1. Introduction
The wetting dynamics of complex fluids, such as polymer or surfactant solutions, can be significantly different with respect to simple liquids. Even in the case of very dilute solutions, the comparison with a Newtonian solvent (e.g., water) reveals significant differences in the behaviour of the moving contact line during the spreading and/or receding phase, in the amplitude of the apparent dynamic contact angle, and in the intrinsic time scale of the phenomenon. A well-known example is the dynamic wetting behaviour of dilute polymer solution droplets impacting on low-energy (hydrophobic) surfaces. When a droplet of water falls on to a hydrophobic surface, such as the waxy leaf of a plant, the drop is often observed to bounce off; however, for about 20 years it has been known that the addition of very small quantities (c ∼ 100 ppm) of a high-molecular weight flexible polymer such as poly-(ethylene oxide) (PEO) can completely prevent rebound, by reducing the recoil velocity of the drop after the inertial spreading of two orders of magnitude [
1,
2]. This is surprising since the shear viscosity and surface tension of such drops are almost identical to those of pure water.
This phenomenon was initially understood as a direct consequence of the nonlinear bulk rheology of the fluid, namely of the elongational viscosity, and normal stresses [
1,
2,
3]. However, the interpretation in terms of bulk elongational viscosity was soon contradicted by a number of different experiments revealing the prevailing role of dynamic wetting [
4,
5,
6,
7,
8]. Remarkably, some of the elongational viscosity measurements used to support the initial understanding of the phenomenon turned out to be highly inaccurate [
9]. Later on, it was proposed to describe the contact line dynamics using a modifieded lubrication equation for thin films including an additional dissipative term proportional to the first normal stress coefficient [
3]. This approach, however, does not consider the elastic force associated with normal stresses, which should accelerate drop retraction instead of slowing it down as observed experimentally. Moreover, in dilute solutions the magnitude of normal stresses is too small, therefore the effect on the contact line dynamics is negligible; a significant reduction of the contact line velocity can be obtained only with normal stress values typical of semi–dilute solutions [
9].
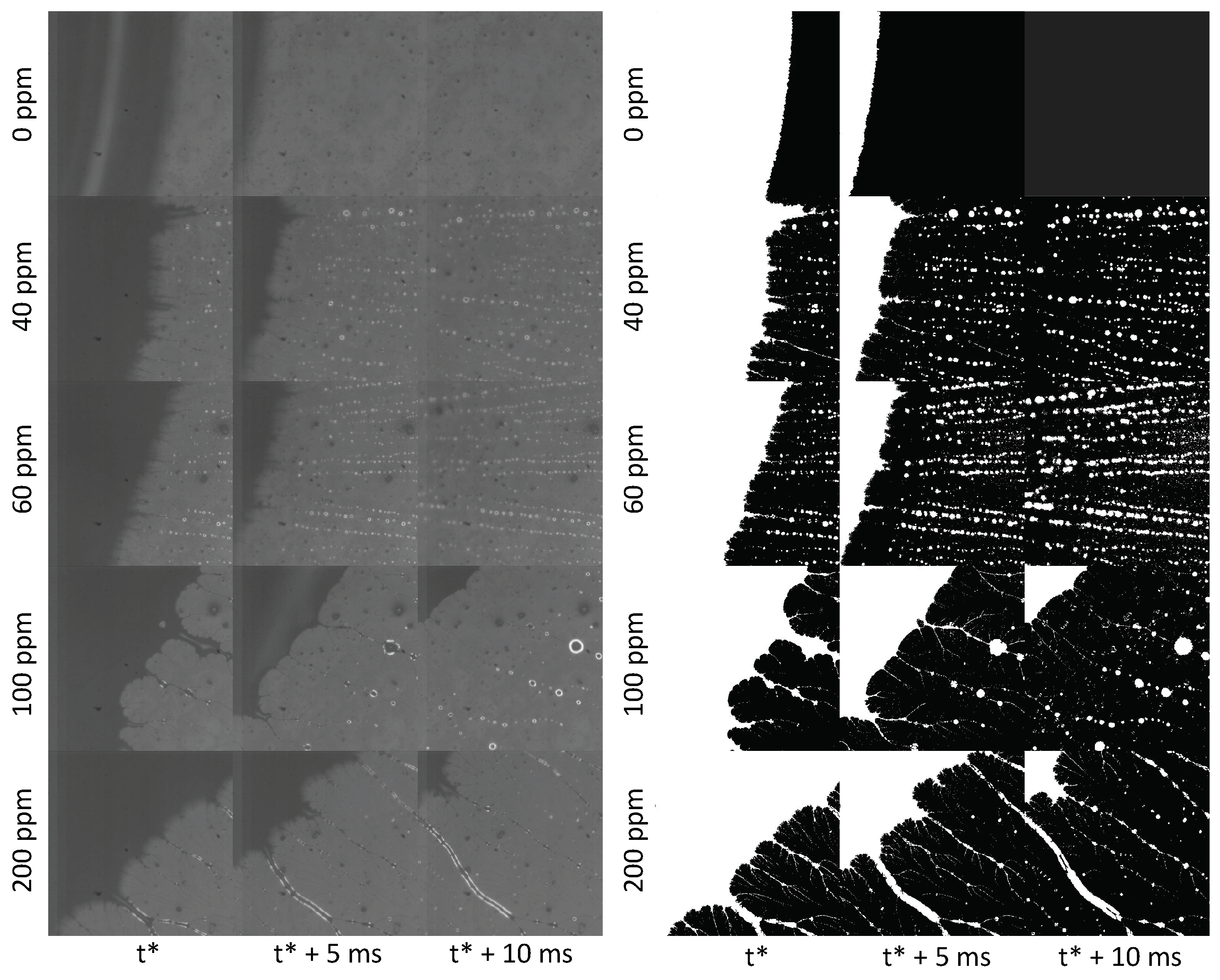
More recently, it was observed that when dilute solution drops are doped with fluorescent
