Study of Interfacial Properties of Anionic–Nonionic Surfactants Based on Succinic Acid Derivatives via Molecular Dynamics Simulations and the IGMH Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Surfactant Molecular Conformation
2.2. Simulation Details
2.3. Cluster Analysis
3. Results and Discussion
3.1. Interfacial Membranes for MD Simulations
3.2. Interfacial Thickness
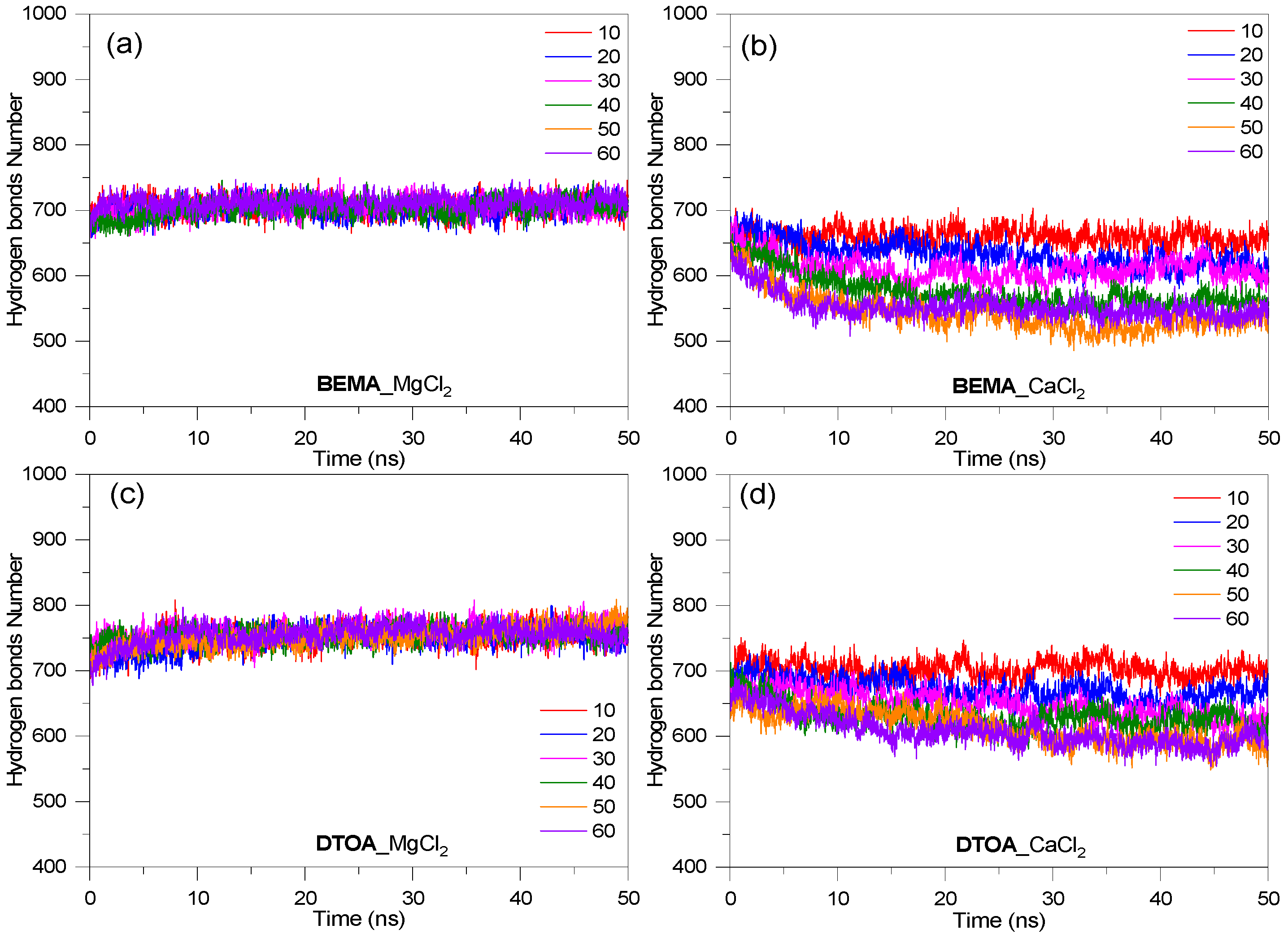
3.3. Hydrogen Bond Distributions
3.4. Order Parameter
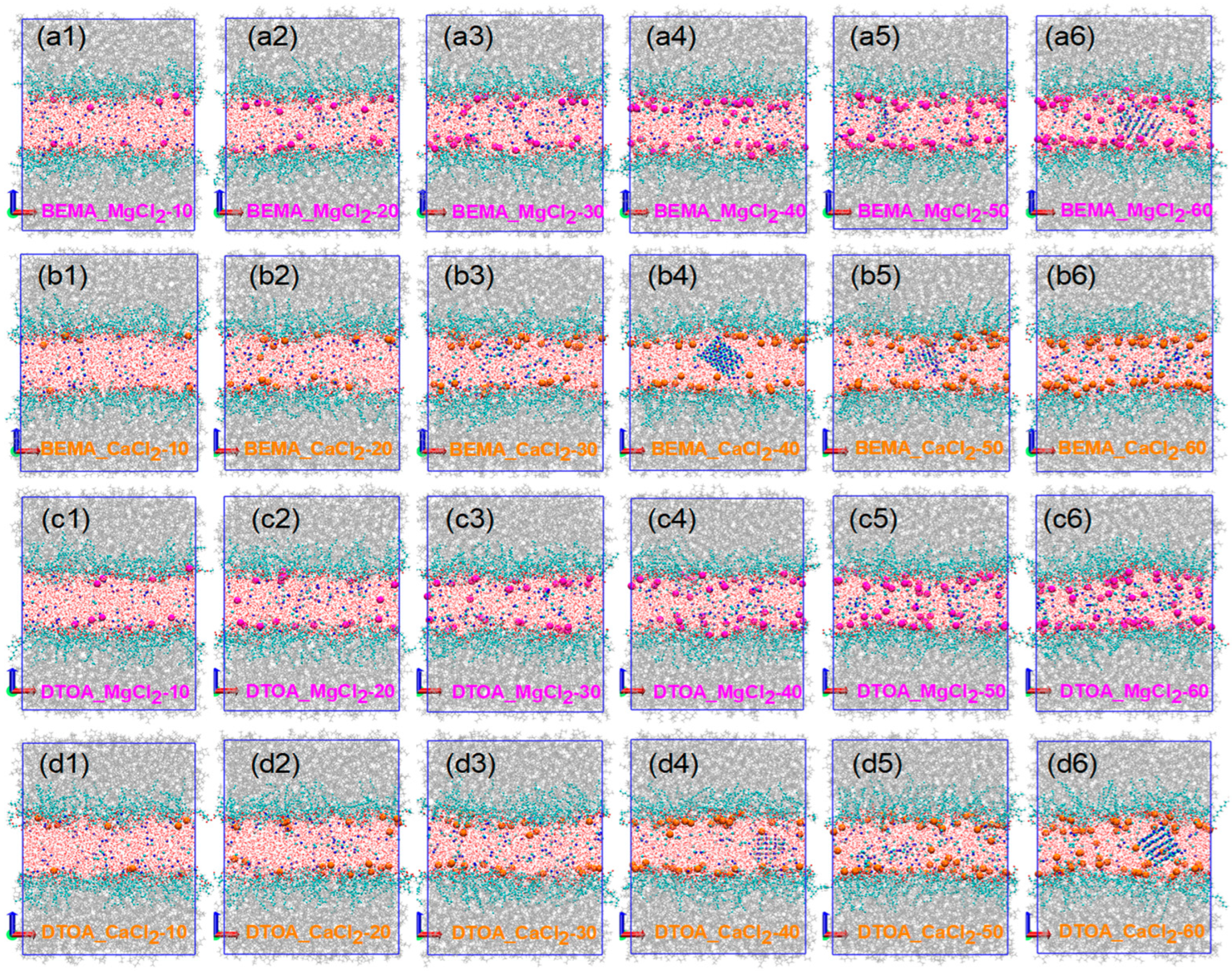
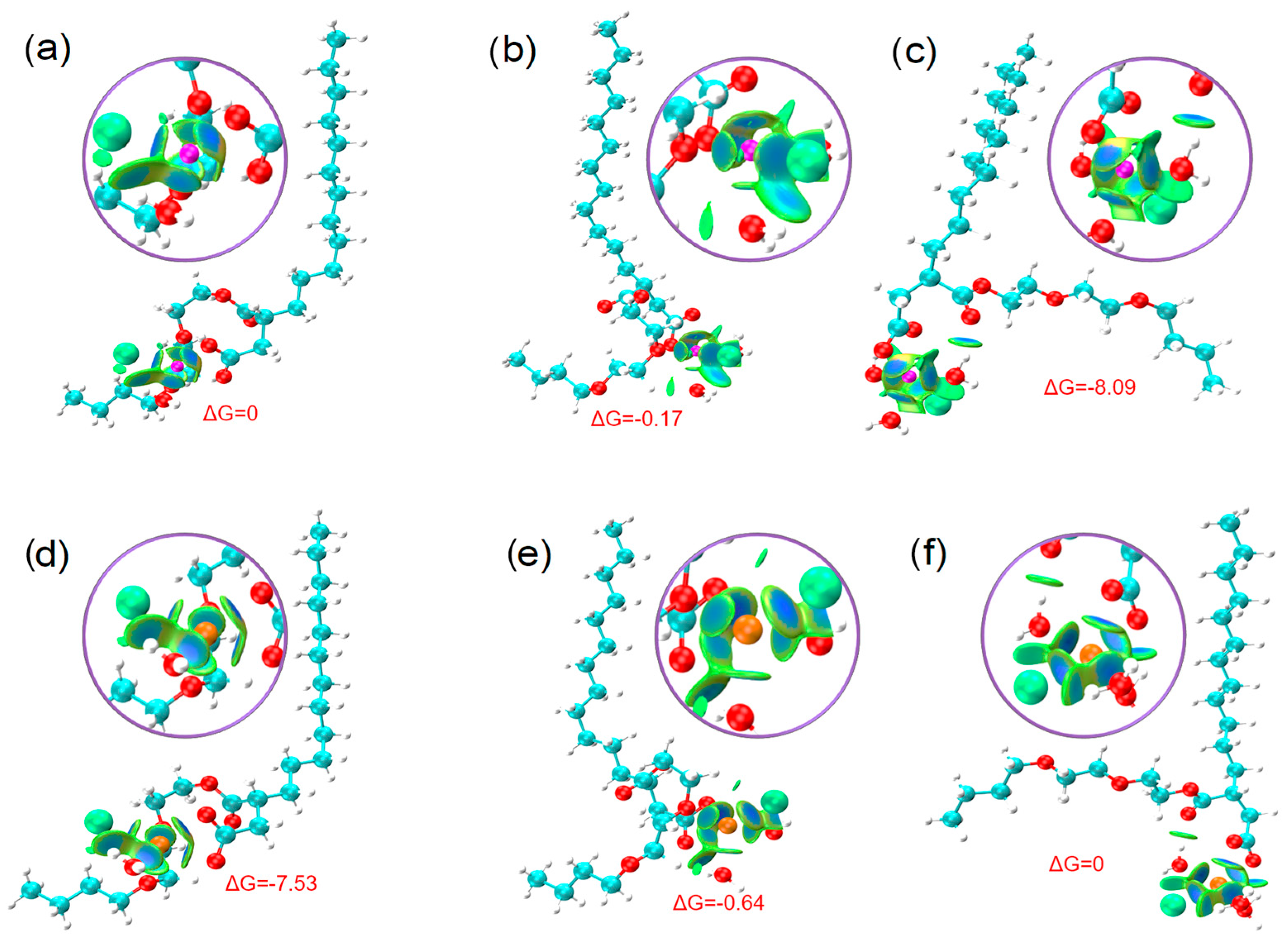
3.5. Salt Resistance of Surfactants
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Su, Y.; Sun, Y.; Zhai, Y.; Gu, L.; Li, J.; Gong, L.; Chang, C.; Yang, Y. Effects of surfactants on activity and structure of egg yolk antibody. Food Bioprod. Process. 2022, 132, 167–176. [Google Scholar] [CrossRef]
- Nitschke, M.; e Silva, S.S. Recent food applications of microbial surfactants. Crit. Rev. Food Sci. Nutr. 2018, 58, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Ji, S.; Ren, Z.; Jiang, S.; Yu, Q.; Guo, J.; Wang, A.X.; Kong, X. Rapid, convenient, and ultrasensitive point-of-care sensing of histamine from fish: A Portable chromatographic platform based on derivatization reaction. J. Chromatogr. A 2023, 1696, 463953. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Fan, Q.; Yu, Q.; Wang, R.; Wang, H.; Kong, X. Facile detection of carbendazim in food using TLC-SERS on diatomite thin layer chromatography. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 247, 119037. [Google Scholar] [CrossRef] [PubMed]
- Karthick, A.; Roy, B.; Chattopadhyay, P. A review on the application of chemical surfactant and surfactant foam for remediation of petroleum oil contaminated soil. J. Environ. Manag. 2019, 243, 187–205. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Gu, F.; Liao, K.; Wen, X.; Jiang, L.; Li, X.; Huang, W.; Shao, M. Molecular dynamics simulation of enhancing surfactant flooding performance by using SiO2 nanoparticles. J. Mol. Liq. 2022, 367, 120404. [Google Scholar] [CrossRef]
