Effect of Size Asymmetry of Latex Nanoparticles on Formation and Properties of Nanocolloidal Gels
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Nanoparticle Synthesis
2.3. Characterization of Latex Nanoparticles
2.3.1. The Hydrodynamic Diameter and ζ Potential of the NPs
2.3.2. Rheological Properties
2.3.3. Imaging of NPs
3. Results and Discussion
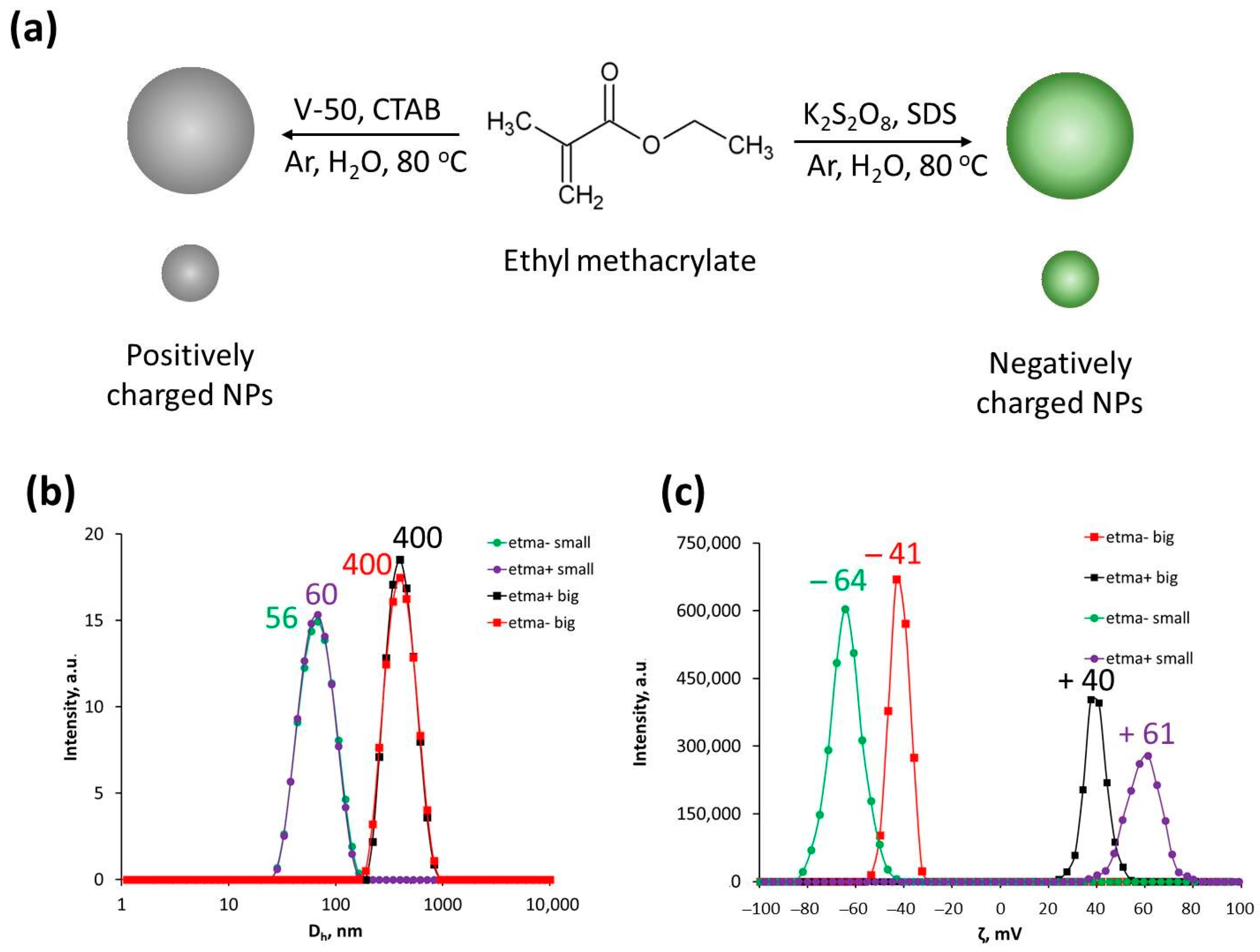
3.1. Nanoparticle Synthesis
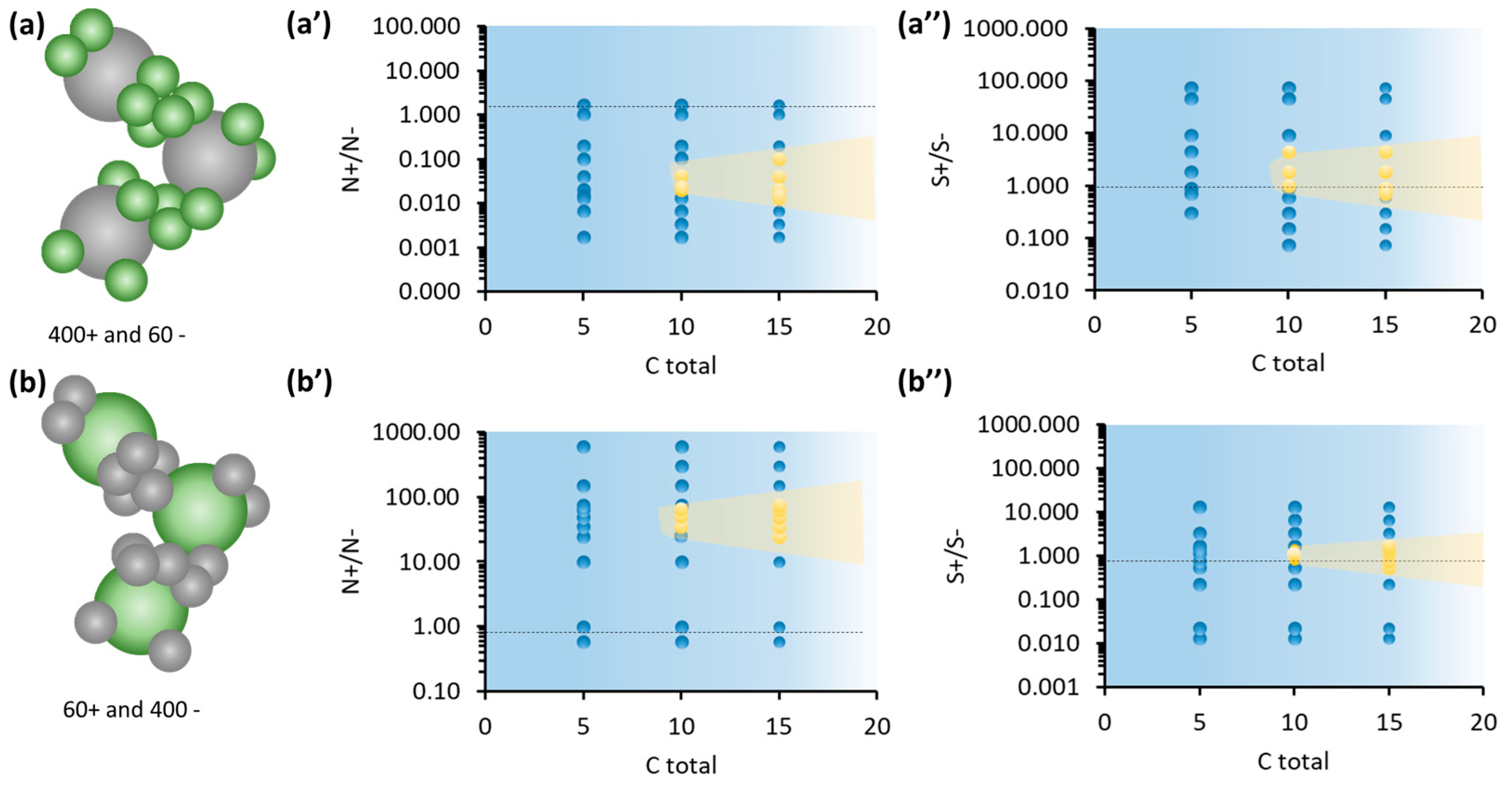
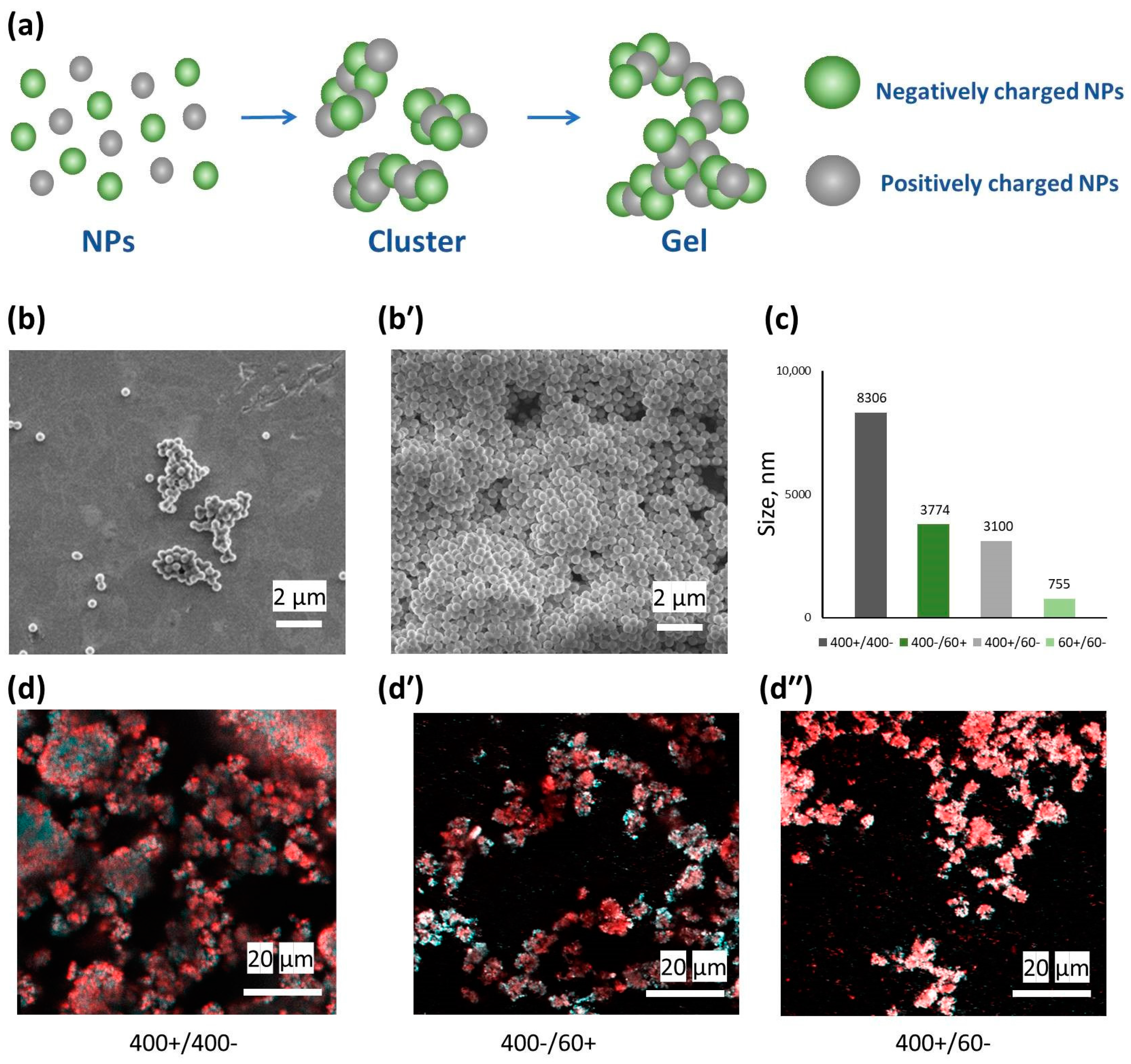
3.2. Gel Formation for Symmetric and Asymmetric Oppositely Charged Nanoparticles
3.3. Gel Properties for Symmetric and Asymmetric Oppositely Charged Nanoparticles
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Morozova, S.M.; Gevorkian, A.; Kumacheva, E. Design, Characterization and Applications of Nanocolloidal Hydrogels. Chem. Soc. Rev. 2023, 52, 5317–5339. [Google Scholar] [CrossRef]
- Lu, P.J.; Weitz, D.A. Colloidal Particles: Crystals, Glasses, and Gels. Annu. Rev. Condens. Matter Phys. 2013, 4, 217–233. [Google Scholar] [CrossRef]
- Ma, Y.; Morozova, S.M.; Kumacheva, E. From Nature-Sourced Polysaccharide Particles to Advanced Functional Materials. Adv. Mater. 2024, 36, e2312707. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Sun, J.; Yao, Q.; Ji, C.; Liu, J.; Zhu, Q. 3D Printing with Cellulose Materials. Cellulose 2018, 25, 4275–4301. [Google Scholar] [CrossRef]
- Zhu, C.; Pascall, A.J.; Dudukovic, N.; Worsley, M.A.; Kuntz, J.D.; Duoss, E.B.; Spadaccini, C.M. Colloidal Materials for 3D Printing. Annu. Rev. Chem. Biomol. Eng. 2019, 10, 17–42. [Google Scholar] [CrossRef]
