Synergistic Integration of Graphene Nanoparticles in Colloidal TiO2 for Grätzel Cells (DSSC)
Abstract
1. Introduction
2. Materials and Methods
- (a)
- FTO Glass Slides (FL2446, Stanford Advanced Materials, Santa Ana, CA, USA): Transparent glass coated with a conductive layer of fluorine-doped tin oxide (FTO) was used. This serves as the base substrate—transparent and conductive—used for the anode, while the counter electrode consists of platinum also deposited on FTO glass. The slides feature one conductive surface and an insulating opposite side, making them suitable for directional electric flow. Dimensions: 2.5 cm × 2.5 cm.
- (b)
- N719 Dye (Alfa Chemical, CAS No. 207347-46-4, Jiaozuo City, China): A ruthenium complex was employed due to its broad visible light absorption spectrum, high electron injection efficiency, and good stability. N719 is one of the most used sensitizers in dye-sensitized solar cells (DSSCs), thanks to its excellent photochemical properties and operational stability. Chemical formula: Di-tetrabutylammonium cis-bis(isothiocyanato)bis(2,2′-bipyridyl-4,4′-dicarboxylate) ruthenium (II). This dye plays a fundamental role in sensitizing TiO2, extending its spectral response from the UV to the visible region—crucial for efficient current production in the cell [31,32,33].
- (c)
- Electrolyte Solution (I−/I3−): Prepared in the lab using lithium iodide (LiI) and molecular iodine (I2) from Alfa Chemical (Zhengzhou, China), with acetonitrile (CH3CN) as the solvent, purchased from Alfasigma (Bologna, Italy). The preparation involves dissolving 0.5 mol/L of LiI in acetonitrile under continuous magnetic stirring. Once fully dissolved, 0.05 mol/L of I2 is gradually added and mixed until completely dissolved. The formation of the triiodide complex (I3−) is indicated by a reddish color shift. The final solution is stored in a sealed dark glass container to prevent photochemical degradation. Electrolyte insertion is done with a fine-needle pipette by introducing 2–3 drops into the open edge between the glass slides. Capillary action distributes the liquid to the center. This iodide/triiodide solution was chosen for its low viscosity, high ionic mobility, and excellent dye regeneration capability [34].
- (d)
- Titanium Dioxide (TiO2), 99% purity (AEROXIDE® P25, Essen, Germany): The anatase crystalline form was selected for its excellent electronic and structural properties. This material acts as a photoactive semiconductor, able to transport photoexcited electrons generated by the light-sensitive dye. Its nanoporous morphology provides a high specific surface area, promoting efficient dye adsorption (e.g., N719) and enhancing photovoltaic performance. Optoelectronically, anatase has a direct band gap of approximately 3.2 eV, corresponding to an absorption peak around 388 nm in the ultraviolet region, which is crucial for capturing sunlight and initiating the photoinduced process inside the DSSC [35].
- (e)
- (f)
- PEG (Polyethylene glycol 20000, Merck Schuchardt (Hohenbrunn, Germany): A dispersing and binding agent that contributes to the formation of the paste by improving the viscosity and colloidal stability of the TiO2 suspension. It promotes a uniform spread on the FTO glass slide and acts as a rheological modifier to regulate the fluidity of the colloidal paste.
- (g)
- TiCl4 (Titanium tetrachloride, CAS: 7550-45-0, Alfa Chemical, Zhengzhou, China): Used as a precursor to promote the formation of a compact TiO2 layer through hydrolysis:This reaction enhances the connectivity between TiO2 nanoparticles, reducing electron recombination.TiCl4 + 2H2O → TiO2 + 4HCl
- (h)
- Triton X-100 (Sigma-Aldrich, Darmstadt, Germany): A non-ionic surfactant from the alkylphenol ethoxylate family. When introduced into the titanium colloidal paste, it functions as a thickening and stabilizing agent.
- (i)
- Acetylacetone (Acac) (Sigma-Aldrich, Darmstadt, Germany): A thickener that plays a complexing role, stabilizing TiO2 nanoparticles and preventing their aggregation. It regulates the pH and slows the subsequent hydrolysis of TiCl4, improving the particle size distribution of the colloidal paste.
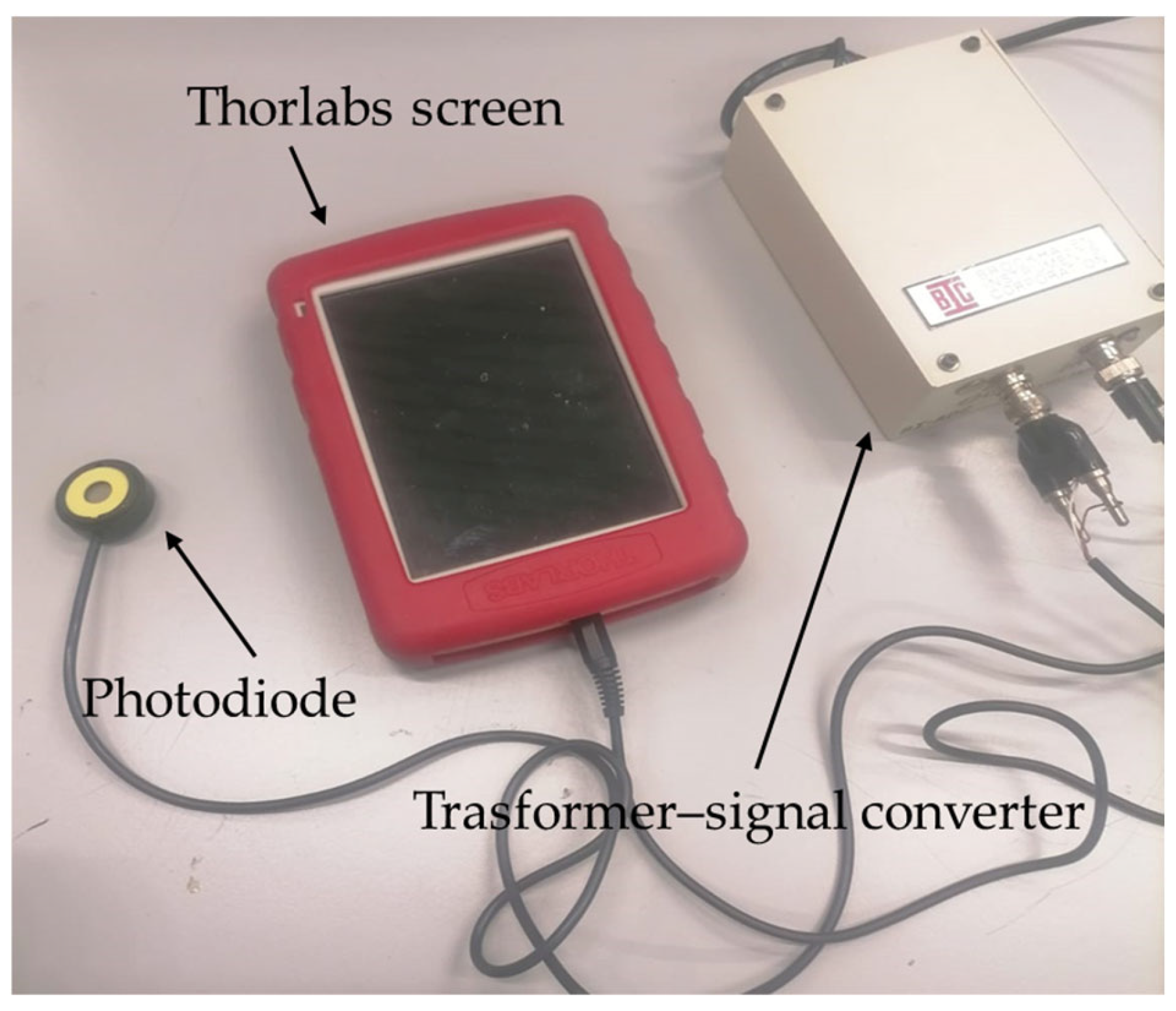
- Potentiometer (Thorlabs Inc., Newton, NJ, USA): Employed to measure the current generated by incident light. The device integrates a high-sensitivity photodiode and a transimpedance amplifier, enabling conversion of the optical current into a proportional voltage. It was used to assess the photovoltaic response of the cell in relation to light intensity and wavelength (Figure 4).