–DNA, the de–wetted substrate is covered with stretched DNA molecules, oriented in the direction perpendicular to the receding contact line [
7]. Independent experiments on forced dewetting showed that polymer deposited on the substrate results into a velocity–dependent force at the contact line [
10]. These results suggest that the receding contact line is slowed down by a force, in the direction opposed to the contact line movement, which arises in the liquid film left behind the drop edge during retraction.
Here, the microscopic contact line morphology during dewetting of dilute polymer solution drops impacting on a hydrophobic surface is studied by high–speed microscopy, to get a deeper understanding of the origin of contact line forces. Experiments reveal the formation of transient microscopic dendritic structures generated by the receding contact line, which evolve in a similar fashion to the well–known beads–on–a–string mechanism [
11]. Fingering and/or dendritic structures on a moving contact line were observed during spreading of surfactant solutions [
12], evaporation of aqueous polymer solutions [
13] and particle-laden droplets [
14]; however, they have never been observed during rapid dewetting following drop impact. It is demonstrated that the shear flow in the liquid wedge near the contact line induces a second order coil–stretch transition of the polymer molecules leading to a significant increase of the local viscosity [
15], which enables a quantitative estimation of the contact line friction. The proposed approach is substantially different from most of the existing studies, which interpret the same phenomenon as a consequence of a hypothetical but unrealistic elongational flow within the impacting drop.
2. Materials and Methods
Polymer solutions were prepared by dissolving polyethylene oxide (PEO) with average molecular weight of 4000 kDa (Sigma-Aldrich, St. Louis, MO, USA) in de-ionised water (Barnstead Easypure), at concentrations of 40, 60, 100 and 200 ppm. Since the overlap concentration of this polymer in water, calculated based on the Mark–Houwink correlation for the characteristic viscosity, is approximately 570 ppm [
9,
16,
17], these solutions fall within the dilute regime. In this regime viscosity,
, and the relaxation time,
, are approximately a linear and a square root function of the polymer concentration, respectively [
18]. Unlike
and
, the surface tension,
, of PEO solutions is approximately the same as the solvent (∼70 mN/m) on the timescale of experiments [
19].