- Highley, C.B.; Song, K.H.; Daly, A.C.; Burdick, J.A. Jammed Microgel Inks for 3D Printing Applications. Adv. Sci. 2019, 6, 1801076. [Google Scholar] [CrossRef]
- Tan, H.W.; An, J.; Chua, C.K.; Tran, T. Metallic Nanoparticle Inks for 3D Printing of Electronics. Adv. Electron. Mater. 2019, 5, 1800831. [Google Scholar] [CrossRef]
- Highley, C.B.; Rodell, C.B.; Burdick, J.A. Direct 3D Printing of Shear-Thinning Hydrogels into Self-Healing Hydrogels. Adv. Mater. 2015, 27, 5075–5079. [Google Scholar] [CrossRef] [PubMed]
- Bishop, K.J.M.; Biswal, S.L.; Bharti, B. Active Colloids as Models, Materials, and Machines. Annu. Rev. Chem. Biomol. Eng. 2023, 14, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Popescu, M.N.; Uspal, W.E.; Domínguez, A.; Dietrich, S. Effective Interactions between Chemically Active Colloids and Interfaces. Acc. Chem. Res. 2018, 51, 2991–2997. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lv, X.; Moran, J.L.; Duan, S.; Zhou, C. A Practical Guide to Active Colloids: Choosing Synthetic Model Systems for Soft Matter Physics Research. Soft Matter 2020, 16, 3846–3868. [Google Scholar] [CrossRef] [PubMed]
- Richtering, W.; Alberg, I.; Zentel, R. Nanoparticles in the Biological Context: Surface Morphology and Protein Corona Formation. Small 2020, 16, e2002162. [Google Scholar] [CrossRef]
- Zhang, M.; Ye, K.; Tao, H.; Cheng, R.; Zhang, D.; Pan, X. Colloid-like Tools Fast Beat Viral Bio-Colloids: Micron-Surface Enrichment and in-Situ Inactivation Induced by Interface-Decorated Microbubbles. Chem. Eng. J. 2024, 483, 149250. [Google Scholar] [CrossRef]