- Spectrophotometer (UV 3100 PC, VWR, West Chester, PA, USA): This instrument was used to analyze the absorption spectrum of the dye and the sensitized TiO2 film. It allowed verification of the actual coverage of the visible spectrum by the N719 dye and helped monitor any changes occurring during the experimental phase.
- Digital Multimeter (CEN-TECH, Harbor Freight Tools Group, Calabasas, CA, USA): This device was used to measure direct current (DC) voltage and current generated by the cell. Additionally, it enabled identification of the conductive surface of the FTO glass slides by detecting a specific resistance in the range of tens of ohms. Finally, the conductivity of the FTO substrates was experimentally verified using a tester applied in configurations A and B to confirm the suitability of the conductive substrates (Figure 5).
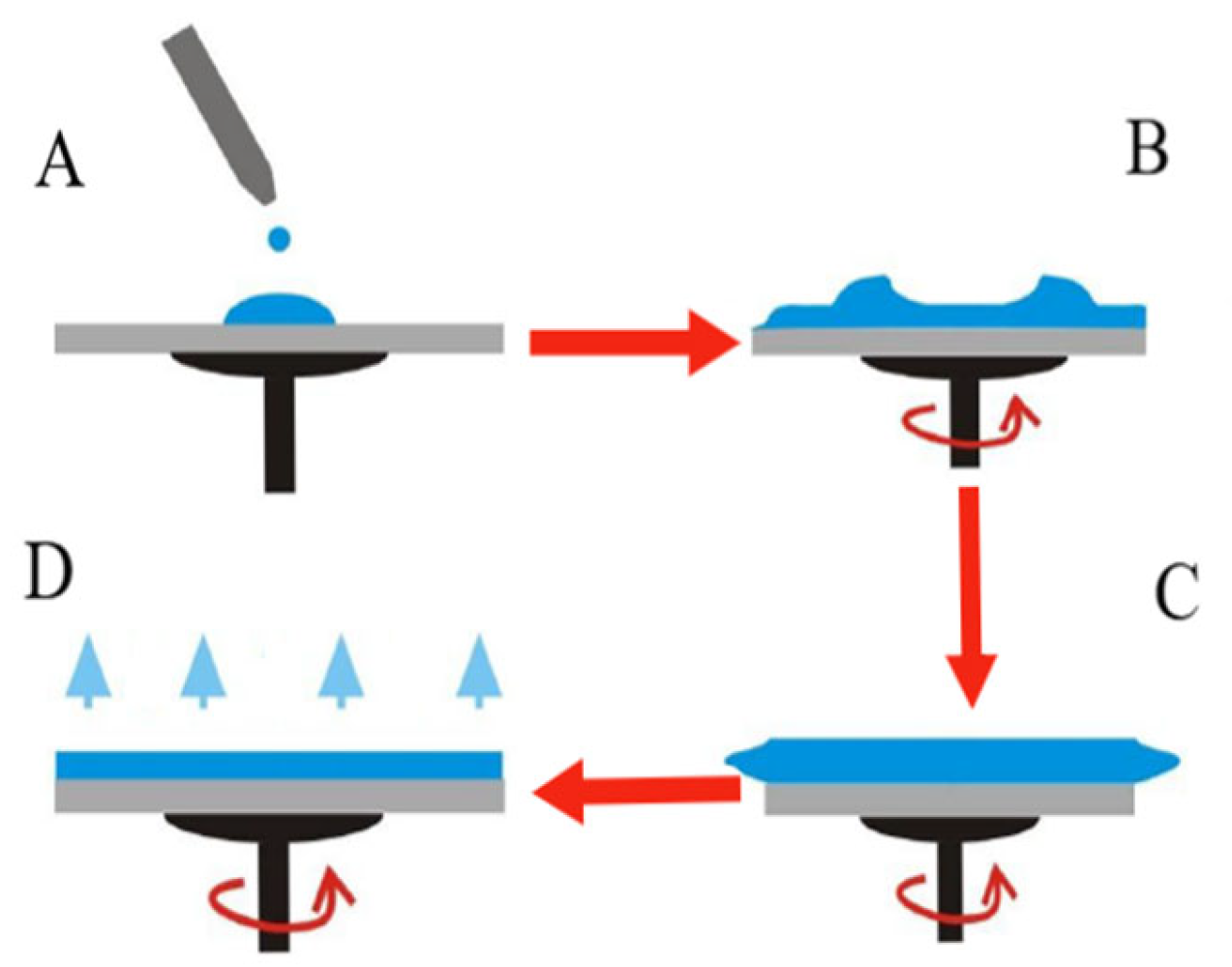
- Spin Coater (WS-650 MZ-23NPP, Laurell Technologies, PA 19446, USA): A fundamental device for the deposition of thin, uniform films on planar substrates such as FTO glass. The graphene-containing film was prepared by spin coating, a widely used technique for DSSC fabrication. Although no direct thickness measurements were performed, the parameters adopted and the nature of the solution suggest that the resulting film likely falls within the optimal range (400–700 nm) reported in the literature, ensuring appropriate transparency, adhesion, and conductivity for photovoltaic application [37]. The spin coating process is based on the centrifugal distribution of a precursor solution dispensed onto a rotating substrate, allowing precise control over the thickness and uniformity of the active layer. The use of nitrogen flow under controlled conditions reduces environmental contamination, promotes solvent evaporation, and improves the quality of the coating. This technique is particularly suitable for depositing semiconductor layers, transparent electrodes, or interfacial layers in thin-film photovoltaic cells, such as perovskite or organic heterojunction cells. Its reproducibility, metrological precision, and compatibility with a wide range of materials make it an essential tool for research and optimization of photovoltaic performance [38,39] (Figure 6).
