Abstract
The dynamic surface tension (DST) data and biochemical parameters (BCP) of animal blood have been obtained. A strong positive correlation was found for goats between σ1, σ2, σ3 (DST) and sodium levels (BCP), λz (DST)—with the levels of lipid and sodium (BCP); whereas a strong negative correlation was found for goats between σ3 (DST) and the level of total protein and chloride (BCP), λz (DST)—with the level of albumin (BCP). A strong positive correlation was found for horses between σ1, σ2, σ3 (DST) and lipid levels (BCP); λ0 (DST)—the level of chloride (BCP); λz (DST)—the level of albumin and chloride (BCP). A strong negative correlation was found for horses between σ1 (DST) and sodium level (BCP); σ2, σ3 (DST) and chloride level (BCP); λ0 (DST) and lipid level (BCP). Some moderate and weak correlations of different types were also found, but these have less importance for practical usage. The particular correlations between BCP and DST data of the same serum samples allowed DST data to be checked directly at the farm, and requiring the use of more expensive and time-consuming biochemical analysis only in cases of high necessity for a particular animal.
1. Introduction
Powerful techniques for dynamic surface tension (DST) measurement have been developed and successfully applied for various biological liquids [1,2,3,4], and this has particular importance for human medicine [5,6], general biochemistry [7,8,9] and veterinary fields [8,9,10,11]. There have been some attempts to estimate a static surface tension of blood serum or plasma samples, but these found no pronounced difference with human age [3,4], no statistical correlations with total serum cholesterol and some enzymes levels [4], or with plasma free haemoglobin levels, cardiopulmonary bypass and oxygenator type [1]. Successful studies of human biological liquids were carried out in detail by the colleagues from the Donetsk Medical University [5,6] with DST devices and technology from the Max Planck Institute of Colloids and Interfaces and “SINTERFACE Technologies e.K.” (Berlin, Germany) [1]. A detailed discussion of the DST measurements of human biological liquids taken from patients with kidney disease (Chapter 4), with rheumatic diseases (Chapter 5), with pulmonary diseases (Chapter 6), with diseases of the central nervous system (Chapter 7), and with neoplasms (Chapter 8) is provided in the book of Kazakov, V.N. et al. [5]. Thus, the DST measurements of human biological liquids can be used for studies of various human disorders [6]. An interest in the fundamental and applied sides of such studies has been growing over recent years, as can be seen from the publications concerning the temperature dependence of blood surface tension [12], the main characteristics of the power spectrum of bubble oscillations for complex analysis [13], the analysis of tensiograms of cerebrospinal fluid [14,15], DST application as a medical diagnostic tool [16,17], etc.
A variety of methods for measuring the surface tension of aqueous solutions are known (here are just a few book summarizing DST terminology [5,6,7,11]). The most reliable methods in this field are based on direct measurement of capillary forces acting on curved or flat surfaces (e.g., the following methods: du Nui, Wilhelmy, Langmuir, capillary rise, static and dynamic bubble drops, etc.) [5,6,7,11].
The reliable correlations between DST measurements and the most important biochemical parameters of animal blood have only recently been presented in the works [7,8,9,10,11]. DST investigations can give valuable information for early diagnosis, estimation of the physiological and biochemical status, for “quick identification” of healthy and ill animals, and treatment monitoring in the veterinary field.
The main aims of the present work are the following: to study the biochemical and DST parameters of the animal serum samples; to obtain the correlations between these biochemical and DST parameters of particular animal groups; and to study the DST parameters of the mixtures based on specific proteins, lipids and salts as model systems of animal blood plasma (serum).
2. Materials and Methods
The blood samples (144 in total) from healthy animals (2 years old) were examined: thirty-six goats (group 1) and twelve horses (group 2). Before the beginning of the experiment, each animal was checked based on indicators characterizing the physiological state of the body: body temperature, heart rate, respiratory rate, etc. [11]. All the animals examined corresponded to the physiological norm. Blood from experimental animals was taken before morning feeding (on an empty stomach) from the vein. After partial retraction of the blood clot, the serum was separated by centrifugation for 15 min 3000 rpm. All procedures were carried out with the approval of the Animal Care Committee of the Moscow State Academy of Veterinary Medicine and Biotechnology and internationally recognized guidelines (EU Directive 2010/63/EU for animal experiments [18], U.K. Animals (Scientific Procedures) Act, 1986 [19], etc.).