- Stradner, A.; Schurtenberger, P. Potential and Limits of a Colloid Approach to Protein Solutions. Soft Matter 2020, 16, 307–323. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Lin, Y.; Qiao, Y. Engineered Colloidosomes as Biomimetic Cellular Models. Giant 2023, 13, 100143. [Google Scholar] [CrossRef]
- Zong, Y.; Yuan, G.; Han, C.C. Asymmetrical Phase Separation and Gelation in Binary Mixtures of Oppositely Charged Colloids. J. Chem. Phys. 2016, 145, 014904. [Google Scholar] [CrossRef]
- Morozova, S.M.; Statsenko, T.G.; Ryabchenko, E.O.; Gevorkian, A.; Adibnia, V.; Lozhkin, M.S.; Kireynov, A.V.; Kumacheva, E. Multicolored Nanocolloidal Hydrogel Inks. Adv. Funct. Mater. 2021, 31, 2105470. [Google Scholar] [CrossRef]
- Morozova, S.M.; López-Flores, L.; Gevorkian, A.; Zhang, H.; Adibnia, V.; Shi, W.; Nykypanchuk, D.; Statsenko, T.G.; Walker, G.C.; Gang, O.; et al. Colloidal Clusters and Networks Formed by Oppositely Charged Nanoparticles with Varying Stiffnesses. ACS Nano 2023, 17, 15012–15024. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, S.J.; Ahn, K.H.; Lee, S.J. Nanoparticle-Induced Gelation of Bimodal Slurries with Highly Size-Asymmetric Particles: Effect of Surface Chemistry and Concentration. Langmuir 2015, 31, 13639–13646. [Google Scholar] [CrossRef]
- Dlamini, N.; Prestipino, S.; Pellicane, G. Self-Assembled Structures of Colloidal Dimers and Disks on a Spherical Surface. Entropy 2021, 23, 585. [Google Scholar] [CrossRef] [PubMed]
- Bremmell, K.E.; Dunstan, D.E. Probe Diffusion Measurements of Polystyrene Latex Particles in Polyelectrolyte Solutions of Varying Ionic Strength. Macromolecules 2002, 35, 1994–1999. [Google Scholar] [CrossRef]