- (A)
- Initial Deposition: The precursor solution is distributed on the substrate, either stationary or rotating. The mode of deposition affects the final film thickness.
- (B)
- Acceleration: The support reaches the set rotation speed. Part of the fluid is expelled from the surface by centrifugal force until the film becomes thin enough to resist rotational force due to its viscosity.
- (C)
- Steady Rotation: The substrate rotates at a constant speed. The film thinning is regulated by the viscosity of the solution.
- (D)
- Evaporation: Rotation continues while the solvent evaporates; the film reaches its final thickness and stabilizes.
- Sonicator (VWR USC 100 TH (Avantor) ultrasonic cleaner, Radnor, PA, USA): This instrument operates through the emission of high-frequency ultrasound, used to promote the dispersion of colloidal systems and the homogenization of liquid solutions. By exploiting the phenomenon of acoustic cavitation, it generates intense pressure waves that break up particle aggregates and enhance molecular mixing. Operating frequencies may range from 20 kHz to 1 MHz depending on the application and the nature of the sample being processed.
- Solar Lamp (LS0106, Quantum Design GmbH, Pfungstadt, Germany): A high-precision instrument designed for photovoltaic testing in laboratory conditions. This xenon solar lamp has a power output of 150 W with a typical irradiance of 90 mW/cm2 ± 20%, featuring an integrated AM 1.5G filter to simulate standard solar radiation. It is tailored for photovoltaic evaluations and scientific applications.
2.1. Preparation of Titanium Paste (TiO2)
2.2. Preparation of Titanium Paste with Graphene (Gh)
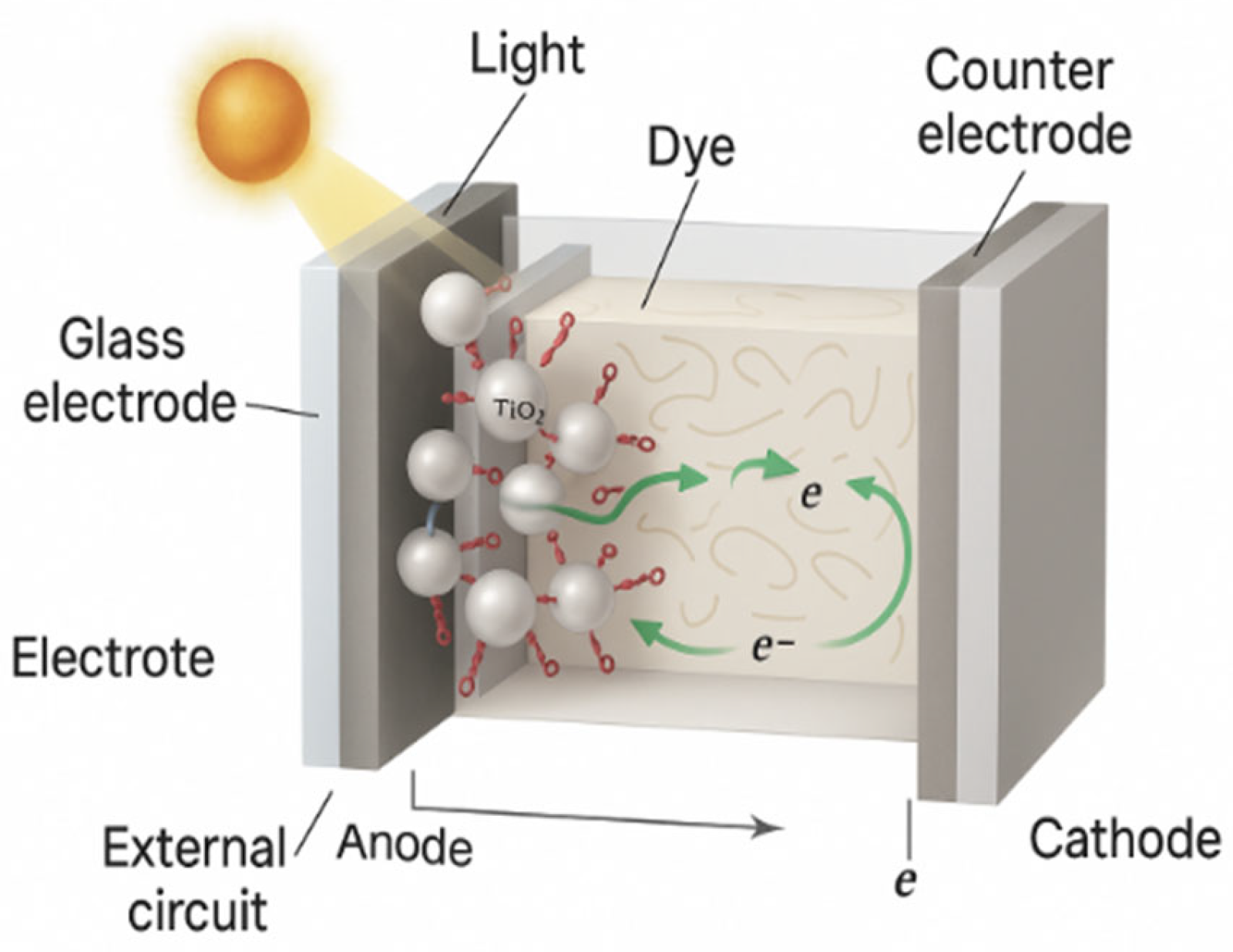