- Guagliardo, R.; Pérez-Gil, J.; De Smedt, S.; Raemdonck, K. Pulmonary surfactant and drug delivery: Focusing on the role of surfactant proteins. J. Control. Release 2018, 291, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Li, J. Molecular Assemblies of Biomimetic Microcapsules. Langmuir 2019, 35, 8557–8564. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, A.; Bhattacharyya, A. Quest for an eco-friendly alternative surfactant: Surface and foam characteristics of natural surfactants. J. Clean. Prod. 2017, 150, 127–134. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Y.; Liu, X.; Chen, H.; Fang, Y. Retrieving Oil and Recycling Surfactant in Surfactant-Enhanced Soil Washing. ACS Sustain. Chem. Eng. 2018, 6, 4981–4986. [Google Scholar] [CrossRef]
- Shao, Q.; He, Y.; White, A.D.; Jiang, S. Difference in Hydration between Carboxybetaine and Sulfobetaine. J. Phys. Chem. B 2010, 114, 16625–16631. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Dong, X.; Ruan, W.; Shang, Y.; Liu, H. A thermo-sensitive OEGMA-based polymer: Synthesis, characterization and interactions with surfactants in aqueous solutions with and without salt. Colloid Polym. Sci. 2017, 295, 327–340. [Google Scholar] [CrossRef]
- Souayeh, M.; Al-Maamari, R.S.; Karimi, M.; Aoudia, M. Wettability alteration and oil recovery by surfactant assisted low salinity water in carbonate rock: The impact of nonionic/anionic surfactants. J. Pet. Sci. Eng. 2021, 197, 108108. [Google Scholar] [CrossRef]
- Zhang, D.; Kang, J.; Zhu, W. Selective flotation separation of fluorite and calcite by utilising a novel anionic/nonionic collector. Colloids Surf. A Physicochem. Eng. Asp. 2022, 642, 128687. [Google Scholar] [CrossRef]
- Liang, X.; Dong, J.; Zhang, W.; Mo, Y.; Li, Y.; Bai, J. Solubilization mechanism and mass-transfer model of anionic-nonionic gemini surfactants for chlorinated hydrocarbons. Sep. Purif. Technol. 2024, 330, 125534. [Google Scholar] [CrossRef]
- Kleinen, J. Will biosurfactants replace conventional surfactants? Curr. Opin. Colloid Interface Sci. 2023, 68, 101764. [Google Scholar] [CrossRef]
- Baccile, N. Are microbial biosurfactants actually only surfactants? Curr. Opin. Colloid Interface Sci. 2023, 68, 101747. [Google Scholar] [CrossRef]