Solutions of three-component systems based on (a) bovine serum albumin (BSA), (b) natural phosphatidylcholine or so-called lecithin (L), and (c) sodium chloride (NaCl) were prepared by mixing components in the following ratios: BSA (40 g/L), L (3 mM), NaCl (140 mM). All manipulations were carried out with these solutions prepared on bidistilled water. All components (BSA, L, NaCl) were obtained from Sigma-Aldrich (St. Louis, MO, USA) at 98–99% purity.
The method of DST measurement of animal serum by tensiometer BPA-1P (Maximum Bubble Pressure Tensiometer) is described elsewhere [1,5,6,7,8,9,10,11,16]. The significant advantages of BPA devices are the following: a small sample volume, high analysis speed, full automation of the measurement process, the computer processing of the information received. The general principle of the device operation (based on the maximum pressure in the bubble method [1,16]) is described below [5,6]. The air from the compressor enters the capillary, which is lowered into the test liquid. The maximal pressure in the system is determined (Figure 1) and used to calculate the surface tension.
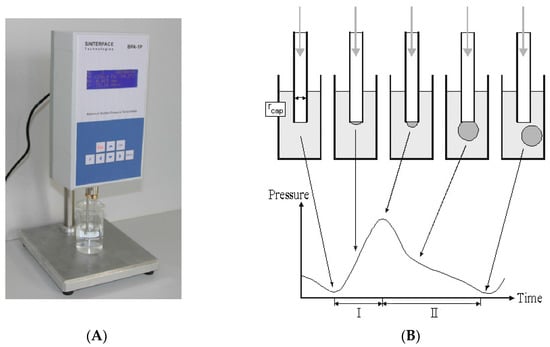
Figure 1.
(A) The tensiometer BPA-1P (left), (B) principle concept of the maximum bubble pressure measurements by this tensiometer (right), adapted from www.sinterface.de and publications [1,6,16].
The pressure required for the separation of the air bubble from the capillary tip, which drops at the air-liquid interfaces, is directly proportional to the surface tension (σ) on this “border”. To overcome the wetting phenomenon in the capillary (dipped into liquid), an excessive air pressure is required. The maximum pressure that occurs during the formation of the air bubble during blowing depends on the capillary radius (Figure 1B, “min” at the left insert). At the moment in which the bubble takes the form of a hemisphere, the capillary radius is equal to the radius of the curvature, and the pressure reaches its maximum value (Figure 1B, “max” at the middle insert). With further bubble growth, the curvature radius increases again, which reduces the pressure inside the bubble (Figure 1B, “min” at the right insert). The division of the interval between the bubbles into the so-called “dead time” and the surface “lifetime” is based on the existence of a critical point, depending on the air flow pressure [6].
The surface tension (σ) of the studied liquid is calculated according to the measured excessive pressure (P) by the Laplace equation:
where r—capillary radius, PН—the hydrostatic pressure in the measuring cell, Pd—the dynamic pressure caused by the “viscoinertial” effects.
It is important to note that Pd must be close to zero for the capillary used in the study of the biological liquids. The time of the surface formation (so-called “lifetime” or “t” in the equation) is calculated according to the equation below:
where tb—measured interval between bubbles, L—air flow (volume per time). The values of Lc and Pc refer to the particular “critical” point for L and P. For the values L > Lc, so-called “continuous air flow regime” exists in the capillary; whereas, for the values L < Lc on the capillary tip, the individual bubbles are formed with a lifetime tf > 0. It should be noted that strict hydrodynamic theory of the method of maximum bubble pressure (taking into account inertia, viscosity, unsteadiness, etc.) has not yet been completely elaborated. However, the simplified Equations (1) and (2) are also adequate in the case of biological liquids. This method of the maximal pressure measurement “in the buoyant bubble” allowed us to obtain the dependences of DST values vs. time (i.e., in the form of “tensiograms” [1,6,11]). The following DST parameters were obtained: σ1—at the “very short” time (0.01 s), σ2—at the “middle time” (1 s), σ3—at the “rather long” time (100 s), as well as the initial (λ0) and final (λz) tilt values of the “tensiograms” [1,6,11]. All measurements were carried out at 20 °C.