2.3. Experimental Application and Cell Structure
2.4. Light Absorption in the Fabricated Samples
2.5. Investigation of Theoretical Characteristic Parameters
3. Results and Discussion
3.1. Characterization of Reagents and the Light Source
3.2. Correlation Between Optical and Electrical Parameters in Grätzel Cells with Varying Graphene Concentrations
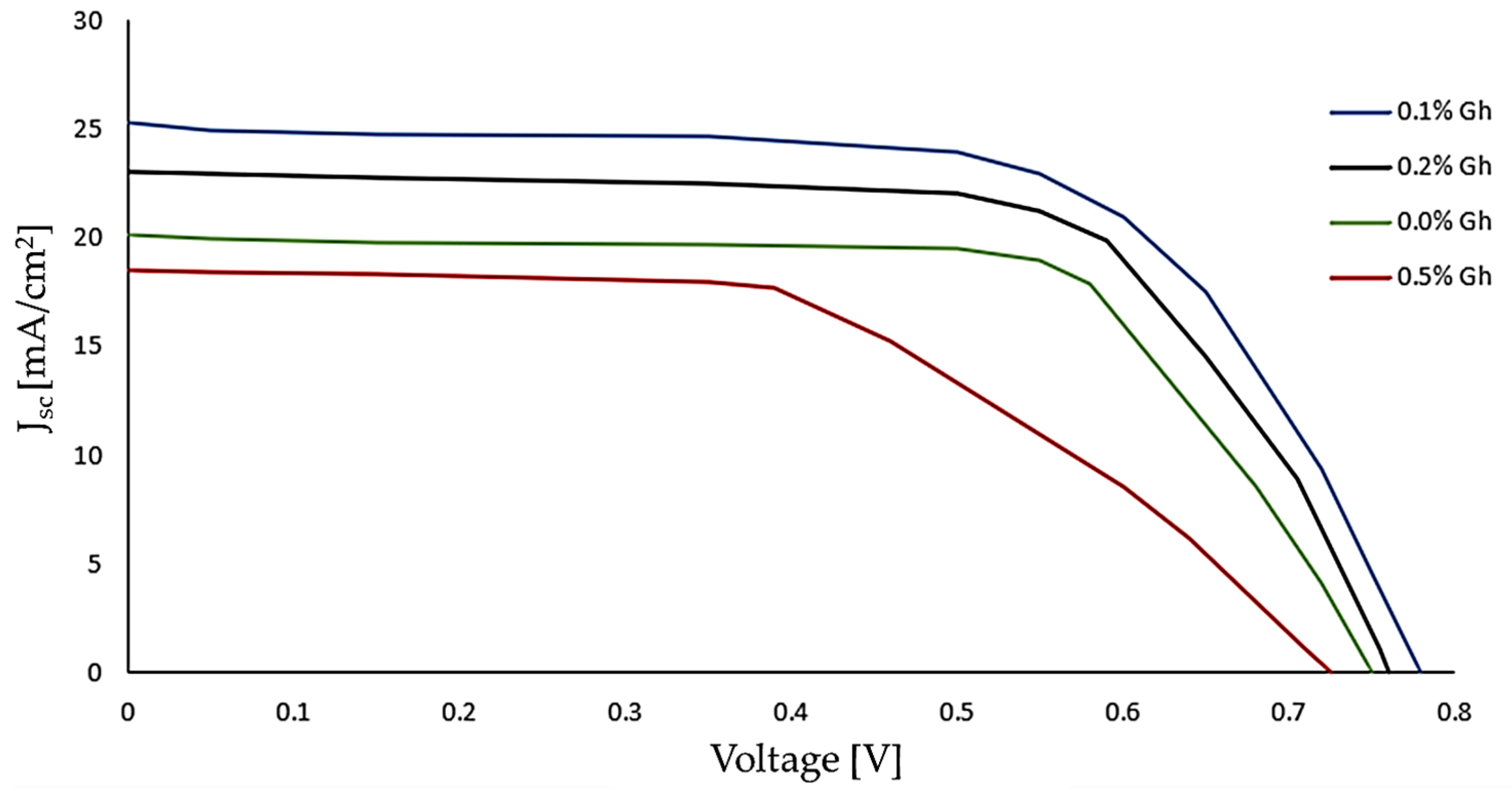
3.2.1. Analysis of Vm in DSSCs
3.2.2. Efficiency as a Function of Maximum Power Voltage
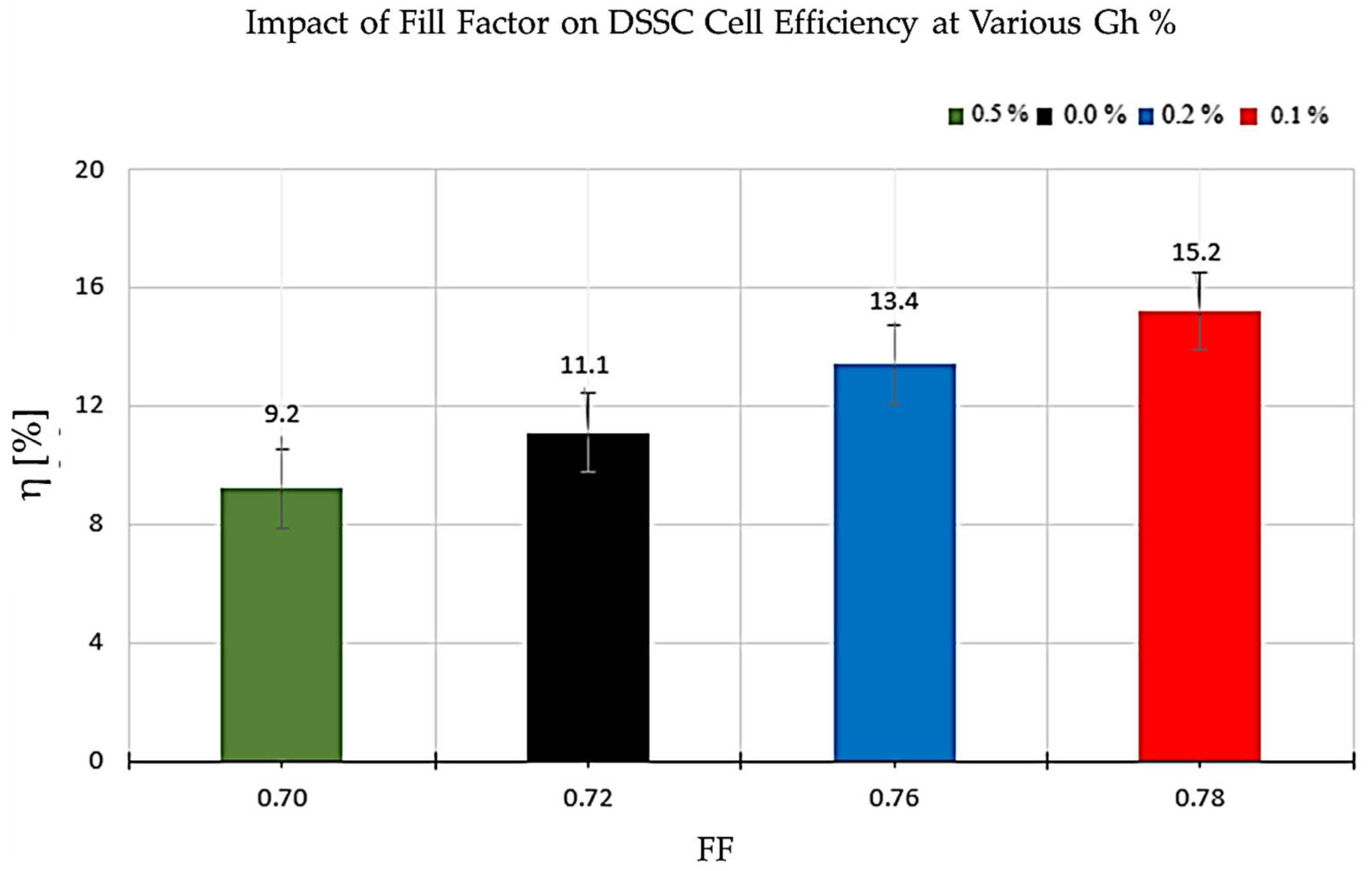
3.2.3. Relationship Between Photovoltaic Efficiency (η) and Fill Factor (FF)
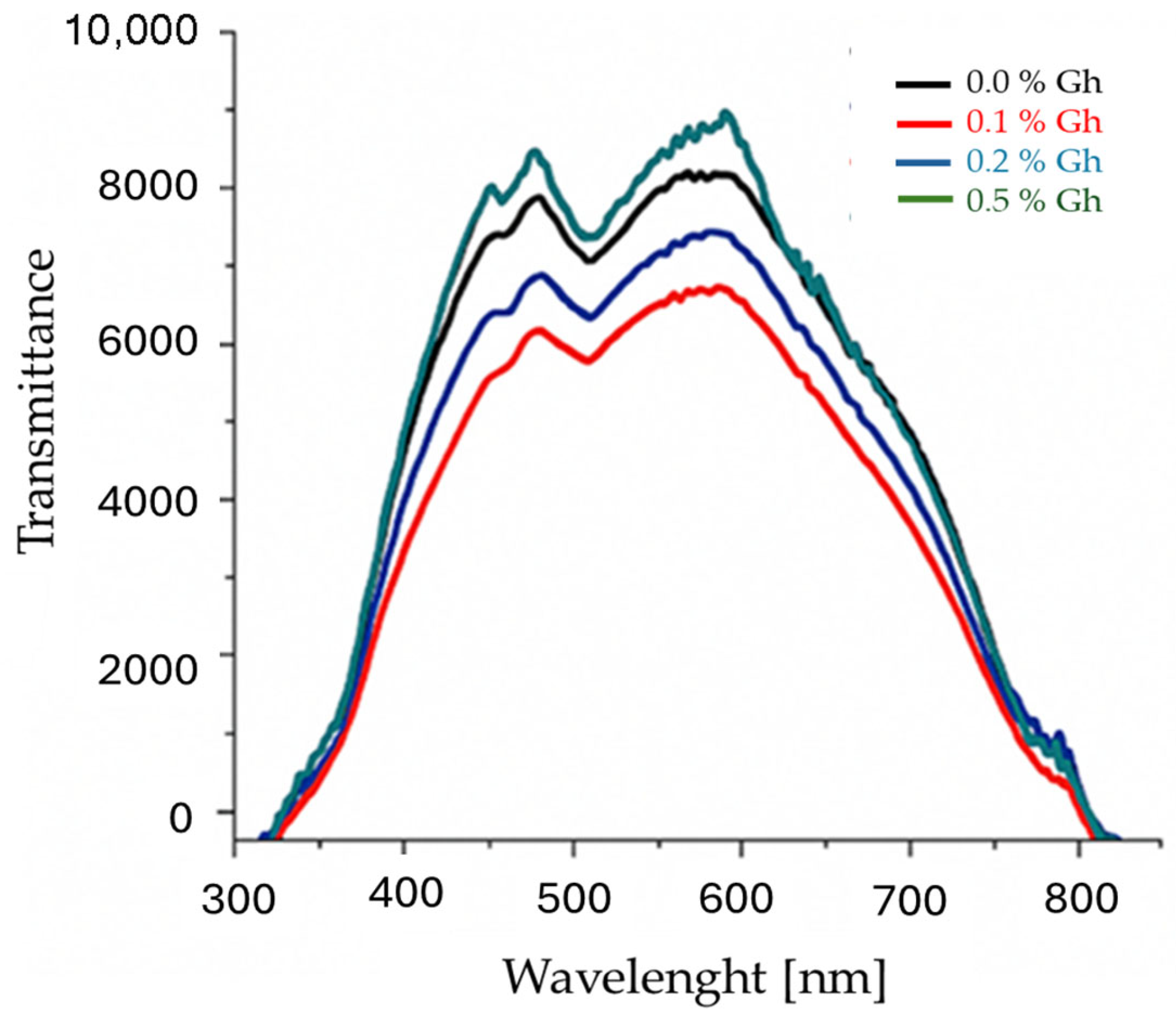
3.2.4. Transmittance
3.2.5. Quantitative Impact of Graphene Concentration on Photovoltaic Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petrović, A.M. Renewable and nonrenewable energy potential. Afford. Clean Energy 2020, 1, 1–10. [Google Scholar]
- Chen, H.; Xie, Z.; Zhang, X.; Hao, J.H. Engine combustion and emission fuelled with natural gas: A review. J. Energy Inst. 2019, 92, 1123–1136. [Google Scholar] [CrossRef]
- Green, M. Photovoltaics: Technology overview. Energy Policy 2000, 28, 989–998. [Google Scholar] [CrossRef]
- Khomenko, S.; Cirach, M.; Pereira-Barboza, E.; Mueller, N.; Barrera-Gómez, J.; Rojas-Rueda, D.; de Hoogh, K.; Hoek, G.; Nieuwenhuijsen, M. Premature mortality due to air pollution in European cities: A health impact assessment. Lancet Planet. Health 2021, 5, 121–134. [Google Scholar] [CrossRef]
- Zito, R. Ionizing radiation hazards: Dangerous goods IV. J. Syst. Saf. 2021, 56, 12–33. [Google Scholar] [CrossRef]
- Kumar, S.; Bharti, P.; Singh, B.P. Performance optimization of efficient PbS quantum dots solar cells through numerical simulation. Sci. Rep. 2023, 13, 10511. [Google Scholar] [CrossRef] [PubMed]