The impact substrates were glass slides coated with Fluoropel PFC1302A (Cytonix Corp., Beltsville, MD, USA), a 2% fluoropolymer solution in low boiling point (135 C) fluorosolvent, with equilibrium contact angle for water of 105 2; the Fluoropel coating was created by dipping glass slides into the liquid, and then dried at 90 C for 10 min to optimize adhesion.
Drops were released from a blunt hypodermic needle (gauge 21, i.d. 0.495 mm) suspended above the target surface. The equilibrium drop diameter (obtained from drop mass measurements) was in the range between 2.92 mm and 3.06 mm for all fluids. The impact velocity was controlled by adjusting the falling height between 20 mm and 140 mm, corresponding to impact Weber numbers between 13 and 110; the Weber number, , where is the fluid density, denotes the vertical impact velocity, and denotes the equilibrium drop diameter prior to impact, is routinely used in the drop impact literature to characterise impacts through the competition between inertial and capillary forces, although it does not take into account the viscous dissipation. To account for viscous effects, one can introduce the Reynolds number , where is the fluid viscosity, representing the ratio of inertial to viscous forces, and the Ohnesorge number , representing the ratio of viscous to capillary forces.
The contact line details during drop impact and recoil were recorded using a high-speed CMOS camera (Phantom v9.1) equipped with a Keyence VH-100ZR zoom lens (magnification range of 100×–1000×), at the speed of 5000 fps and a resolution of pixels, corresponding to 1.46 m/pixel; the camera and the lens were arranged vertically looking at the substrate from below, while illumination was provided by an optic fiber halogen illuminator (ThorLabs, Newton, NJ, USA).
4. Discussion
To understand how the theoretical framework outlined in
Section 3.2 above can be applied to the case of the receding contact line of a polymer solution drop after impacting onto a solid surface, one must observe the drop dynamics at the very begenning of the recoil stage after maximum spreading.