Biochemical blood analyses were made using spectrophotometer “KFK-2-UChL” and standard biochemical kits (DIACOM, Moscow, Russia) [7,11]. Statistical and correlation analysis was performed using general statistics programs [5,6,7].
3. Results and Discussion
The maximum DST values for goat or horse serum samples were obtained at a very short time (σ1) for both studied groups of animals: 70.3 ± 0.4 mN/m (group 1) and 72.2 ± 0.5 mN/m (group 2). Further, the DST values of the blood serum of these animals gradually reduced (goats by 12%, and horses by 15%) with an increase in time of the air-serum interface “existence”. For example, the DST values at the middle (σ2) and long times (σ3) were as follows: goats—66.9 ± 0.4 mN/m, horses—67.7 ± 0.9 mN/m and goats—62.2 ± 0.3 mN/m, horses—62.2 ± 0.9 mN/m. The tilt values of the initial part of the tensiograms (λ0) for goats were 3.7 ± 0.1 mN∙m−1∙s−½, while for horses were 4.7 ± 0.7 mN∙m−1∙s−½. The final tilt values of the tensiograms (λz) for goats were 5.1 ± 0.2 mN∙m−1∙s½, whereas for horses, these were again slightly higher—about 5.7 ± 0.3 mN∙m−1∙s½.
The major parameters of the animal blood plasma, such as total proteins, albumin, phospholipids, sodium, chloride (which have the greatest influence on the DST values of biological fluids [5,6,7]) were obtained by biochemical analysis of the goat or horse serum samples (Table 1). It is important to highlight that our data summarized in the (a) columns are in agreement with the values in the (b) columns cited by different authors in the available literature for goats and horses [8,11,20].

Table 1.
The average values of some biochemical parameters of animal blood serum according to (a) our data and (b) those of different authors [20].
The general correlations (the values region was more important than the direction—positive or negative) between the DST and biochemical parameters were calculated (Table 2 and Table 3). In particular, the strong correlations had correlation coefficients ≥0.69, the middle correlations had correlation coefficients from 0.30 to 0.69, and the weak correlations had correlation coefficients from 0.01 to 0.29. A strong positive correlation was found for goats (Table 2) between σ1, σ2, σ3 and sodium levels, λz—with the levels of lipid and sodium; whereas a strong negative correlation was found for goats between σ3 and the level of total protein and chloride, λz—with the level of albumin. Some middle and weak correlations of different types were also found, but these are not as important for practical usage as strong positive (negative) correlations.

Table 2.
Results of the correlation analysis between the parameters of the dynamic surface tension (DST) and biochemical indices of the goat serum samples.

Table 3.
Results of the correlation analysis between the parameters of the dynamic surface tension (DST) and biochemical indices of the horse serum samples.
A strong positive correlation was found for horses (Table 3) between σ1, σ2, σ3 and lipid levels; λ0—the level of chloride; λz—the level of albumin and chloride. A strong negative correlation was found for horses between σ1 and sodium level; σ2, σ3 and chloride level; λ0 and lipid level. The same correlations were found for all types of the studied ruminants: σ1 and albumin level (weak negative), σ2 and lipid level (positive medium) and sodium (strong positive), λ0 and chloride level (negative medium), λz and lipid level (strong positive).
The main systems modelling animal serum (or blood plasma) are the mixtures of their major components, such as proteins, lipids and salts. It is important to evaluate the tensiometry measurements starting from the individual serum components in order to study the behaviour of such complicated systems by DST method [6,21]. Therefore, the DST data of BSA solutions at 30–80 g/L concentrations obtained in order to model the total proteins content in animal serum. The DST parameters (σ2) of the protein solutions at each BSA concentration decreased by 4–6%, as compared to those at very short times (σ1). Finally, the DST parameters reached the minimal values of from 55.9 mN/m to 58.4 mN/m at long times (σ3). These DST data (σ3) were about 25–30% lower, as compared to those at very short times (σ1). These values corresponded to the DST values of the BSA solutions with the lowest concentrations. The tilt values of the initial (λ0) and final (λz) parts of the tensiograms differed by 1.5–2 times, which could be valuable for general estimation of the overall DST parameters. The observed changes can be explained by the influence of uncompensated electric charge of the protein molecules adsorbed at the interface.
