1. Introduction
Moderate exposure to ultraviolet (UV) radiation promotes cutaneous synthesis of vitamin D [
1] and provides antimicrobial benefits, but excessive exposure produces acute damage—sunburn, erythema, and hyperpigmentation—and long-term health risks. Chronic over-exposure accelerates photoaging, leading to transepidermal water loss, loss of elasticity, and wrinkle formation, and it is strongly implicated in the development of skin cancer [
2,
3,
4]. Rising awareness of these hazards has driven rapid growth of sunscreen products in the global cosmetics market [
5].
Formulating an effective sunscreen is inherently complex, requiring simultaneous attention to scientific performance, consumer sensorial expectations, and regulatory safety. Users demand safe, highly efficient protection delivered in a premium texture with excellent skin affinity [
6]. Equally critical is broad-spectrum coverage: an ideal sunscreen must shield both UVB (290–320 nm) and UVA (320–400 nm) wavelengths while minimizing dermal penetration [
6,
7,
8]. Achieving this typically necessitates blending multiple UVB and UVA filters within a single formulation [
9,
10,
11]. OMC and BP-3 are widely used UV filters in commercial sunscreen products with established safety records. OMC primarily absorbs UVB radiation, whereas BP-3 absorbs both UVB and part of the UVA spectrum. Co-encapsulation of these two filters can provide complementary spectral coverage, achieving broad-spectrum protection while enhancing SPF and reducing transdermal penetration.
Because sunscreens are applied widely and frequently, their safety profile has become an intense focus of research. Recent strategies include reformulating vehicle systems [
12], adding antioxidants or natural extracts [
13,
14,
15,
16,
17,
18,
19,
20], and—most prominently—employing carrier technologies such as hydrotalcite (HTlc), cyclodextrin (CD), solid–lipid nanoparticles (SLN), nanostructured lipid carriers (NLCs), and mesoporous silica (MS). Each carrier presents distinct advantages and limitations. In terms of loading capacity, HTlc typically reaches 20–25%, CD 2–21%, SLN, and NLC 15–20%, whereas MS achieves 30–65%. The superior loading afforded by MS enables high photoprotective efficacy with minimal carrier addition, preserving formulation aesthetics and aligning with current commercial sunscreen requirements. Reports on simultaneous encapsulation of two UV filters are scarce; to date, only one patent (EP1656199A1, Scalia et al., 2016) describes such co-loading, with no experimental sunscreen evaluation, and no study has examined mesoporous silica as a co-encapsulation platform. Combining OMC (UVB filter) and BP-3 (UVB/UVA filter) in the same mesoporous silica matrix can provide complementary spectral coverage, enhance SPF, and improve loading efficiency through the solubilizing effect of liquid OMC on solid BP-3.
Our laboratory has previously demonstrated that mesoporous silica (MS) can individually encapsulate either OMC or BP-3, sharply decreasing their skin permeation while raising their photoprotective indices. To date, however, no study has addressed the co-encapsulation of both filters within a single MS matrix. Accordingly, the present work explores simultaneous loading of OMC and BP-3 into the same MS carrier. Housing both actives in one platform should provide complementary UVA and UVB protection, prevent direct filter–skin contact, and further curtail transdermal penetration.
2. Experimental Section
2.1. Materials
Tetraethyl orthosilicate (TEOS), methanol, ethanol, 1-chlorobutane, acetonitrile, sodium tetrafluoroborate, isopropyl alcohol, and 1-methyl-imidazole were obtained from Acros Organics (Geel, Belgium). Octyl methoxycinnamate (OMC), cyclomethicone (TSF-405), glyceryl stearate/PEG-100 stearate (Arlacel 165), glyceryl monostearate (GMS), propylene glycol (PG), 1,3-butylene glycol (1,3-BG), Carbopol® 940, disodium EDTA, and triethanolamine (TEA) were supplied by Top-Thyme (Taipei, Taiwan). Cetyl octanoate and Phenonip-XP were purchased from Kosfarm Corporation (Taipei, Taiwan), while Myritol® 318 (caprylic/capric triglyceride) and benzophenone-3 (BP-3) were sourced from Eugeni Company (New Taipei, Taiwan) and Essence Plus Company (Hsinchu, Taiwan), respectively. All chemicals were of analytical grade and used as received, without further purification.