- Cao, X.J.; Cummins, H.Z.; Morris, J.F. Structural and Rheological Evolution of Silica Nanoparticle Gels. Soft Matter 2010, 6, 5425. [Google Scholar] [CrossRef]
- Ghaffari, Z.; Rezvani, H.; Khalilnezhad, A.; Cortes, F.B.; Riazi, M. Experimental Characterization of Colloidal Silica Gel for Water Conformance Control in Oil Reservoirs. Sci. Rep. 2022, 12, 9628. [Google Scholar] [CrossRef] [PubMed]
- Ilyin, S.O.; Arinina, M.P.; Malkin, A.Y.; Kulichikhin, V.G. Sol–Gel Transition and Rheological Properties of Silica Nanoparticle Dispersions. Colloid J. 2016, 78, 608–615. [Google Scholar] [CrossRef]
- Neterebskaia, V.O.; Goncharenko, A.O.; Morozova, S.M.; Kolchanov, D.S.; Vinogradov, A.V. Inkjet Printing Humidity Sensing Pattern Based on Self-Organizing Polystyrene Spheres. Nanomaterials 2020, 10, 1538. [Google Scholar] [CrossRef] [PubMed]
- Lazzari, S.; Maggioni, G.M.; Soos, M.; Wu, H.; Morbidelli, M. Shear-Stability and Gelation of Inverse Latexes. Soft Matter 2013, 9, 10866. [Google Scholar] [CrossRef]
- de Oliveira Reis, G.; Menut, P.; Bonfils, F.; Vaysse, L.; Hemar, Y.; Sanchez, C. Acid-Induced Aggregation and Gelation of Natural Rubber Latex Particles. Colloids Surf. A Physicochem. Eng. Asp. 2015, 482, 9–17. [Google Scholar] [CrossRef]
- Desire, C.T.; Lotierzo, A.; Arrua, R.D.; Hilder, E.F.; Bon, S.A.F. Robust Open Cellular Porous Polymer Monoliths Made from Cured Colloidal Gels of Latex Particles. Green Chem. 2018, 20, 2499–2511. [Google Scholar] [CrossRef]
- Russell, E.R.; Sprakel, J.; Kodger, T.E.; Weitz, D.A. Colloidal Gelation of Oppositely Charged Particles. Soft Matter 2012, 8, 8697. [Google Scholar] [CrossRef]