- Magliano, E.; De Carlo, A.; Lamberti, C.M.; Tresso, E.; Penna, M.P.; Pugliese, A.M. Semitransparent perovskite solar cells with ultrathin protective buffer layers. ACS Appl. Energy Mater. 2023, 6, 10340–10353. [Google Scholar] [CrossRef] [PubMed]
- Coppola, C.; Visibelli, A.; Parisi, M.L.; Santucci, A.; Zani, L.; Spiga, O.; Sinicropi, A. A combined ML and DFT strategy for the prediction of dye candidates for indoor DSSCs. npj Comput. Mater. 2025, 11, 28. [Google Scholar] [CrossRef]
- Grätzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, D.; Suo, J.; Cao, Y.; Eickemeyer, F.T.; Vlachopoulos, N.; Zakeeruddin, S.M.; Hagfeldt, A.; Grätzel, M. Hydroxamic acid preadsorption raises efficiency of cosensitized solar cells. Nature 2022, 610, 614–620. [Google Scholar] [CrossRef]
- Arkan, F.; Pakravesh, F.; Barati Darband, F.; Sabagh, S.; Izadyar, M. Recent Progress toward High-Performance Dye-Sensitized Solar Cells: A Review. J. Iran. Chem. Soc. 2024, 21, 577–638. [Google Scholar] [CrossRef]
- Casaluci, S.; Gemmi, M.; Pellegrini, V.; Di Carlo, A.; Bonaccorso, F. Graphene-based large area dye-sensitized solar cell modules. Nanoscale 2016, 8, 5368–5378. [Google Scholar] [CrossRef]
- Habibi Jetani, G.; Rahmani, M.B. TiO2/GO Nanocomposites: Synthesis, Characterization, and DSSC Application. Eur. Phys. J. Plus 2020, 135, 720. [Google Scholar] [CrossRef]
- Verified Market Reports. Graphene Market Size and Forecast (2023–2033); ID Report: 554963; Verified Market Reports: Washington, DC, USA, 2025; Available online: https://www.verifiedmarketreports.com/it/product/graphene-market-size-and-forecast/ (accessed on 9 July 2025).