2.2. Preparation of Mesoporous Silica Co-Loaded with OMC and BP-3 (Sample Code S4M1B1)
BP-3 (1.45 g) and OMC (1.45 g) were first magnetically stirred with tetraethyl orthosilicate (TEOS, 5.8 g) until a homogeneous mixture was obtained. Methanol (1.9 g), the ionic liquid 1-butyl-3-methylimidazolium tetrafluoroborate ([BMIM][BF4], 16.7 g), and de-ionized water (3.4 g) were then added sequentially under continuous stirring. The sealed mixture was subjected to sol–gel hydrolysis–condensation at room temperature for 72 h, after which it was uncapped and air-dried for at least two weeks. The resulting xerogel was Soxhlet-extracted with de-ionized water to remove residual [BMIM][BF4] and unbound organics and then freeze-dried at −50 °C for seven days to yield a pale, free-flowing powder. This process achieved up to 72 wt% sunscreen loading, with a 1:1 mass ratio of OMC to BP-3 identified as optimal for maximizing encapsulation while avoiding viscous gels or incomplete entrapment.
2.3. Characterization of the Encapsulation Product
Fourier transform infrared spectra were obtained on a JASCO FTIR-4200 spectrometer (JASCO Corporation, Tokyo, Japan) (KBr pellet technique, 32 scans, 4 cm−1 resolution) using either neat S4M1B1 powder or equimolar amounts of free BP-3 and OMC for reference. Thermogravimetric profiles were recorded with a Seiko TG/DTA-220 (Seiko Instruments Inc., Chiba, Japan) under nitrogen (50 mL min−1), heating from 30 °C to 200 °C at 10 °C min−1 and then to 600 °C at 5 °C min−1 after a 15 min isothermal purge at ambient temperature. Differential scanning calorimetry was carried out on a TA Instruments Q20 (TA Instruments, New Castle, DE, USA); 3–6 mg samples containing an identical BP-3 mass were sealed in aluminum pans and scanned from 30 °C to 100 °C at 10 °C min−1 under nitrogen. UV–visible absorption between 200 nm and 500 nm was measured on a Thermo Evolution 60S spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) using compressed powders of BP-3 or S4M1B1 with equivalent BP-3 content. Surface morphology of S4M1B1 was examined on a JEOL JSM-6300 scanning electron microscope (JEOL Ltd., Tokyo, Japan) after gold sputtering. In every test, the sample mass of BP-3 was held constant to ensure direct comparison between the free and encapsulated states. The nitrogen adsorption isotherms of the powders were measured using an Accelerated Surface Area and Porosimetry System (ASAP 2010, Micromeritics Instrument Corporation, Norcross, GA, USA).
2.4. Photostability Test
Liquid OMC (1 g) was dissolved in 100 mL of isopropyl alcohol, whereas BP-3 and S4M1B1 were evaluated in the solid state. Each sample was spread onto a glass plate at a surface density of 2 mg cm−2 and irradiated with a solar simulator (Abet Technologies, Milford, CT, USA) delivering 500 W m−2. The simulator was equipped with an optical filter that removed wavelengths below 290 nm and an IR-block filter to minimize thermal artefacts. To further suppress heat effects, the loaded glass plates were placed on a stage cooled by a 4 °C circulating water bath. After 30, 60, 90, and 120 min of exposure, UV–visible absorption spectra (290–500 nm) were recorded with a spectrophotometer. All samples were protected from ambient light before and immediately after irradiation.
