Song, R.; Yang, J.; Han, X.; Wang, Y.; Wang, L. Covalently Functionalized Nanopores for Highly Selective Separation of Monovalent Ions. Adv. Mater. 2023, 36, e2307242. [Google Scholar] [CrossRef]
- Qiu, M.; Zhu, Z.; Wang, D.; Xu, Z.; Xia, F.; Jiang, L.; Tian, Y. Superspreading-Confined Assembly of Oriented 2D MOF Membranes for Biomimetic Cation-Regulated Ion Transport. Adv. Funct. Mater. 2024, 34, 2316040. [Google Scholar] [CrossRef]
- Cao, L.; Chen, I.-C.; Li, Z.; Liu, X.; Mubashir, M.; Al Nuaimi, R.; Lai, Z. Switchable Na+ and K+ selectivity in an amino acid functionalized 2D covalent organic framework membrane. Nat. Commun. 2022, 13, 7894. [Google Scholar] [CrossRef]
- Rastgar, M.; Moradi, K.; Burroughs, C.; Hemmati, A.; Hoek, E.; Sadrzadeh, M. Harvesting Blue Energy Based on Salinity and Temperature Gradient: Challenges, Solutions, and Opportunities. Chem. Rev. 2023, 123, 10156–10205. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Chen, X.; Liang, Z.; Fang, Y.; Yao, J.; Lu, C.; Cai, Y.; Jiang, L. Two-Dimensional Nanofluidic Membranes with Intercalated In-Plane Shortcuts for High-Performance Blue Energy Harvesting. Small 2022, 19, e2205003. [Google Scholar] [CrossRef] [PubMed]
- Zhi, H.; Yan, P.; Wang, D.; Liu, Y.; Tang, J.; Yang, X.; Liu, Z.; Zhang, Y.; Li, N.; An, M.; et al. Large-Area Graphene-Based Ion-Selective Membranes with Micro/Meso-Pores for Osmotic Energy Harvesting. Adv. Funct. Mater. 2024, 34, 2401922. [Google Scholar] [CrossRef]
- Wang, H.; Su, L.; Yagmurcukardes, M.; Chen, J.; Jiang, Y.; Li, Z.; Quan, A.; Peeters, F.M.; Wang, C.; Geim, A.K.; et al. Blue Energy Conversion from Holey-Graphene-like Membranes with a High Density of Subnanometer Pores. Nano Lett. 2020, 20, 8634–8639. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, P.; Niu, B.; Liu, Y.; Xin, W.; Chen, W.; Kong, X.-Y.; Zhang, Z.; Jiang, L.; Wen, L. Metallic Two-Dimensional MoS2 Composites as High-Performance Osmotic Energy Conversion Membranes. J. Am. Chem. Soc. 2021, 143, 1932–1940. [Google Scholar] [CrossRef]
- Tong, X.; Liu, S.; Zhao, Y.; Huang, L.; Crittenden, J.; Chen, Y. MXene Composite Membranes with Enhanced Ion Transport and Regulated Ion Selectivity. Environ. Sci. Technol. 2022, 56, 8964–8974. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Z.; Qi, H.; Ortega-Guerrero, A.; Wang, L.; Xu, K.; Wang, M.; Park, S.; Hennersdorf, F.; Dianat, A.; et al. On-water surface synthesis of charged two-dimensional polymer single crystals via the irreversible Katritzky reaction. Nat. Synth. 2021, 1, 69–76. [Google Scholar] [CrossRef]
- Zhang, Z.; Bhauriyal, P.; Sahabudeen, H.; Wang, Z.; Liu, X.; Hambsch, M.; Mannsfeld, S.C.B.; Dong, R.; Heine, T.; Feng, X. Cation-selective two-dimensional polyimine membranes for high-performance osmotic energy conversion. Nat. Commun. 2022, 13, 3935. [Google Scholar] [CrossRef]