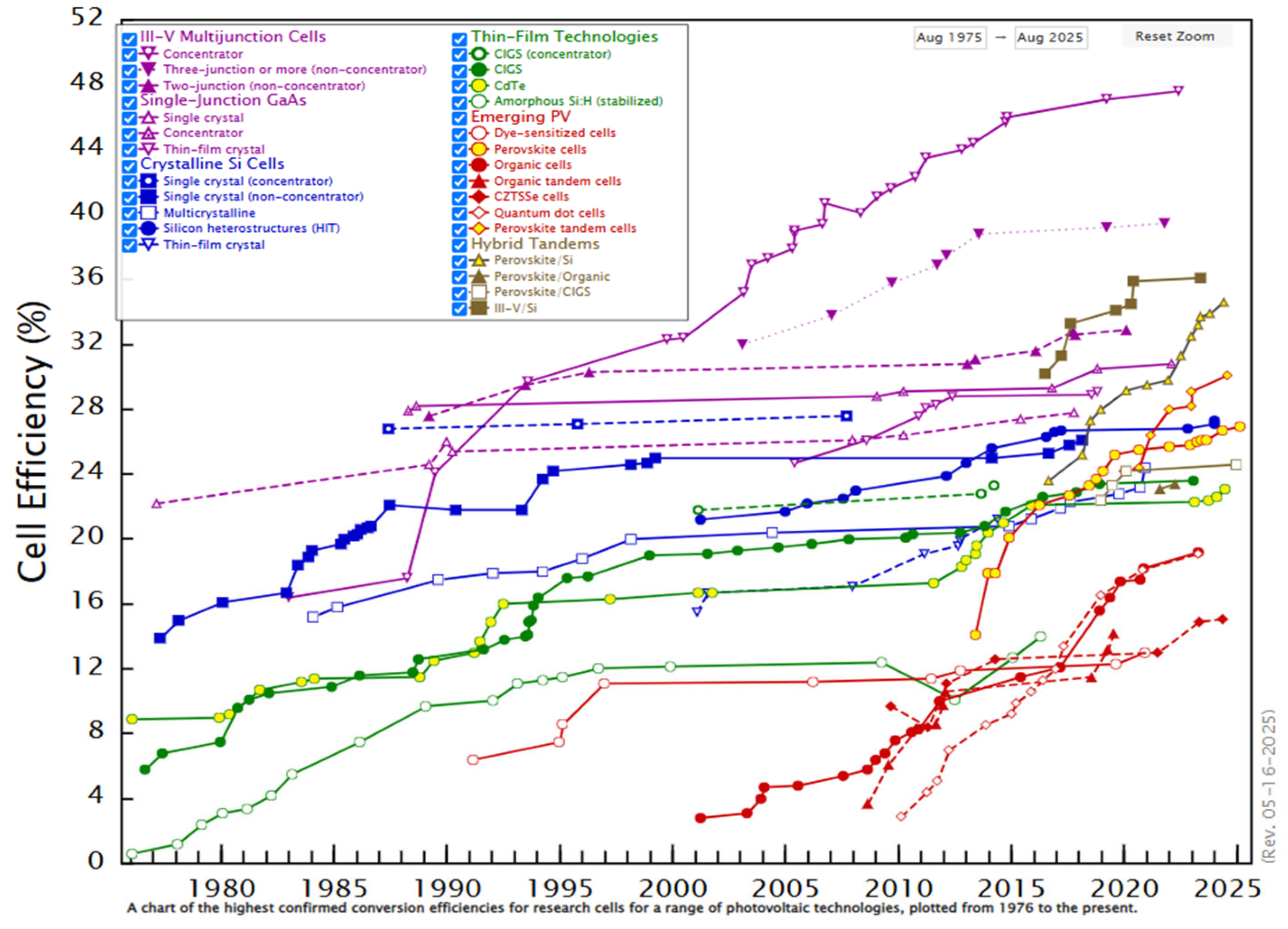
- National Renewable Energy Laboratory (NREL). Best Research-Cell Efficiency Chart. Available online: https://www.nrel.gov/pv/cell-efficiency (accessed on 9 July 2025).
- National Renewable Energy Laboratory (NREL). Interactive Best Research-Cell Efficiency Chart. Last Updated: September 2025. Maintained by: U.S. Department of Energy, Office of Energy Efficiency and Renewable Energy. Available online: https://www.nrel.gov/pv/interactive-cell-efficiency (accessed on 13 October 2025).
- Wang, H.; Hu, Y.H. Graphene as a counter electrode material for dye-sensitized solar cells. Energy Environ. Sci. 2012, 5, 8182–8188. [Google Scholar] [CrossRef]
- Muchuweni, E.; Martincigh, B.S.; Nyamori, V.O. Recent advances in graphene-based materials for dye-sensitized solar cell fabrication. RSC Adv. 2020, 10, 44453–44469. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, J. Unusual enhancement in efficiency of DSSCs upon modifying photoanodes with reduced graphene oxide. RSC Adv. 2022, 12, 45577–45586. [Google Scholar] [CrossRef] [PubMed]
- Bett, A.W.; Dimroth, F.; Stollwerck, G.; Siefer, G.; Schöne, J.; Krause, R.; Wiesenfarth, M.; Philipps, S.P. Metamorphic Epitaxy for Multijunction Solar Cells. MRS Bull. 2016, 41, 183–188. [Google Scholar] [CrossRef]
- Beenakker, C.W.J.; van Houten, H. Quantum transport in semiconductor nanostructures. Solid. State Phys. 1991, 44, 1–228. [Google Scholar] [CrossRef]
- Xu, F.; Madeo, L.; Sapia, P.; Versace, C. Photocatalytic activities of WO3@TiO2 nanoparticles coated on a limestone surface. Nanotechnol. Environ. Eng. 2025, 10, 41. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, D. Electrochemical synthesis of graphene oxide and its application as counter electrode in DSSC. J. Renew. Sustain. Energy 2014, 6, 013125. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, Q.; Liang, Q.; Li, W.; Li, X.; Liu, S.; Shuai, J. α-Fe2O3/Reduced Graphene Oxide Composites as Cost-Effective Counter Electrode for Dye-Sensitized Solar Cells. Catalysts 2022, 12, 645. [Google Scholar] [CrossRef]
- Hatib, R.; Anwar, K.; Magga, R.; Astak, M.A.; Widhiyanuriyawan, D.; Wardoyo, W. Performance Enhancement of Dye Sensitized Solar Cell (DSSC) through TiO2/rGO Hybrid: Comprehensive Study on Synthesis and Characterization. J. Mech. Eng. Sci. Technol. (JMEST) 2024, 8, Article 11. Available online: https://citeus.um.ac.id/jmest/vol8/iss1/11 (accessed on 2 November 2025). [CrossRef]
- Rahman, M.Y.A.; Islam, R. Review of graphene and its modification as cathode for dye-sensitized solar cells. J. Mater. Sci. Mater. Electron. 2021, 32, 1465–1480. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z. Degradation mechanisms of I−/I3− electrolyte in dye-sensitized solar cells under thermal stress. J. Power Sources 2015, 285, 93–100. [Google Scholar]
- Grätzel, M. Stability issues in electrolytes of dye-sensitized solar cells. Acc. Chem. Res. 2017, 50, 448–455. [Google Scholar]
- Cai, C.-Y.; Tseng, S.-K.; Kuo, M.; Lin, K.-Y.A.; Yang, H.; Lee, R.-H. Photovoltaic Performance of a N719 Dye-Based Dye-Sensitized Solar Cell with Transparent Macroporous Anti-Ultraviolet Photonic Crystal Coatings. RSC Adv. 2015, 5, 102803–102810. [Google Scholar] [CrossRef]
- Tanifuji, N.; Shimizu, T.; Shimizu, A.; Shimizu, K.; Abe, K.; Tanaka, M.; Wang, H.; Yoshikawa, H. Stability Modification of Dye-sensitized Solar Cells by Ruthenium Dyes Embedded on Eggshell Membranes. Materials 2023, 16, 6654. [Google Scholar] [CrossRef]