- Johnson, P.; Trybala, A.; Starov, V.; Pinfield, V.J. Effect of synthetic surfactants on the environment and the potential for substitution by biosurfactants. Adv. Colloid Interface Sci. 2021, 288, 102340. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, M.T.; Khan, M.N. Eco-friendly, biodegradable natural surfactant (Acacia concinna): An alternative to the synthetic surfactants. J. Clean. Prod. 2018, 188, 678–685. [Google Scholar] [CrossRef]
- Dix, L.R. Sodium Salts of Bis(1-dodecenylsuccinamic Acids): A Simple Route to Anionic Gemini Surfactants. J. Colloid Interface Sci. 2001, 238, 447–448. [Google Scholar] [CrossRef]
- Dix, L.R.; Gilblas, R. Lyotropic and interfacial behaviour of an anionic gemini surfactant. J. Colloid Interface Sci. 2006, 296, 762–765. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Cheng, Y.; Han, C.; Lai, J.; Sui, L.; Luo, G. Synthesis and Surface Properties of Anionic Gemini Surfactants having N-acylamide and Carboxylate Groups. J. Surfactants Deterg. 2014, 17, 727–732. [Google Scholar] [CrossRef]
- Sun, R.; Hong, K.H.; Lv, Z.; Ding, X.; Wu, X.; Yu, F. Synthesis and Surface Properties of a Novel Anionic Gemini Surfactant using Dioxaoctane as a Linking Group. J. Surfactants Deterg. 2018, 21, 461–466. [Google Scholar] [CrossRef]
- Penfold, J.; Thomas, R.K. The adsorption and self-assembly of biosurfactants and biosurfactant/surfactant mixtures using neutron scattering techniques. Curr. Opin. Colloid Interface Sci. 2023, 68, 101766. [Google Scholar] [CrossRef]
- Fu, Z.; Shen, Z.; Fan, Q.; Hao, S.; Wang, Y.; Liu, X.; Tong, X.; Kong, X.; Yang, Z. Preparation of multi-functional magnetic–plasmonic nanocomposite for adsorption and detection of thiram using SERS. J. Hazard. Mater. 2020, 392, 122356. [Google Scholar] [CrossRef] [PubMed]
- Russell Lewis, B.; Uddin, M.R.; Moniruzzaman, M.; Kuo, K.M.; Higgins, A.J.; Shah, L.M.N.; Sobott, F.; Parks, J.M.; Hammerschmid, D.; Gumbart, J.C.; et al. Conformational restriction shapes the inhibition of a multidrug efflux adaptor protein. Nat. Commun. 2023, 14, 3900. [Google Scholar] [CrossRef] [PubMed]