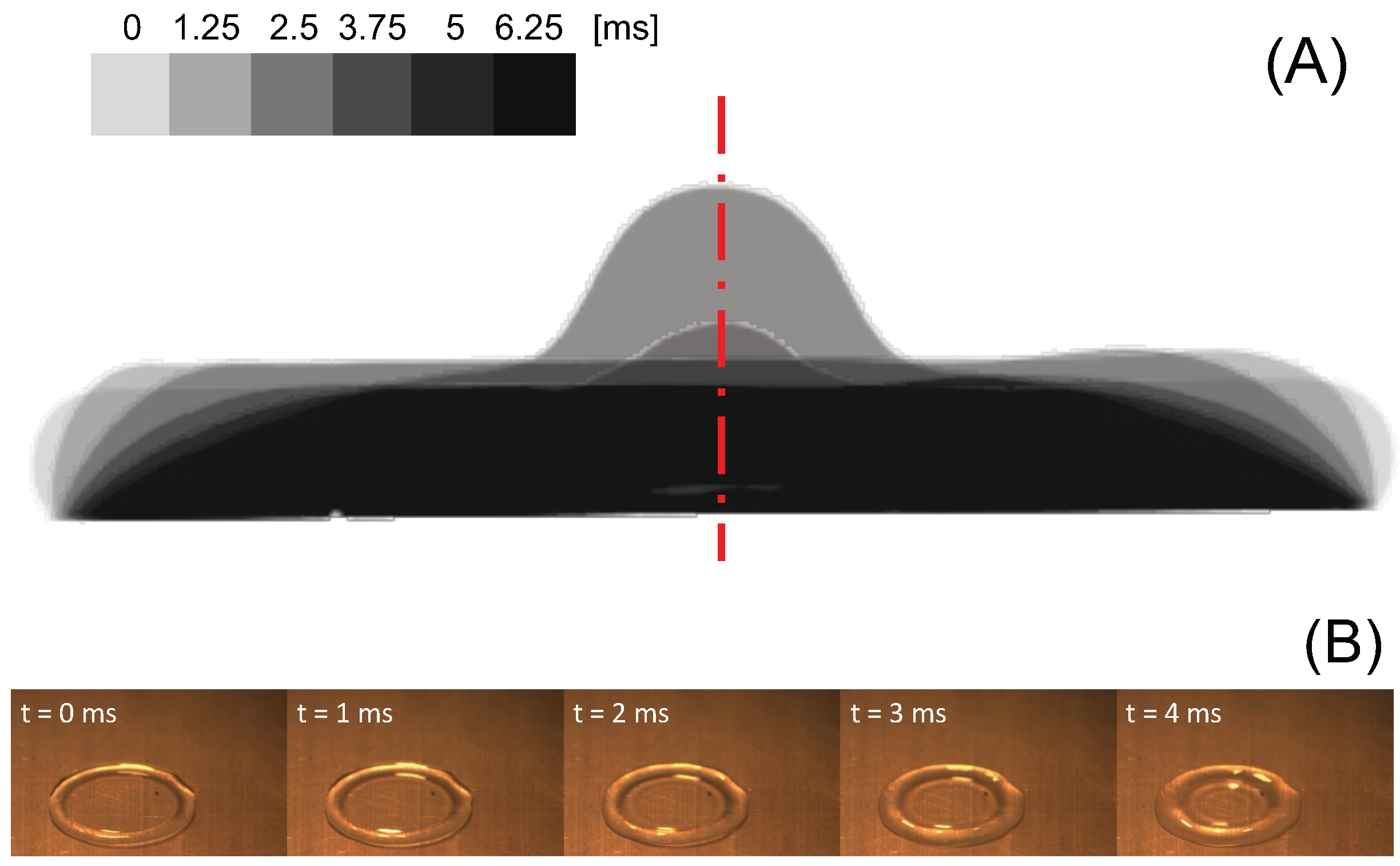
Figure 3A shows that at the beginning of recoil the contact line moves very slowly (although it is not pinned on the surface) compared to the displacement of the liquid free surface that defines the apparent contact angle, and causes the liquid in the rim, visible from the top vies displayed in
Figure 3B, to flow back towards the centre of the drop. Previous works [
7,
8,
9] show that while in water drops the fluid velocity is the same as the velocity of the receding contact line, in dilute polymer solution drops the bulk velocity of the fluid during retraction is two or three orders of magnitude larger than the contact line velocity. In particular, particle velocimetry measurements of the radial velocity in the lamella of a 200 ppm polyethylene oxide solution drop during retraction show the recoil velocity is approximately 300 mm/s, and grows linearly from the centre to the rim [
8].
The drop dynamics illustrated in
Figure 3 suggests the simple two-dimensional shear flow described in
Figure 2a does not describe the flow field in the retracting drop adequately, but one should consider the unsteady boundary layer flow on the target surface, with a shear velocity gradient that can be approximated as:
where
and
are the instantaneous free stream velocity in the radial direction and the boundary layer thickness at a distance
x from the contact point, respectively.
Since the fluid is radially flowing back towards the drop centre, conservation of mass implies that in the first stages of recoil the velocity magnitude increases, so that
. Thus, the ratio between the anti-symmetric and the symmetric part of the velocity gradient tensor is smaller than unity, which triggers the second-order coil-stretch transition as discussed above [
15,
23]. Moreover, in a boundary layer
, therefore the polymer molecule fractional stretching given by Equation (
1) does not depend significantly on the radial velocity gradient of the vertical velocity component,
.