- Yang, G.; Liu, D.; Chen, C.; Qian, Y.; Su, Y.; Qin, S.; Zhang, L.; Wang, X.; Sun, L.; Lei, W. Stable Ti3C2Tx MXene–Boron Nitride Membranes with Low Internal Resistance for Enhanced Salinity Gradient Energy Harvesting. ACS Nano 2021, 15, 6594–6603. [Google Scholar] [CrossRef]
- Duan, R.; Zhou, J.; Ma, X.; Hao, J.; Zhao, D.; Teng, C.; Zhou, Y.; Jiang, L. High Strength MXene/PBONF Heterogeneous Membrane with Excellent Ion Selectivity for Efficient Osmotic Energy Conversion. Nano Lett. 2023, 23, 11043–11050. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Hao, J.; Wu, R.; Su, L.; Wang, J.; Qiu, M.; Bao, B.; Ning, C.; Teng, C.; Zhou, Y.; et al. Maximizing Ion Permselectivity in MXene/MOF Nanofluidic Membranes for High-Efficient Blue Energy Generation. Adv. Funct. Mater. 2022, 32, 2209767. [Google Scholar] [CrossRef]
- Lin, X.; Liu, P.; Xin, W.; Teng, Y.; Chen, J.; Wu, Y.; Zhao, Y.; Kong, X.; Jiang, L.; Wen, L. Heterogeneous MXene/PS-b-P2VP Nanofluidic Membranes with Controllable Ion Transport for Osmotic Energy Conversion. Adv. Funct. Mater. 2021, 31, 2105013. [Google Scholar] [CrossRef]
- Yang, J.; Liu, P.; He, X.; Hou, J.; Feng, Y.; Huang, Z.; Yu, L.; Li, L.; Tang, Z. Photodriven Active Ion Transport Through a Janus Microporous Membrane. Angew. Chem. 2020, 132, 6303–6307. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, X.; Huang, X.; Sun, K.; Chen, S.; Xu, Y.; Xu, F. Synergistic Photoelectric/Photothermal Effects Guided Ion Transport for Enhancing Multiple Climatic Osmotic Energy Conversion Efficiency. Small 2025, 21, e2500366. [Google Scholar] [CrossRef]
- Liu, P.; Zhou, T.; Teng, Y.; Fu, L.; Hu, Y.; Lin, X.; Kong, X.-Y.; Jiang, L.; Wen, L. Light-Induced Heat Driving Active Ion Transport Based on 2D MXene Nanofluids for Enhancing Osmotic Energy Conversion. CCS Chem. 2021, 3, 1325–1335. [Google Scholar] [CrossRef]
- Wang, J.; Song, Z.; He, M.; Qian, Y.; Wang, D.; Cui, Z.; Feng, Y.; Li, S.; Huang, B.; Kong, X.; et al. Light-responsive and ultrapermeable two-dimensional metal-organic framework membrane for efficient ionic energy harvesting. Nat. Commun. 2024, 15, 2125. [Google Scholar] [CrossRef]
- Fan, C.; Zhang, P.; Wang, R.; Xu, Y.; Sun, X.; Zhang, J.; Cheng, J.; Xu, C. Applications of Two Dimensional Material-MXene for Proton Exchange Membrane Fuel Cells (PEMFCs) and Water Electrolysis. Curr. Nanosci. 2021, 17, 2–13. [Google Scholar] [CrossRef]
- Ye, C.; Wang, A.; Breakwell, C.; Tan, R.; Bezzu, C.G.; Hunter-Sellars, E.; Williams, D.R.; Brandon, N.P.; Klusener, P.A.A.; Kucernak, A.R.; et al. Development of efficient aqueous organic redox flow batteries using ion-sieving sulfonated polymer membranes. Nat. Commun. 2022, 13, 3184. [Google Scholar] [CrossRef]
- Sun, P.Z.; Yang, Q.; Kuang, W.J.; Stebunov, Y.V.; Xiong, W.Q.; Yu, J.; Nair, R.R.; Katsnelson, M.I.; Yuan, S.J.; Grigorieva, I.V.; et al. Limits on gas impermeability of graphene. Nature 2020, 579, 229–232. [Google Scholar] [CrossRef]