- Nikolaienko, T.Y. Interaction of anticancer drug doxorubicin with sodium oleate bilayer: Insights from molecular dynamics simulations. J. Mol. Liq. 2017, 235, 31–43. [Google Scholar] [CrossRef]
- Sharma, A.; Bhargava, B.L. Self-Assembly of Cations in Aqueous Solutions of Multiheaded Cationic Surfactants: All Atom Molecular Dynamics Simulation Studies. J. Phys. Chem. B 2018, 122, 10943–10952. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, W.; Liu, N.; Hu, D.; Yu, F.; He, Y.-P. Methionine-Derived Organogels as Lubricant Additives Enhance the Continuity of the Oil Film through Dynamic Self-Healing Assembly. Langmuir 2022, 38, 11492–11501. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Z.; Zhao, S.; Hong, K.H.; Zhang, M.-Y.; Song, L.; Yu, F.; Luo, G.; He, Y.-P. Pyromellitic-Based Low Molecular Weight Gelators and Computational Studies of Intermolecular Interactions: A Potential Additive for Lubricant. Langmuir 2021, 37, 2954–2962. [Google Scholar] [CrossRef]
- Zhu, X.; Jiang, Y.; Yang, D.; Zhang, L.; Li, Y.; Liu, M. Homochiral nanotubes from heterochiral lipid mixtures: A shorter alkyl chain dominated chiral self-assembly. Chem. Sci. 2019, 10, 3873–3880. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yuan, C.; Yan, X. Computational approaches for understanding and predicting the self-assembled peptide hydrogels. Curr. Opin. Colloid Interface Sci. 2022, 62, 101645. [Google Scholar] [CrossRef]
- Liu, P.-Y.; Zhao, S.-C.; Zhang, M.-Y.; Song, L.; Wang, C.; Yu, F.; Meng, Q.; Zhang, Z.; He, Y.-P. Pd-Catalyzed γ-Acetoxylation of Alkylamides: Structural Influence of Directing Groups. J. Org. Chem. 2022, 87, 6378–6386. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.-Y.; Zhao, Y.-M.; Xu, Y.; Wang, S.; Song, L.; Meng, Q.; Zhang, Z.; Yu, F.; He, Y.-P. Experiment and Computational Study on Pd-Catalyzed Methoxyiminoacyl-Directed γ-Alkoxylation of Alkylamides. J. Org. Chem. 2023, 88, 14445–14453. [Google Scholar] [CrossRef]
- Yuan, M.; Nie, W.; Yu, H.; Yan, J.; Bao, Q.; Zhou, W.; Hua, Y.; Guo, L.; Niu, W. Experimental and molecular dynamics simulation study of the effect of different surfactants on the wettability of low-rank coal. J. Environ. Chem. Eng. 2021, 9, 105986. [Google Scholar] [CrossRef]