- Singh, K.; Raghav, A.; Jha, P.K.; Satapathi, S. Effect of Size and Charge Asymmetry on Aggregation Kinetics of Oppositely Charged Nanoparticles. Sci. Rep. 2019, 9, 3762. [Google Scholar] [CrossRef] [PubMed]
- Sanz, E.; Valeriani, C.; Vissers, T.; Fortini, A.; Leunissen, M.E.; van Blaaderen, A.; Frenkel, D.; Dijkstra, M. Out-of-Equilibrium Processes in Suspensions of Oppositely Charged Colloids: Liquid-to-Crystal Nucleation and Gel Formation. J. Phys. Condens. Matter 2008, 20, 494247. [Google Scholar] [CrossRef]
- Manoharan, V.N. Colloidal Matter: Packing, Geometry, and Entropy. Science 2015, 349, 1253751. [Google Scholar] [CrossRef]
- Kim, Y.; Moon, J.; Hwang, H.; Kim, Y.S.; Yi, G. Advances in Colloidal Building Blocks: Toward Patchy Colloidal Clusters. Adv. Mater. 2023, 35, e2203045. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Franco, J.; Zaccarelli, E. On the Role of Competing Interactions in Charged Colloids with Short-Range Attraction. Annu. Rev. Condens. Matter Phys. 2021, 12, 51–70. [Google Scholar] [CrossRef]
- Matter, F.; Luna, A.L.; Niederberger, M. From Colloidal Dispersions to Aerogels: How to Master Nanoparticle Gelation. Nano Today 2020, 30, 100827. [Google Scholar] [CrossRef]
- Vasconcelos, D.C.L.; Campos, W.R.; Vasconcelos, V.; Vasconcelos, W.L. Influence of Process Parameters on the Morphological Evolution and Fractal Dimension of Sol–Gel Colloidal Silica Particles. Mater. Sci. Eng. A 2002, 334, 53–58. [Google Scholar] [CrossRef]
- Anderson, V.J.; Lekkerkerker, H.N.W. Insights into Phase Transition Kinetics from Colloid Science. Nature 2002, 416, 811–815. [Google Scholar] [CrossRef]