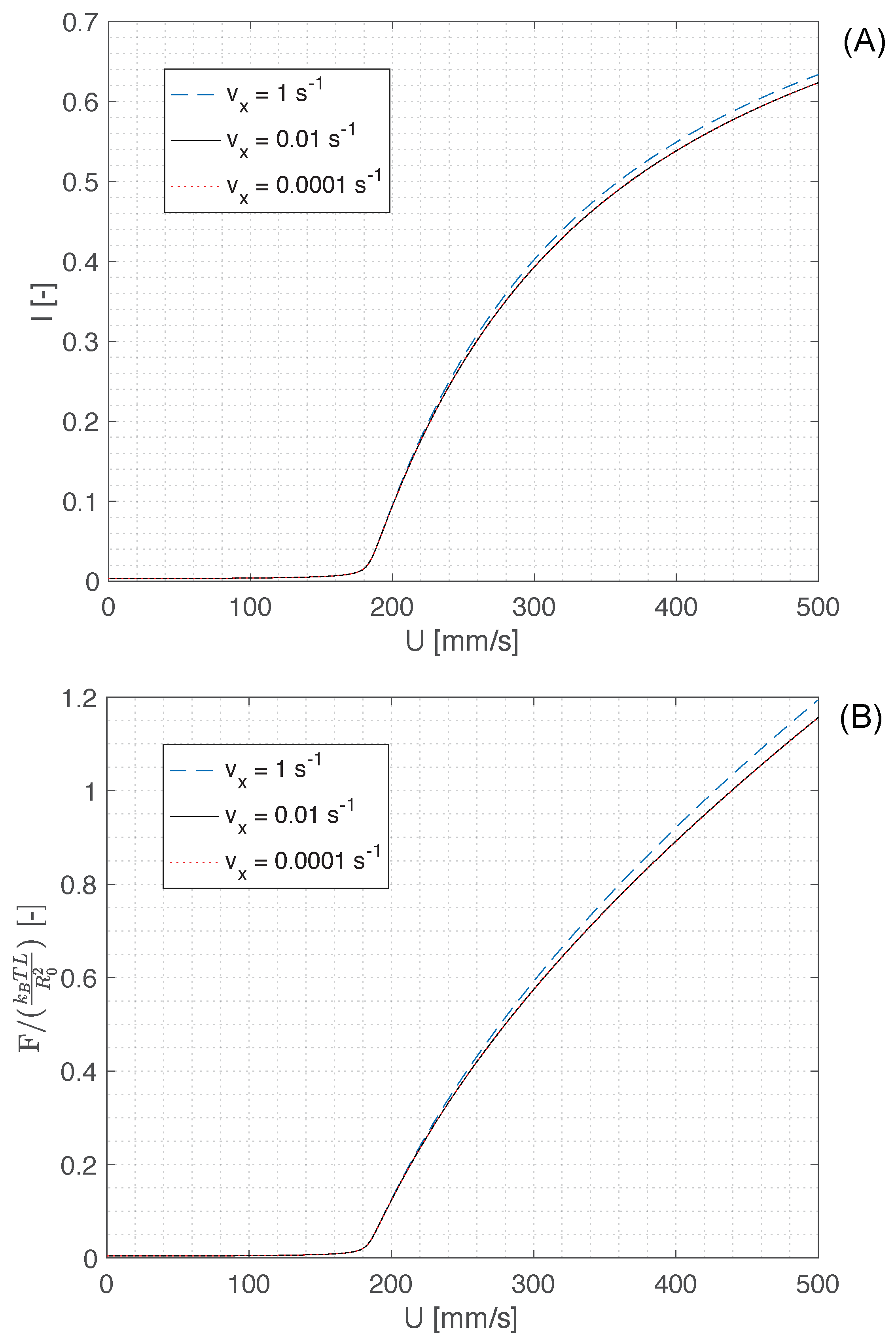
The numerical solution of Equation (
1) for a boundary layer of thickness
m (i.e., just above the polymer coil size
), and the corresponding recall force of a single polymer molecule (Equation (
3)) are displayed in
Figure 4A,B, respectively. These figures suggest that, irrespective of the magnitude of the gradient of the vertical velocity component
, an appreciable stretching of polymer molecules and consequently a buildup of the recall force occur for velocities
mm/s. Since the radial velocity in the lamella is of the order of 300 mm/s [
8], and the velocity of the fluid in the rim is even faster as the rim flows over the lamella during recoil (see
Figure 3B), one can conclude the shear flow near the contact line of the recoiling drop is sufficient to trigger the supercritical coil-stretch transition and cause a large deformation of polymer molecules, which can reach a mean fractional extension
.