- Lu, C.; Liu, W.; Yuan, Z.; Wang, L.; Zhang, Z.; Gao, Q.; Ding, W. Study on the Behavior of Saturated Cardanol-Based Surfactants at the Crude Oil/Water Interface through Molecular Dynamics Simulations. J. Phys. Chem. B 2023, 127, 8938–8949. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Xing, R.; Cui, J.; Fan, W.; Li, J.; Yan, X. Multistep Desolvation as a Fundamental Principle Governing Peptide Self-Assembly Through Liquid–Liquid Phase Separation. CCS Chem. 2024, 6, 255–265. [Google Scholar] [CrossRef]
- Moosavi, S.S.; Zolghadr, A.R. Effect of quaternary ammonium surfactants on biomembranes using molecular dynamics simulation. RSC Adv. 2023, 13, 33175–33186. [Google Scholar] [CrossRef]
- Poorsargol, M.; Sohrabi, B.; Dehestani, M. Study of the Gemini Surfactants’ Self-Assembly on Graphene Nanosheets: Insights from Molecular Dynamic Simulation. J. Phys. Chem. A 2018, 122, 3873–3885. [Google Scholar] [CrossRef]
- Su, T.; Hong, K.H.; Zhang, W.; Li, F.; Li, Q.; Yu, F.; Luo, G.; Gao, H.; He, Y.-P. Scaleable two-component gelator from phthalic acid derivatives and primary alkyl amines: Acid–base interaction in the cooperative assembly. Soft Matter 2017, 13, 4066–4073. [Google Scholar] [CrossRef]
- Roy, S.; Skoff, D.; Perroni, D.V.; Mondal, J.; Yethiraj, A.; Mahanthappa, M.K.; Zanni, M.T.; Skinner, J.L. Water Dynamics in Gyroid Phases of Self-Assembled Gemini Surfactants. J. Am. Chem. Soc. 2016, 138, 2472–2475. [Google Scholar] [CrossRef] [PubMed]
- Naullage, P.M.; Bertolazzo, A.A.; Molinero, V. How Do Surfactants Control the Agglomeration of Clathrate Hydrates? ACS Cent. Sci. 2019, 5, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Z.; Zeng, G.; Chen, M.; Jiang, Y.; Shao, B.; Li, Z.; Liu, Y. Effect of surfactants on the interaction of phenol with laccase: Molecular docking and molecular dynamics simulation studies. J. Hazard. Mater. 2018, 357, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, G.; Nielsen, S.O.; Moore, P.B.; Klein, M.L. Molecular Dynamics Simulations of Surfactant Self-Organization at a Solid-Liquid Interface. J. Am. Chem. Soc. 2006, 128, 848–853. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, Y.; Tian, S.; Yan, H. Molecular Dynamics Simulation of Emulsification/Demulsification with a Gas Switchable Surfactant. J. Phys. Chem. C 2019, 123, 25246–25254. [Google Scholar] [CrossRef]