- Thérien-Aubin, H.; Lukach, A.; Pitch, N.; Kumacheva, E. Structure and Properties of Composite Films Formed by Cellulose Nanocrystals and Charged Latex Nanoparticles. Nanoscale 2015, 7, 6612–6618. [Google Scholar] [CrossRef] [PubMed]
- Vandegaer, J.E. Latex Growth. J. Appl. Polym. Sci. 1965, 9, 2929–2938. [Google Scholar] [CrossRef]
- Zaccarelli, E. Colloidal Gels: Equilibrium and Non-Equilibrium Routes. J. Phys. Condens. Matter 2007, 19, 323101. [Google Scholar] [CrossRef]
- Lu, P.J.; Conrad, J.C.; Wyss, H.M.; Schofield, A.B.; Weitz, D.A. Fluids of Clusters in Attractive Colloids. Phys. Rev. Lett. 2006, 96, 028306. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morozova, S.M.; Statsenko, T.G. Effect of Size Asymmetry of Latex Nanoparticles on Formation and Properties of Nanocolloidal Gels. Colloids Interfaces 2025, 9, 11. https://doi.org/10.3390/colloids9010011
Morozova SM, Statsenko TG. Effect of Size Asymmetry of Latex Nanoparticles on Formation and Properties of Nanocolloidal Gels. Colloids and Interfaces. 2025; 9(1):11. https://doi.org/10.3390/colloids9010011
Chicago/Turabian StyleMorozova, Sofia M., and Tatiana G. Statsenko. 2025. "Effect of Size Asymmetry of Latex Nanoparticles on Formation and Properties of Nanocolloidal Gels" Colloids and Interfaces 9, no. 1: 11. https://doi.org/10.3390/colloids9010011
APA StyleMorozova, S. M., & Statsenko, T. G. (2025). Effect of Size Asymmetry of Latex Nanoparticles on Formation and Properties of Nanocolloidal Gels. Colloids and Interfaces, 9(1), 11. https://doi.org/10.3390/colloids9010011