These results are confirmed both qualitatively and quantitatively by the work of Smith et al. [
26], who directly observed the conformational dynamics of individual, flexible polymers in steady shear flow by the use of video fluorescence microscopy. In particular, it was found polymers reach an asymptotic mean fractional extension
, characterised by a practically flat probability density between
and
[
26]; this is also consistent with the direct observation of stretched DNA molecules [
7] and of thin liquid filaments behind the receding contact line (
Figure 1).
Thus, at the beginning of drop recoil, which occurs when the contact angle is still
, the partially stretched polymer molecules on the de-wetted substrate induce a recall force on the receding contact line, opposed to the contact line velocity; this can be interpreted, from a macroscopic point of view, as an additional, dissipative force acting on the contact line and opposed to its movement, or an effective contact line friction.
Figure 5 displays a schematic of the contact line forces in case of a drop of a pure fluid (
Figure 5A) and in case of a dilute polymer solution (
Figure 5B). Since the drop-surface system is not at thermodynamic equilibrium, the Young-Laplace equation
(where
and
are the solid-vapour, solid-liquid, and liquid-vapour interfacial tensions, respectively, and
is the equilibrium contact angle) is not applicable. However, the contact line displacement is driven by surface forces, therefore one can write the following inequality:
where
is the apparent contact angle observed during drop retraction.
In other words, the net force on the contact line in the radial direction determines whether the drop spreads (
) or recoils (
), as shown schematically in
Figure 5A. In the case of polymer solutions (
Figure 5B), during drop recoil there is an additional resistive force due to the polymer chains stretching,
, so that the condition for recoil becomes:
If the magnitude of the polymer force (per unit length of the contact line) is comparable to the liquid surface tension,
, the additional resistive force on the contact line is compensated by a significant reduction of the apparent contact angle, which is precisley what one can observe experimentally [
6,
27].
In order to estimate the magnitude of the polymer force per unit lenght of the contact line, one can evaluate an average value of the recall force of a single polymer molecule, given by Equation (
3), and multiply it by the number of stretched molecules in a vertical liquid wedge near the contact line.
Figure 4A suggests that for the measured fluid velocity during recoil [
7,
8] the fracional stretching of polymer molecules is approximately 50%, and the same value can be estimated on the basis of the direct observation of the conformational dynamics of individual polymers in steady shear flow [
26]. The corresponding value of the inverse Langevin function is
.
The bulk number density of polymer coils in the fluid wedge is , where is the polymer density, Avogadro’s number, the volume concentration of the polymer, and M its molecular mass. However, the polymer coils that are stretched as the contact line sweeps the substrate align in a thin layer at the bottom of the fluid wedge, therefore their number scales as .