- He, M.; Zheng, W.; Wang, N.; Gao, H.; Ouyang, D.; Huang, Z. Molecular Dynamics Simulation of Drug Solubilization Behavior in Surfactant and Cosolvent Injections. Pharmaceutics 2022, 14, 2366. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, M.-Y.; Wang, K.; Sun, R.; Zhao, S.; Zhang, Z.; He, Y.-P.; Yu, F. Geometry transformation of ionic surfactants and adsorption behavior on water/n-decane-interface: Calculation by molecular dynamics simulation and DFT study. RSC Adv. 2021, 11, 28286–28294. [Google Scholar] [CrossRef] [PubMed]
- Arbabi, S.; Deuar, P.; Denys, M.; Bennacer, R.; Che, Z.; Theodorakis, P.E. Molecular dynamics simulation of the coalescence of surfactant-laden droplets. Soft Matter 2023, 19, 8070–8080. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liao, M.; Ma, K.; Wang, Z.; Demé, B.; Penfold, J.; Lu, J.R.; Webster, J.R.P.; Li, P. Implications of surfactant hydrophobic chain architecture on the Surfactant-Skin lipid model interaction. J. Colloid Interface Sci. 2022, 608, 405–415. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, K.; Wang, C.; Zhao, S.; Zhang, Z.; Yu, F.; He, Y.-P. Enhanced oil recovery: QM/MM based descriptors for anionic surfactant salt-resistance. Colloids Surf. A Physicochem. Eng. Asp. 2022, 641, 128422. [Google Scholar] [CrossRef]
- Alonso, G.; Gamallo, P.; Mejía, A.; Sayós, R. Assessing salt-surfactant synergistic effects on interfacial tension from molecular dynamics simulations. J. Mol. Liq. 2020, 299, 112223. [Google Scholar] [CrossRef]
- Pereira, F.; Xiao, K.; Latino, D.A.R.S.; Wu, C.; Zhang, Q.; Aires-de-Sousa, J. Machine Learning Methods to Predict Density Functional Theory B3LYP Energies of HOMO and LUMO Orbitals. J. Chem. Inf. Model. 2017, 57, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.; Schauperl, M.; Nerenberg, P.S. Benchmarking Electronic Structure Methods for Accurate Fixed-Charge Electrostatic Models. J. Chem. Inf. Model. 2020, 60, 249–258. [Google Scholar] [CrossRef]