- King, R.R.; Law, D.C.; Edmondson, K.M.; Fetzer, C.M.; Kinsey, G.S.; Yoon, H.; Sherif, R.A.; Karam, N.H. 40% Efficient Metamorphic GaInP/GaInAs/Ge Multijunction Solar Cells. Appl. Phys. Lett. 2007, 90, 183516. [Google Scholar] [CrossRef]
- Katsnelson, M.I. Graphene: Carbon in Two Dimensions. Mater. Today 2007, 10, 20–27. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Ryndyk, D.A. Theory of Quantum Transport at Nanoscale: An Introduction; Springer Series in Solid-State Sciences; Springer: Berlin/Heidelberg, Germany, 2016; Volume 184. [Google Scholar] [CrossRef]
- Tsega, M. Structural and Optical Properties of Mn-Doped Anatase-Rutile TiO2 Nanoparticles. Indian J. Phys. 2025, 99, 177–189. [Google Scholar] [CrossRef]
- Montes-Zavala, J.; Méndez-Hernández, C.; Martínez-Magadán, J.M.; Cruz-Sosa, L.; Guerrero-Sánchez, C. Colloidal Stability of Graphene in Aqueous Medium: A Theoretical Approach Through Molecular Dynamics. J. Mol. Model. 2023, 29, 220. [Google Scholar] [CrossRef]
- Li, M.; Xie, L.-J.; Yi, Z.-L.; Liu, D.; Wang, Z.; Niu, R.-H.; Jia, H.; Kong, Q.-Q. Preparation of Reduced Graphene Oxide Films with High and Uniform Thickness for Electromagnetic Interference Shielding. Crystals 2024, 14, 322. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Jiang, D.; Zhang, Y.; Morozov, S.V.; Stormer, H.L.; Zeitler, U.; Maan, J.C.; Boebinger, G.S.; Kim, P.; Geim, A.K. Electronic Properties of Graphene. Phys. Status Solidi B 2007, 244, 4106–4111. [Google Scholar] [CrossRef]
- Wei, L.; Kuo, P.K.; Thomas, R.L.; Anthony, T.R.; Banholzer, W.F. Thermal Conductivity of Isotopically Modified Single Crystal Diamond. Phys. Rev. Lett. 1993, 70, 3764–3767. [Google Scholar] [CrossRef]
- Wu, T.-C.; Huang, W.-M.; Meen, T.-H.; Tsai, J.-K. Performance Improvement of Dye-Sensitized Solar Cells with Pressed TiO2 Nanoparticles Layer. Coatings 2023, 13, 907. [Google Scholar] [CrossRef]
- Class for Physics of the Royal Swedish Academy of Sciences. Scientific Background on the Nobel Prize in Physics 2010: Graphene; Royal Swedish Academy of Sciences: Stockholm, Sweden, 2010; Available online: https://www.nobelprize.org/uploads/2018/06/advanced-physicsprize2010.pdf (accessed on 2 November 2025).
- Nair, R.R.; Blake, P.; Grigorenko, A.N.; Novoselov, K.S.; Booth, T.J.; Stauber, T.; Peres, N.M.R.; Geim, A.K. Fine Structure Constant Defines Visual Transparency of Graphene. Science 2008, 320, 1308. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Cao, G. Nanostructured Photoelectrodes for Dye-Sensitized Solar Cells. Nano Today 2011, 6, 91–109. [Google Scholar] [CrossRef]
- Jankauskas, Š.; Gudaitis, R.; Vasiliauskas, A.; Guobienė, A.; Meškinis, Š. The Graphene Structure’s Effects on the Current-Voltage and Photovoltaic Characteristics of Directly Synthesized Graphene/n-Si (100) Diodes. Nanomaterials 2022, 12, 1640. [Google Scholar] [CrossRef]
- Antuch, M.; Grigoriev, S.A.; El Rouby, W.M.A.; Millet, P. Implementation of a TiO2/N719-Dye Photo-Anode in a DSSC and Performance Analysis. Russ. J. Electrochem. 2020, 56, 929–937. [Google Scholar] [CrossRef]
- Bendoni, R. Processi di Deposizione di Blocking Layers di TiO2 per Dye-Sensitized Solar Cells (DSSC). Master’s Thesis, Università di Bologna, Bologna, Italy, 2012. Available online: https://amslaurea.unibo.it/id/eprint/3041/ (accessed on 2 November 2025).