- Griffin, E.; Mogg, L.; Hao, G.-P.; Kalon, G.; Bacaksiz, C.; Lopez-Polin, G.; Zhou, T.; Guarochico, V.; Cai, J.; Neumann, C.; et al. Proton and Li-Ion Permeation through Graphene with Eight-Atom-Ring Defects. ACS Nano 2020, 14, 7280–7286. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Song, R.; Zhang, S.; Han, X.; Zhu, Z.; Chen, X.; Wang, L. Biomimetic N-Doped Graphene Membrane for Proton Exchange Membranes. Nano Lett. 2021, 21, 4314–4319. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Hao, G.-P.; Tan, Y.-T.; Xiong, W.; Liu, Y.; Zhou, W.; Tang, D.-M.; Ma, R.; Yuan, S.; Sasaki, T.; et al. High proton conductivity through angstrom-porous titania. Nat. Commun. 2024, 15, 10546. [Google Scholar] [CrossRef] [PubMed]
- Mogg, L.; Hao, G.-P.; Zhang, S.; Bacaksiz, C.; Zou, Y.-C.; Haigh, S.J.; Peeters, F.M.; Geim, A.K.; Lozada-Hidalgo, M. Atomically thin micas as proton-conducting membranes. Nat. Nanotechnol. 2019, 14, 962–966. [Google Scholar] [CrossRef]
- Li, J.; Cao, H.; Wang, Q.; Zhang, H.; Liu, Q.; Chen, C.; Shi, Z.; Li, G.; Kong, Y.; Cai, Y.; et al. Space-Confined Synthesis of Monolayer Graphdiyne in MXene Interlayer. Adv. Mater. 2023, 36, e2308429. [Google Scholar] [CrossRef]
- Li, J.; Wang, C.; Su, J.; Liu, Z.; Fan, H.; Wang, C.; Li, Y.; He, Y.; Chen, N.; Cao, J.; et al. Observing Proton–Electron Mixed Conductivity in Graphdiyne. Adv. Mater. 2024, 36, e2400950. [Google Scholar] [CrossRef]
- Xu, J.; Jiang, H.; Shen, Y.; Li, X.-Z.; Wang, E.G.; Meng, S. Transparent proton transport through a two-dimensional nanomesh material. Nat. Commun. 2019, 10, 3971. [Google Scholar] [CrossRef]
- Li, W.; Xu, C.; Xiong, T.; Jiang, Y.; Ma, W.; Yu, P.; Mao, L. Giant Water Uptake Enabled Ultrahigh Proton Conductivity of Graphdiyne Oxide. Angew. Chem. Int. Ed. Engl. 2022, 62, e202216530. [Google Scholar] [CrossRef]
- Chakraborty, G.; Park, I.-H.; Medishetty, R.; Vittal, J.J. Two-Dimensional Metal-Organic Framework Materials: Synthesis, Structures, Properties and Applications. Chem. Rev. 2021, 121, 3751–3891. [Google Scholar] [CrossRef]
- Shi, B.; Pang, X.; Li, S.; Wu, H.; Shen, J.; Wang, X.; Fan, C.; Cao, L.; Zhu, T.; Qiu, M.; et al. Short hydrogen-bond network confined on COF surfaces enables ultrahigh proton conductivity. Nat. Commun. 2022, 13, 6666. [Google Scholar] [CrossRef]
- Wang, X.; Shi, B.; Yang, H.; Guan, J.; Liang, X.; Fan, C.; You, X.; Wang, Y.; Zhang, Z.; Wu, H.; et al. Assembling covalent organic framework membranes with superior ion exchange capacity. Nat. Commun. 2022, 13, 1020. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Zhou, Y.; Wang, W.; Zhai, Y.; Liu, X.; He, W.; Ou, W.; Ding, R.; Zhang, H.-L.; Wu, M.; et al. Interlayer confinement toward short hydrogen bond network construction for fast hydroxide transport. Sci. Adv. 2025, 11, eadr5374. [Google Scholar] [CrossRef] [PubMed]