- Case, D.A.; Aktulga, H.M.; Belfon, K.; Cerutti, D.S.; Cisneros, G.A.; Cruzeiro, V.W.D.; Forouzesh, N.; Giese, T.J.; Götz, A.W.; Gohlke, H.; et al. AmberTools. J. Chem. Inf. Model. 2023, 63, 6183–6191. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Martínez, L.; Andrade, R.; Birgin, E.G.; Martínez, J.M. PACKMOL: A package for building initial configurations for molecular dynamics simulations. J. Comput. Chem. 2009, 30, 2157–2164. [Google Scholar] [CrossRef]
- Ivanova, A.A.; Cheremisin, A.N.; Barifcani, A.; Iglauer, S.; Phan, C. Molecular insights in the temperature effect on adsorption of cationic surfactants at liquid/liquid interfaces. J. Mol. Liq. 2020, 299, 112104. [Google Scholar] [CrossRef]
- Páll, S.; Zhmurov, A.; Bauer, P.; Abraham, M.; Lundborg, M.; Gray, A.; Hess, B.; Lindahl, E. Heterogeneous parallelization and acceleration of molecular dynamics simulations in GROMACS. J. Chem. Phys. 2020, 153, 134110. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Lu, T.; Chen, Q. Independent gradient model based on Hirshfeld partition: A new method for visual study of interactions in chemical systems. J. Comput. Chem. 2022, 43, 539. [Google Scholar] [CrossRef]
- Lu, T.; Chen, Q. Visualization Analysis of Weak Interactions in Chemical Systems. In Comprehensive Computational Chemistry, 1st ed.; Yáñez, M., Boyd, R.J., Eds.; Elsevier: Oxford, UK, 2024; pp. 240–264. [Google Scholar]
- Jang, S.S.; Lin, S.-T.; Maiti, P.K.; Blanco, M.; Goddard, W.A.; Shuler, P.; Tang, Y. Molecular Dynamics Study of a Surfactant-Mediated Decane-Water Interface: Effect of Molecular Architecture of Alkyl Benzene Sulfonate. J. Phys. Chem. B 2004, 108, 12130–12140. [Google Scholar] [CrossRef]
- Yu, F.; Miao, Y.; Wang, M.; Liu, Q.; Yuan, L.; Geng, R.; Qiu, Q.; Ni, C.; Kay, M. Predicting nanoemulsion formulation and studying the synergism mechanism between surfactant and cosurfactant: A combined computational and experimental approach. Int. J. Pharm. 2022, 615, 121473. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Luo, H.; Tan, X.; Lu, Y.; Zhang, H.; Yang, X. Molecular dynamics simulation study of adsorption of anionic–nonionic surfactants at oil/water interfaces. RSC Adv. 2022, 12, 27330–27343. [Google Scholar] [CrossRef] [PubMed]