It is known [6,21] that at concentrations of globular proteins less than 10 g/L (depending on the pH and electrolyte additives), they can be present in such solutions as “separate” or “non-associated” molecules. Such “non-associated” protein molecules capable of fast adsorption at the interface. In contrast, at higher protein concentrations, only part of the protein molecules are able to reach the interface. Therefore, lower amounts of protein molecules are able to adsorb at the interface in the latter case. This explains the rather “weak” influence of the BSA concentrations on dynamic surface tension values.
Sodium chloride did not adsorb at the interface, and caused almost no changes in DST parameters of NaCl solutions at 110–160 mM concentrations. It was important to check an effect of lipids on the DST values of the model systems that can be considered for example as the aqueous lecithin (L) dispersions at concentrations close to those in the animal serum (Table 4). To our surprise, lecithin has no significant effect on the DST parameters of the distilled water, regardless of the lecithin concentration and time range. These results were unexpected, because of the high surface activity of any lipid molecules, including lecithin.

Table 4.
Dynamic surface tension parameters of the aqueous lecithin (L, 3 mM) dispersions and its mixtures with an aqueous solution of sodium chloride (NaCl, 140 mM) and protein (BSA, 40 g/L).
The parameter σ1 changes insignificantly in the majority of the mixtures. Whereas parameter σ2 increases by 8% in the lecithin-protein mixture, by 7% in the protein-NaCl mixture and by 15% in mixture with all three components as compared to the lecithin-protein mixture. Parameter σ3 increases by 26% in the lecithin-protein mixture, by 15% in the protein-NaCl mixture and by 10% in the three-components mixture as compared to the lecithin-protein mixture. Furthermore, the parameter λ0 decreases by 80% in the lecithin-protein mixture, by 26% in the protein-NaCl mixture and by 15% in mixture with all three components as compared to the lecithin-protein mixture. It turned out that parameter λz decreased by 45% in the lecithin-protein mixture, by 20% in the protein-NaCl mixture and by 48% in the three-components mixture as compared to the lecithin-protein mixture (Table 4).
Thus, the DST parameters were influenced by the simultaneous adsorption of complex protein and lecithin molecules at the interfaces, whereas saline only slightly influenced the parameters of lecithin dispersions as compared to the protein molecules (Table 4).
4. Conclusions
Thus, DST measurements can be considered a powerful approach for the study of animal blood samples and comparison with the model solutions. The different correlations were found for particular animal types that showed the close correlation between biochemical parameters of blood serum and the corresponding dynamic surface tension values. The general features of the DST values for the animal groups are: (1) decreasing during the measurement time; (2) becoming lower—at high protein and lipid content; (3) becoming higher—at high salt content in the blood (serum). The particular correlations between BCP and DST data of the same serum samples made it possible to check DST data directly at farm, and to only use more expensive and time-consuming biochemical analysis in cases of high necessity for a particular animal.
Acknowledgements
This work was supported by the Russian Foundation for Basic Research (grant 18-016-00207). The author is thankful to Irina V. Milaeva and Ekaterina N. Zarudnaya for technical assistance and valuable discussions.
Conflicts of Interest
The author declares no conflict of interest.