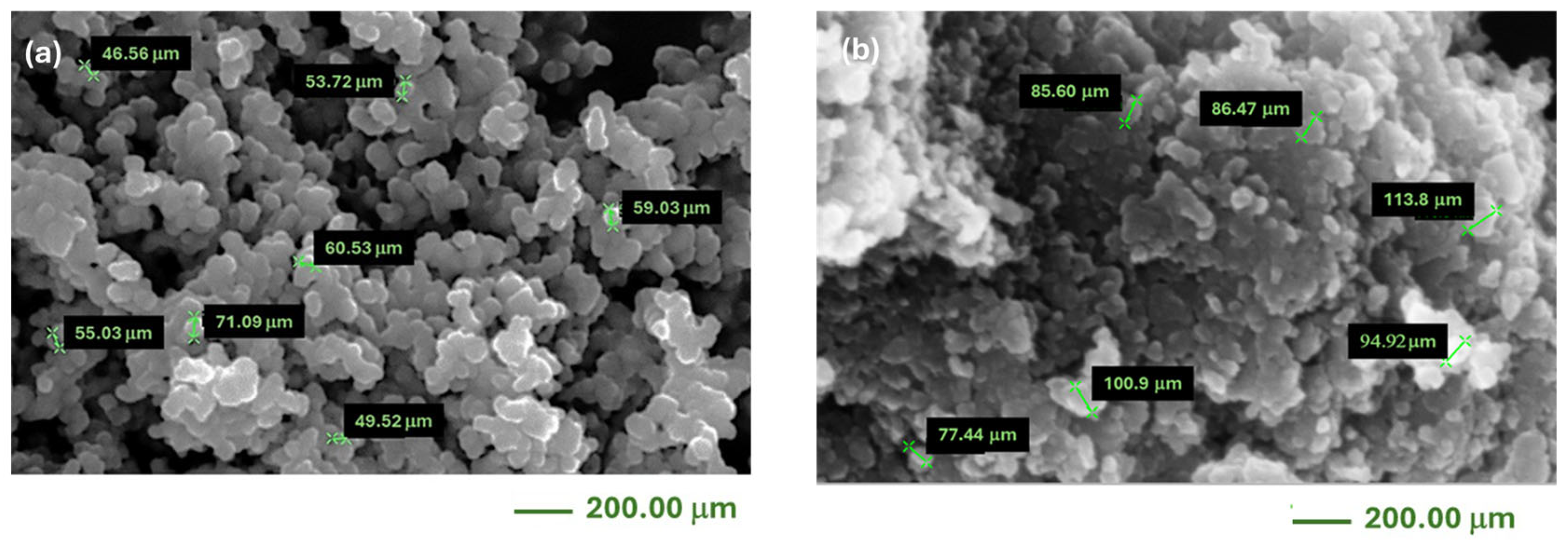



| Properties of Graphene | Effect in DSSC |
|---|---|
| High electrical conductivity | Promotes rapid electron transport in TiO2 |
| UV-vis absorption | It contributes to the collection of light in synergy with the dye |
| Barrier effect | Reduces the recombination of electrons with the electrolyte |
| Two-dimensional structure | Improves connectivity between TiO2 nanoparticles |
| TiO2 [g] ± 0.0001 | Gh [g] ± 0.0001 | Gh [%] |
|---|---|---|
| 3.1001 | – | 0.0 |
| 3.1005 | 0.0031 | 0.1 |
| 3.1004 | 0.0065 | 0.2 |
| 3.1003 | 0.0155 | 0.5 |
| Cells Type | Quantity of Solution [mL] | Acceleration [rpm/s] | Speed [rpm] | Baking [°C × min] |
|---|---|---|---|---|
| TiO2 + 0% Gh | 5 | 700 | 1500 | 60 °C × 30 min |
| TiO2 + 0.1% Gh | 5 | 700 | 1500 | 60 °C × 30 min |
| TiO2 + 0.2% Gh | 5 | 700 | 1500 | 60 °C × 30 min |
| TiO2 + 0.5% Gh | 5 | 700 | 1500 | 60 °C × 30 min |
| Samples on FTO Slides | Description | Functionality |
|---|---|---|
| Anode—FTO + TiO2 + Dye N719 | FTO (conductive and transparent) slide coated with TiO2 and organic dye | Absorbs light and generates electrons |
| Anode—FTO + TiO2 + Gh + Dye N719 | FTO (conductive and transparent) slide coated with TiO2, graphene and organic dye | It generates electrons and the addition of graphene should improve light absorption |
| Counter electrode—FTO + Platinum | FTO slide with thin platinum layer | Facilitates electron transfer and regeneration by dye |
| Characteristic Parameters | Symbol | Unit |
|---|---|---|
| Current density | Jsc | mA/cm2 |
| Short-circuit current | Isc | mA |
| Open-circuit voltage | Voc | V |
| Voltage and current at maximum power per unit area | Vm, Im | V, mA/cm2 |
| Active cell area | A | cm2 |
| Incident power | Pin | mW/cm2 |
| % Gh | Voc [V] | FF | Vm [V] | Jsc [mA/cm2] | Im * [mA/cm2] |
|---|---|---|---|---|---|
| 0.0 | 0.75 ± 0.02 | 0.72 | 0.54 ± 0.04 | 20.20 ± 2.62 | 14.54 ± 2.62 |
| 0.1 | 0.78 ± 0.03 | 0.78 | 0.61 ± 0.05 | 25.30 ± 2.74 | 19.73 ± 2.54 |
| 0.2 | 0.76 ± 0.02 | 0.76 | 0.58 ± 0.05 | 23.10 ± 2.51 | 17.56 ± 2.60 |
| 0.5 | 0.72 ± 0.03 | 0.70 | 0.50 ± 0.04 | 18.50 ± 2.71 | 12.95 ± 2.69 |
| DSSC Type | η [%] | Percentage Increase in Efficiency Δη [%] | Voc [V] | Δ Voc [%] | Jsc [mA/cm2] | Δ Jsc [%] | FF | Δ FF [%] |
|---|---|---|---|---|---|---|---|---|
| 0.0% Gh | 11.1 | — | 0.75 ± 0.02 | — | 20.20 ± 2.62 | — | 0.72 | — |
| 0.1% Gh | 15.2 | +37% | 0.78 ± 0.03 | 4% | 25.30 ± 2.74 | 25% | 0.78 | 8% |
| 0.2% Gh | 13.4 | +21% | 0.76 ± 0.02 | 1% | 23.10 ± 2.51 | 14% | 0.76 | 6% |
| 0.5% Gh | 9.2 | –17% | 0.72 ± 0.03 | –4% | 18.50 ± 2.71 | –8% | 0.7 | –3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madeo, L.; Macario, A.; Sapia, P.; De Luca, P. Synergistic Integration of Graphene Nanoparticles in Colloidal TiO2 for Grätzel Cells (DSSC). J. Compos. Sci. 2025, 9, 612. https://doi.org/10.3390/jcs9110612
Madeo L, Macario A, Sapia P, De Luca P. Synergistic Integration of Graphene Nanoparticles in Colloidal TiO2 for Grätzel Cells (DSSC). Journal of Composites Science. 2025; 9(11):612. https://doi.org/10.3390/jcs9110612
Chicago/Turabian StyleMadeo, Luigi, Anastasia Macario, Peppino Sapia, and Pierantonio De Luca. 2025. "Synergistic Integration of Graphene Nanoparticles in Colloidal TiO2 for Grätzel Cells (DSSC)" Journal of Composites Science 9, no. 11: 612. https://doi.org/10.3390/jcs9110612
APA StyleMadeo, L., Macario, A., Sapia, P., & De Luca, P. (2025). Synergistic Integration of Graphene Nanoparticles in Colloidal TiO2 for Grätzel Cells (DSSC). Journal of Composites Science, 9(11), 612. https://doi.org/10.3390/jcs9110612