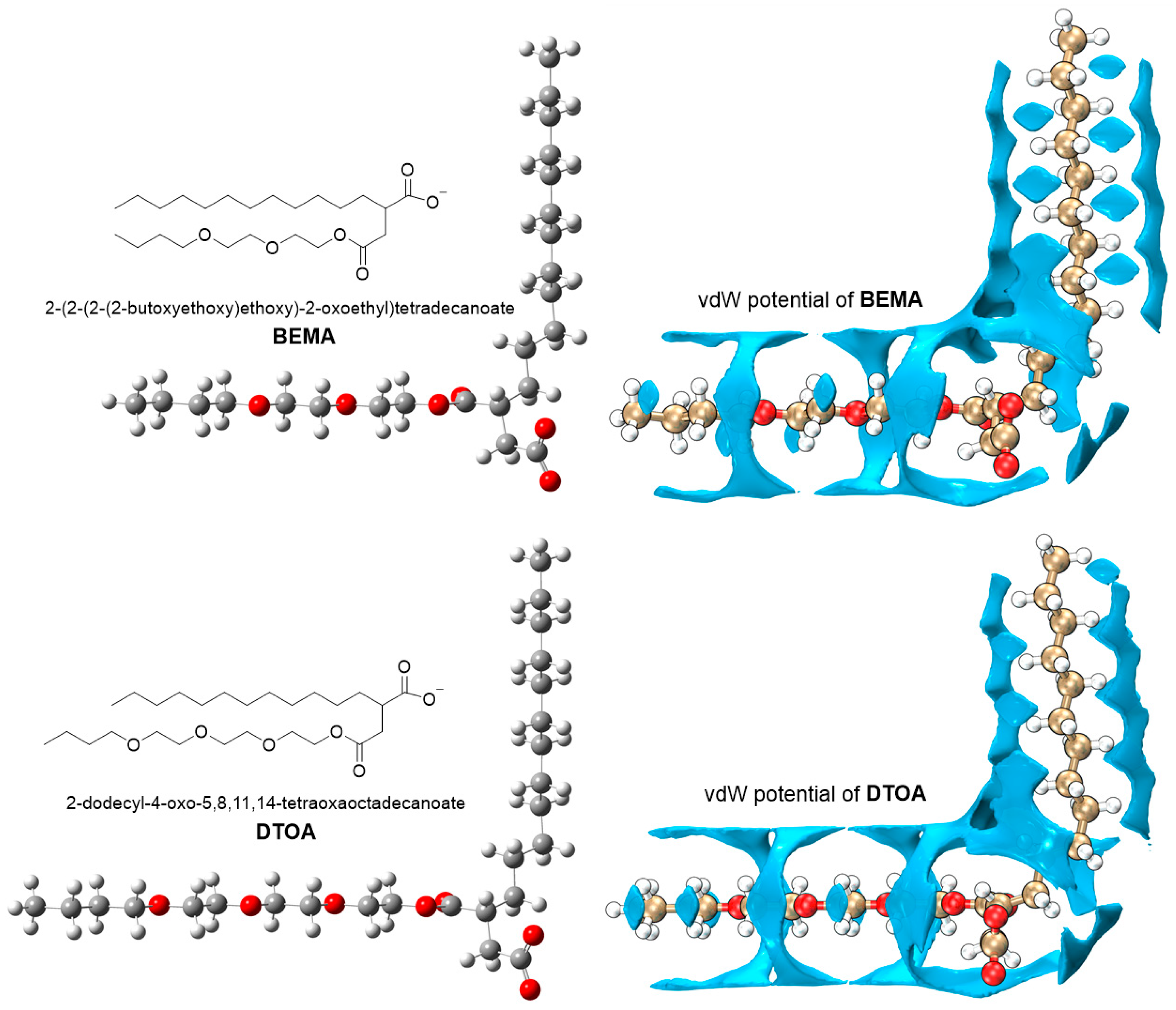
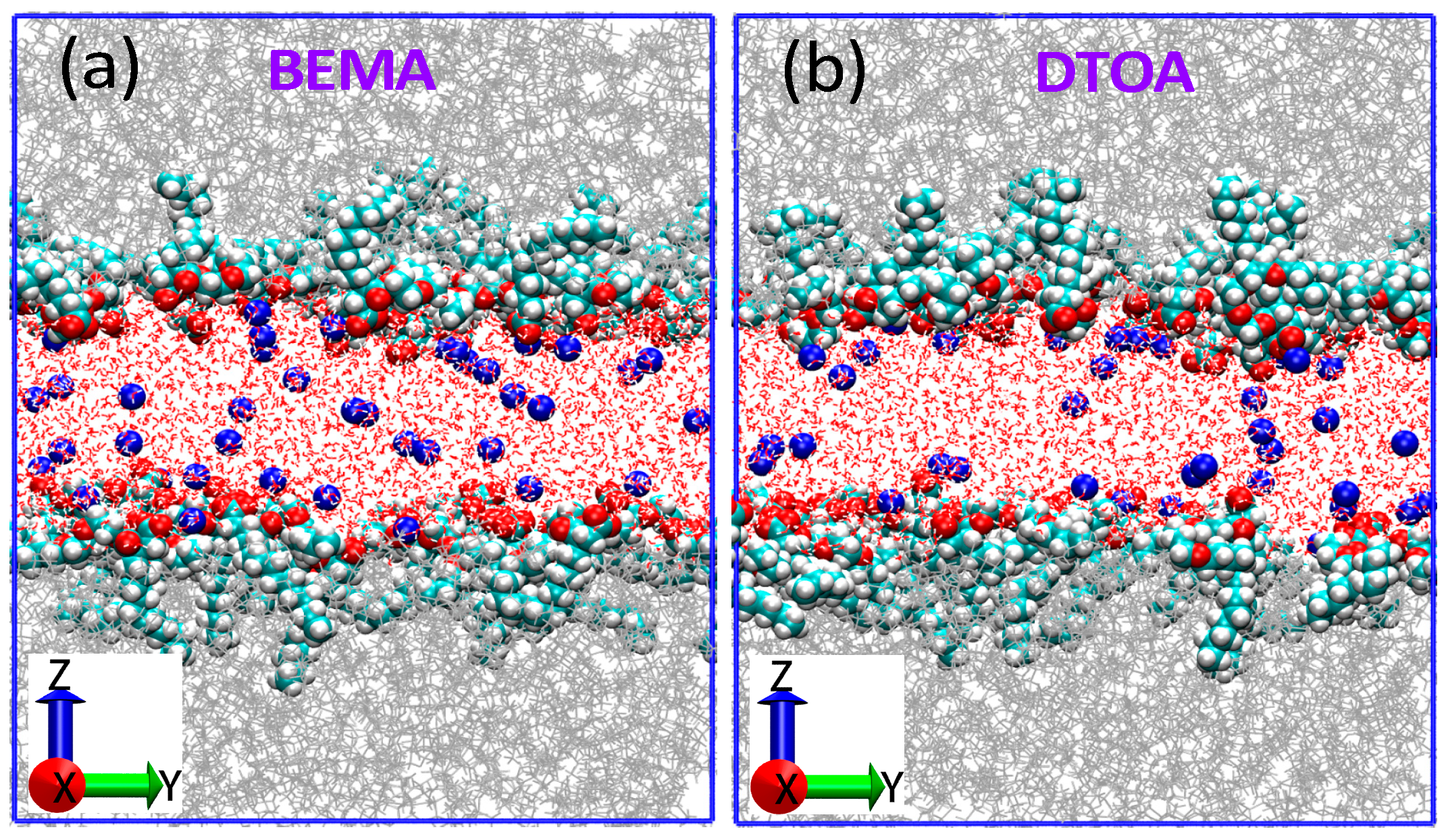
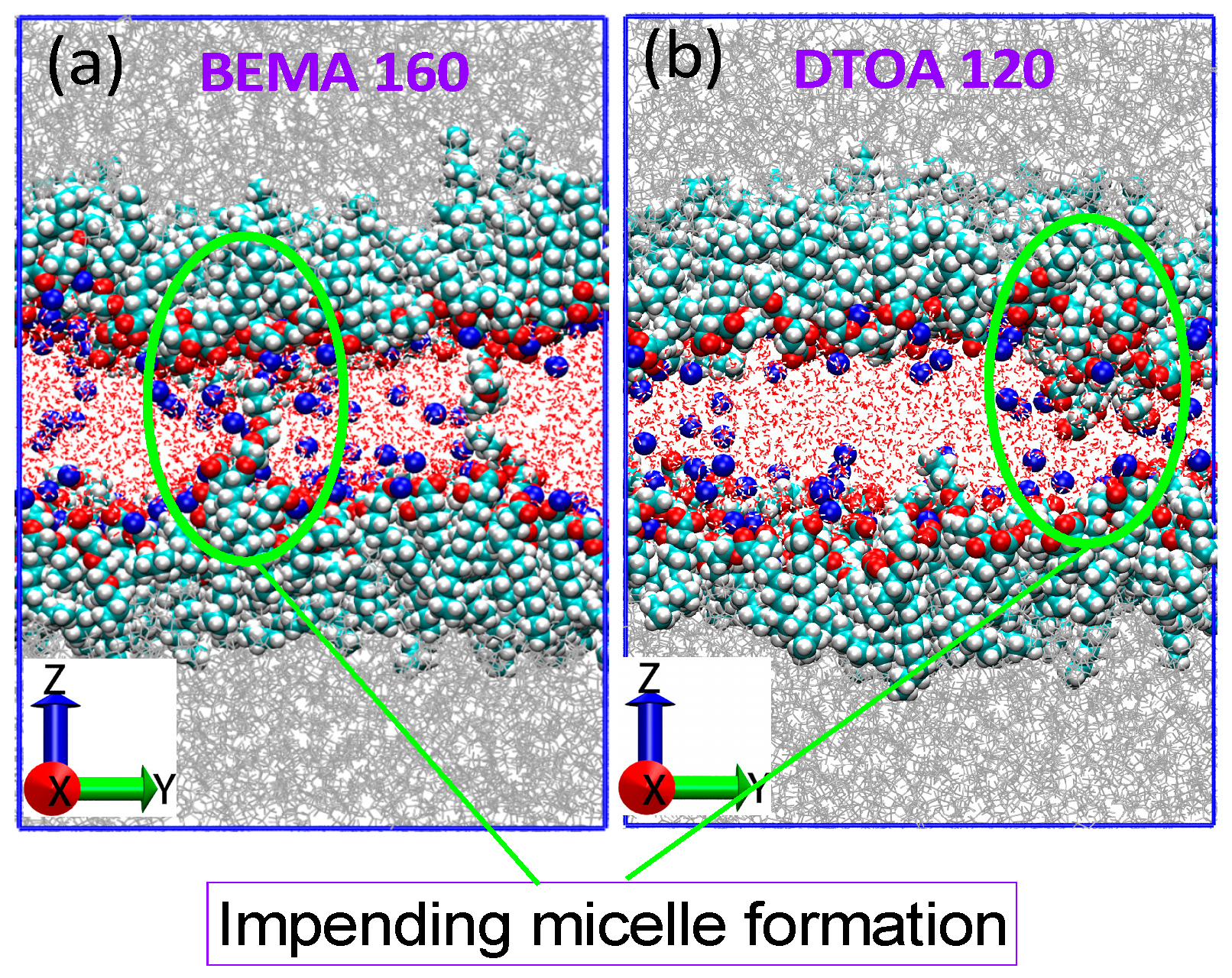
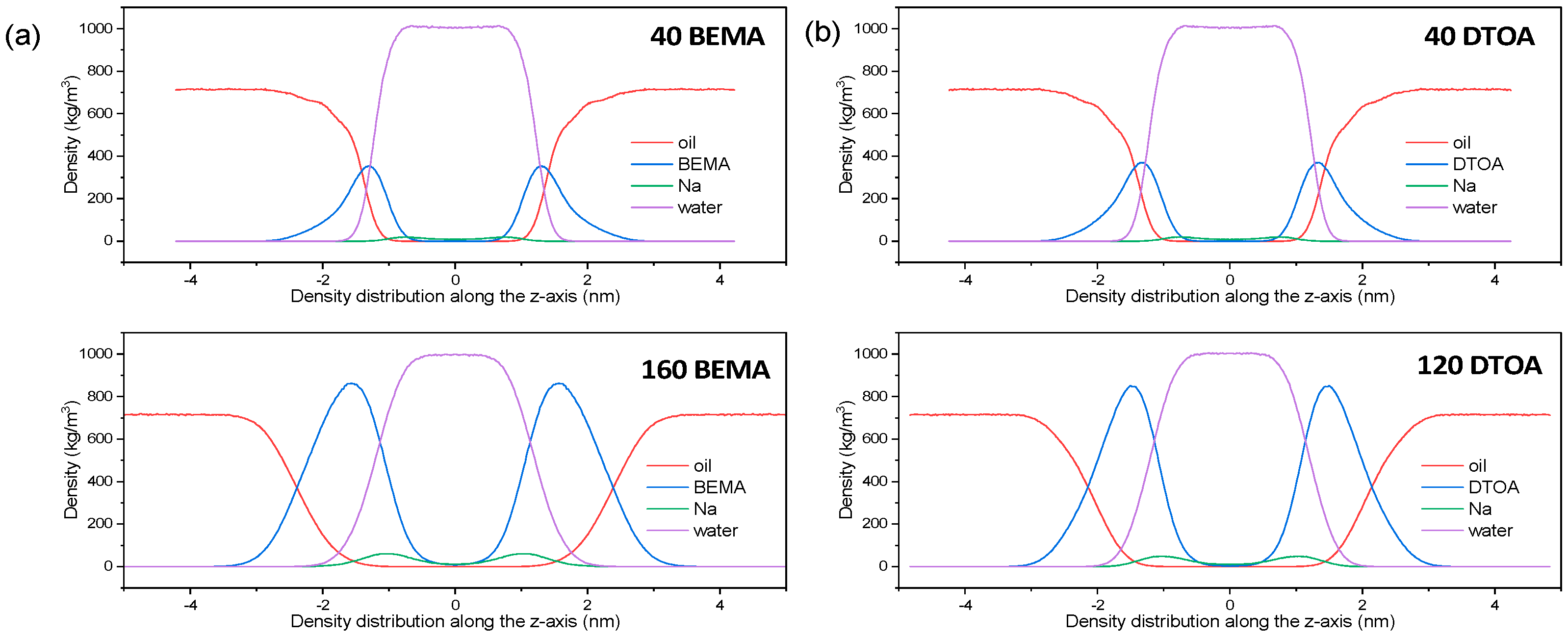


| Entry | Numbers | BEMA (nm) 2 | DTOA (nm) 2 |
|---|---|---|---|
| 1 | 20 1 | 8.19 | 8.20 |
| 2 | 40 | 8.46 | 8.50 |
| 3 | 60 | 8.72 | 8.76 |
| 4 | 80 | 8.99 | 9.03 |
| 5 | 100 | 9.31 | 9.46 |
| 6 | 120 | 9.57 | 9.88 |
| 7 | 140 | 9.85 | 10.15 |
| 8 | 160 | 10.09 | 10.42 |
| 9 | 180 | 10.37 | – |
| 10 | 200 | 10.68 | – |
| Model | Numbers | Interfacial Thickness |
|---|---|---|
| BEMA | 40 | 1.06 |
| BEMA | 160 | 2.14 |
| DTOA | 40 | 1.09 |
| DTOA | 120 | 1.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Luo, F.; Gao, Z.; Chi, H.; Wang, J.; Yu, F.; He, Y.-P. Study of Interfacial Properties of Anionic–Nonionic Surfactants Based on Succinic Acid Derivatives via Molecular Dynamics Simulations and the IGMH Method. Colloids Interfaces 2024, 8, 41. https://doi.org/10.3390/colloids8040041
Zhang W, Luo F, Gao Z, Chi H, Wang J, Yu F, He Y-P. Study of Interfacial Properties of Anionic–Nonionic Surfactants Based on Succinic Acid Derivatives via Molecular Dynamics Simulations and the IGMH Method. Colloids and Interfaces. 2024; 8(4):41. https://doi.org/10.3390/colloids8040041
Chicago/Turabian StyleZhang, Wannian, Feng Luo, Zhigang Gao, Haizhu Chi, Jinlong Wang, Fang Yu, and Yu-Peng He. 2024. "Study of Interfacial Properties of Anionic–Nonionic Surfactants Based on Succinic Acid Derivatives via Molecular Dynamics Simulations and the IGMH Method" Colloids and Interfaces 8, no. 4: 41. https://doi.org/10.3390/colloids8040041
APA StyleZhang, W., Luo, F., Gao, Z., Chi, H., Wang, J., Yu, F., & He, Y.-P. (2024). Study of Interfacial Properties of Anionic–Nonionic Surfactants Based on Succinic Acid Derivatives via Molecular Dynamics Simulations and the IGMH Method. Colloids and Interfaces, 8(4), 41. https://doi.org/10.3390/colloids8040041