References
- Fainerman, V.B.; Miller, R. The maximum bubble pressure technique. In Studies in Interface Science; Möbius, D., Miller, R., Eds.; Elsevier: Amsterdam, The Netherlands, 1998; pp. 279–326. [Google Scholar]
- Butler, B.D.; Conti, V.R. Postbypass plasma surface tension and its role in bubble filtration. Perfusion 1986, 1, 187–191. [Google Scholar] [CrossRef]
- Harkins, H.N.; Harkin, W.D. The surface tension of blood serum and the determination of the surface tension of biological liquids. J. Clin. Investig. 1929, 7, 263–281. [Google Scholar] [CrossRef] [PubMed]
- Hrncir, E.; Rosina, J. Surface tension of blood. Physiol. Res. 1997, 46, 319–321. [Google Scholar] [PubMed]
- Kazakov, V.N.; Sinyachenko, O.V.; Fainerman, V.B.; Pison, U.; Miller, R. Dynamic Surface Tension of Biological Liquids in Medicine, Studies in Interface Science; Möbius, D., Miller, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2000; pp. 34–56. [Google Scholar]
- Vozianov, A.F.; Kazakov, V.N.; Sinyachenko, O.V.; Fainerman, V.B.; Miller, R. Interfacial Tensiometry and Rheometry in Nephrology; Donetsk Medical University Publishing House: Donetsk, Ukraine, 1999. [Google Scholar]
- Zaitsev, S.Y. Supramolecular Nanodimensional Systems at the Interfaces, Concepts and Perspectives for the Bionanotechnology; LENAND Publishing House: Moscow, Russia, 2010. [Google Scholar]
- Zaitsev, S.Y.; Milaeva, I.V.; Zarudnaya, E.N.; Maximov, V.I. Investigation of dynamic surface tension of biological liquids for animal blood diagnostics. Colloids Surf. A Physicochem. Eng. Asp. 2011, 383, 109–113. [Google Scholar] [CrossRef]
- Zaitsev, S.Y. Interfacial tensiometry method for the analysis of model systems in comparison with blood as the most important biological liquid. Mosc. Univ. Chem. Bull. 2016, 71, 205–208. [Google Scholar] [CrossRef]
- Zaitsev, S.Y. Dynamic surface tension measurements as general approach to the analysis of animal blood plasma and serum. Adv. Colloid Interface Sci. 2016, 235, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Zaitsev, S.Y. Tensiometry and Biochemical Analysis of the Blood of Animals: Fundamental and Applied Aspects; “Agricultural Technology” Publishing House: Moscow, Russia, 2016. [Google Scholar]
- Rosina, J.; Kvašňák, E.; Šuta, D.; Kolářová, H.; Málek, J.; Krajči, L. Temperature dependence of blood surface tension. Physiol. Res. 2007, 56 (Suppl. 1), 93–98. [Google Scholar]
- Fathi-Azarbayjani, A.; Jouyban, A. Surface tension in human pathophysiology and its application as a medical diagnostic tool. Bioimpacts 2015, 5, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Gerasimov, G.I.; Zaitsev, I.A.; Kotelnitsky, M.N.; Fainerman, V.B. Analysis of tensiograms of cerebrospinal fluid using a kinetic model. Bull. Exp. Biol. Med. 2004, 137, 597–600. [Google Scholar] [CrossRef]
- Kazakov, V.N.; Vozianov, A.F.; Sinyachenko, O.V.; Trukhin, D.V.; Kovalchuk, V.I.; Pison, U. Studies on the application of dynamic surface tensiometry of serum and cerebrospinal liquid for diagnostics and monitoring of treatment in patients who have rheumatic, neurological or oncological diseases. Adv. Colloid Interface Sci. 2000, 86, 1–38. [Google Scholar] [CrossRef]
- Fainerman, V.B. Accurate analysis of the bubble formation process in maximum bubble pressure tensiometry. Rev. Sci. Instrum. 2004, 75, 213–221. [Google Scholar] [CrossRef]
- Shpak, O.; Verweij, M.; de Jong, N.; Versluis, M. Droplets, bubbles and ultrasound interactions. In Therapeutic Ultrasound, Advances in Experimental Medicine and Biology; Escoffre, J.-M., Bouakaz, A., Eds.; Springer: Cham, Switzerland, 2016; Volume 880, pp. 157–174. ISBN 978-3-319-22535-7. [Google Scholar]
- Legislation for the Protection of Animals Used for Scientific Purposes. Available online: http://ec.europa.eu/environment/chemicals/lab_animals/legislation_en.htm (accessed on 8 June 2016).
- Animals (Scientific Procedures) Act 1986. Available online: https://www.legislation.gov.uk/ukpga/1986/14/contents (accessed on 6 May 1999).
- Holod, V.M.; Ermolaev, G.F. Handbook of Veterinary Biochemistry; Uradzhay: Minsk, Belarus, 1988. [Google Scholar]
- Ybert, C.; di Meglio, J.-M. Study of Protein Adsorption by Dynamic Surface Tension Measurements: Diffusive Regime. Langmuir 1998, 14, 471–475. [Google Scholar] [CrossRef]
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).