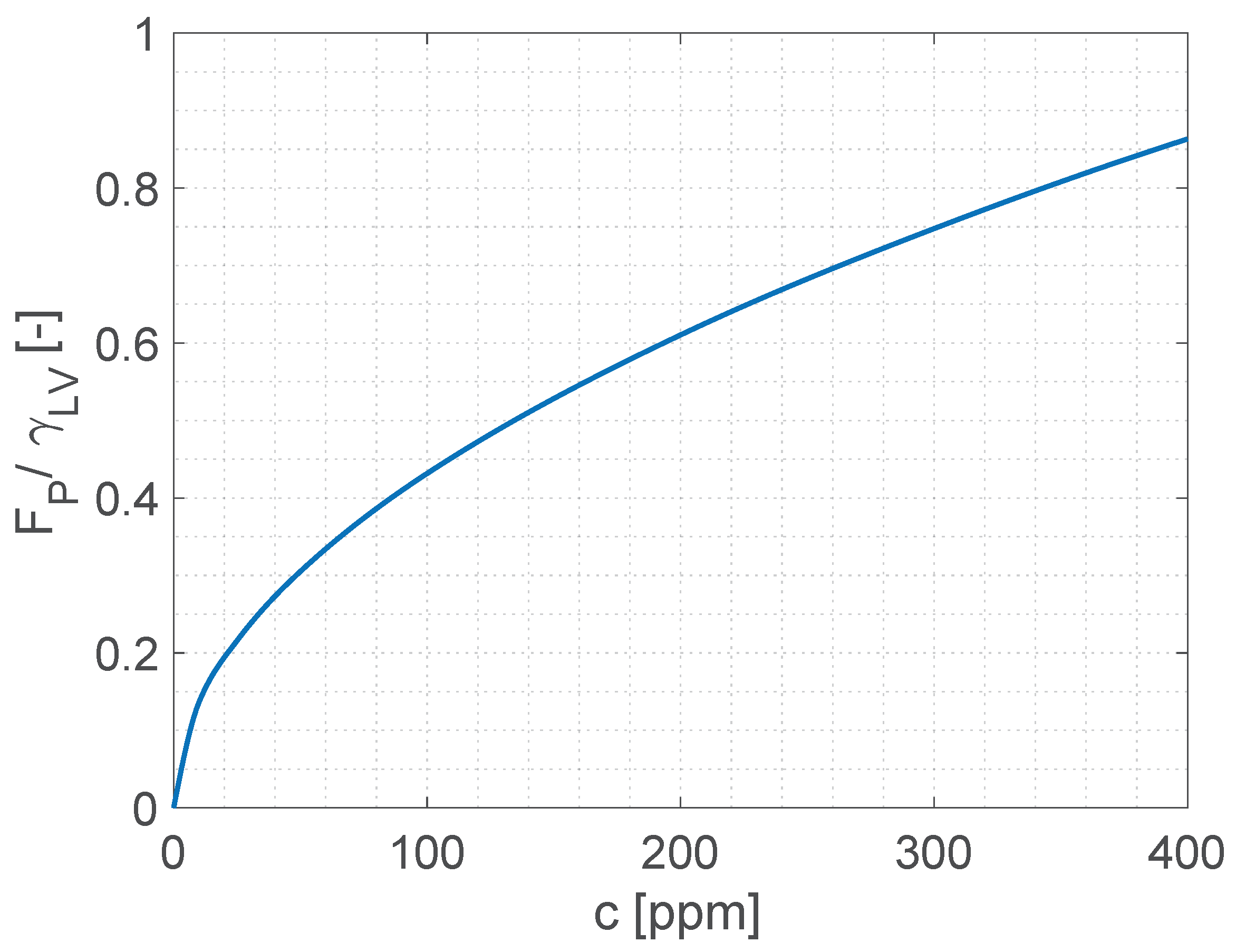
In conclusion, the overall average polymer force per unit contact line length is given by:
Figure 6 shows that the average polymer force per unit length, calculated using Equation (
7) for polymer concentrations corresponding to dilute solutions, is comparable in magnitude with the surface tension of the polymer solution, therefore it can explain the reduction of the contact line retraction velocity observed experimentally. We note the force given by Equation (
7) cannot be used directly as an additional term in a Young-Laplace force balance because the system is very far from equilibrium, therefore the apparent contact angle is not thermodynamically significant. However, the proposed approach provides a quantitative explanation of the phenomenon from first principles without any empirical parameters, and without the need to introduce fictitious elongational flows or other artefacts.