- Asghar, M.R.; Zhang, W.; Su, H.; Zhang, J.; Liu, H.; Xing, L.; Yan, X.; Xu, Q. A review of proton exchange membranes modified with inorganic nanomaterials for fuel cells. Energy Adv. 2024, 4, 185–223. [Google Scholar] [CrossRef]
- Radenahmad, N.; Afif, A.; Petra, P.I.; Rahman, S.M.; Eriksson, S.-G.; Azad, A.K. Proton-conducting electrolytes for direct methanol and direct urea fuel cells—A state-of-the-art review. Renew. Sustain. Energy Rev. 2016, 57, 1347–1358. [Google Scholar] [CrossRef]
- Wang, F.; Zuo, Z.; Li, L.; Li, K.; He, F.; Jiang, Z.; Li, Y. Large-Area Aminated-Graphdiyne Thin Films for Direct Methanol Fuel Cells. Angew. Chem. 2019, 131, 15152–15157. [Google Scholar] [CrossRef]
- Li, L.; Zuo, Z.; He, F.; Jiang, Z.; Li, Y. Nitrogen-rich Graphdiyne Film for Efficiently Suppressing the Methanol Crossover in Direct Methanol Fuel Cells. Chem. Res. Chin. Univ. 2021, 37, 1275–1282. [Google Scholar] [CrossRef]
- Tempelman, C.; Jacobs, J.; Balzer, R.; Degirmenci, V. Membranes for all vanadium redox flow batteries. J. Energy Storage 2020, 32, 101745. [Google Scholar] [CrossRef]
- Kim, S.; Choi, J.; Choi, C.; Heo, J.; Kim, D.W.; Lee, J.Y.; Hong, Y.T.; Jung, H.-T.; Kim, H.-T. Pore-Size-Tuned Graphene Oxide Frameworks as Ion-Selective and Protective Layers on Hydrocarbon Membranes for Vanadium Redox-Flow Batteries. Nano Lett. 2018, 18, 3962–3968. [Google Scholar] [CrossRef]
- Di, M.; Xiu, Y.; Dong, Z.; Hu, L.; Gao, L.; Dai, Y.; Yan, X.; Zhang, N.; Pan, Y.; Jiang, X.; et al. Two-dimensional MoS2 nanosheets constructing highly ion-selective composite membrane for vanadium redox flow battery. J. Membr. Sci. 2021, 623, 119051. [Google Scholar] [CrossRef]
- Pang, B.; Du, R.; Chen, W.; Cui, F.; Wang, N.; Zhao, H.; Xie, G.; Tiantian, L.; He, G.; Wu, X. Self-supporting sulfonated covalent organic framework as a highly selective continuous membrane for vanadium flow battery. Energy Storage Mater. 2024, 67, 103293. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Z.; Zhang, S.; Xu, J.; Liu, Y.; Zhang, Y.; Liu, J.; Zuo, Z. Two-Dimensional Materials for Selective Ion Transport Membrane: Synthesis and Application Advances. Colloids Interfaces 2025, 9, 63. https://doi.org/10.3390/colloids9050063
Jiang Z, Zhang S, Xu J, Liu Y, Zhang Y, Liu J, Zuo Z. Two-Dimensional Materials for Selective Ion Transport Membrane: Synthesis and Application Advances. Colloids and Interfaces. 2025; 9(5):63. https://doi.org/10.3390/colloids9050063
Chicago/Turabian StyleJiang, Zhijian, Shining Zhang, Jianzhi Xu, Ying Liu, Yuanyuan Zhang, Jianguo Liu, and Zicheng Zuo. 2025. "Two-Dimensional Materials for Selective Ion Transport Membrane: Synthesis and Application Advances" Colloids and Interfaces 9, no. 5: 63. https://doi.org/10.3390/colloids9050063
APA StyleJiang, Z., Zhang, S., Xu, J., Liu, Y., Zhang, Y., Liu, J., & Zuo, Z. (2025). Two-Dimensional Materials for Selective Ion Transport Membrane: Synthesis and Application Advances. Colloids and Interfaces, 9(5), 63. https://doi.org/10.3390/colloids9050063