2.5. Preparation of Sunscreen Emulsion
Aqueous phase (Component 1): propylene glycol (4 wt%), Carbopol 940 (0.16 wt%), disodium EDTA (0.20 wt%), and de-ionized water (62.34 wt% for the formulation containing free filters and 59.54 wt% for the MS-encapsulated filter) were blended and heated to 80 °C with gentle agitation. Oil phase (Component 2): cyclomethicone (10 wt%), cetyl octanoate (10 wt%), Arlacel® 165 (2.5 wt%), glyceryl monostearate (1.5 wt%), cetearyl alcohol (1.5 wt%), and either (i) free octyl methoxycinnamate (2.6 wt%) plus benzophenone-3 (4.6 wt%) or (ii) S4M1B1 powder (10 wt%) were combined and likewise heated to 80 °C until homogeneous. While maintaining both phases at 80 °C, the aqueous phase was slowly poured into the oil phase under high-shear mixing and stirred until the mixture cooled to 40 °C. Post-addition (Component 3): triethanolamine 0.10 wt % was added to neutralize the carbomer and adjust the pH, followed by Phenonip-XP (0.50 wt%) as a preservative; stirring continued to ambient temperature to yield a stable cream. The emulsion containing free OMC and BP-3 is designated S4M1B1-Mix-E, whereas the emulsion containing the mesoporous silica–encapsulated filters is designated S4M1B1-E.
2.6. In Vitro Measurement of UV Protection Factor
The in vitro sun protection factor (SPF) was determined using a computer-controlled SPF-290S analyzer (Optometrics Corp., Leeds, UK). A surgical Transpore™ tape (3M Company, St. Paul, MN, USA) with a surface area of 50 cm2 served as the substrate, following the procedure established by Diffey and Robson (1989). Test formulations were applied at a dosage of 2 mg/cm2 using a syringe, followed by manual spreading with a latex-gloved hand (Global), using three longitudinal back-and-forth strokes to achieve even distribution.
After a 15 min equilibration period, six distinct, non-overlapping spots on the tape were exposed to a simulated UV light source. The in vitro SPF, indicative of the product’s UVB and UVA protective efficacy, was calculated based on the monochromatic protection factor (MPF), solar spectral irradiance, and erythemal weighting factors, according to the following equation:
where
Eλ: spectral irradiance of terrestrial sunlight;
Bλ: erythemal effectiveness at wavelength λ;
MPFλ: monochromatic protection factor, defined as the ratio of the detected UV intensity without versus with sunscreen.
A higher SPF value reflects stronger protection against UVB radiation (Kockler et al., 2012).
For UVA protection (320–400 nm), the same equation is applied within the defined wavelength range to calculate the in vitro UVA protection factor (UVA-PF):
2.7. Skin Permeation Test of Sunscreens
In this study, in vitro skin permeation was evaluated using Franz glass diffusion cells. Before the experiment, the cellulose membrane was pre-soaked for 1 h, and the whole assembly was preheated in a circulating water bath for 30 min, stabilizing the system at 37 ± 0.5 °C and ensuring complete membrane–receptor contact without air bubbles. Subsequently, 500 mg of sunscreen lotion (≈2 mg cm−2) was evenly spread in the donor chamber, whereas the receptor chamber was filled with 15 mL of pH 7.4 phosphate-buffered saline containing 2% Tween 20 and magnetically stirred at 350 rpm to keep the permeated sunscreen uniformly dispersed.
Samples (1 mL) were withdrawn from the receptor chamber at 1, 2, 3, 4, 5, 6, and 24 h, and each withdrawal was immediately replaced with an equal volume of fresh buffer to maintain a constant volume. Each aliquot was diluted with 2 mL of buffer, centrifuged for 10 min, and the supernatant was analyzed by UV–vis spectrophotometry at 291 nm for BP-3 and 310 nm for OMC. Absorbance values at each time point were converted into cumulative permeation (μg cm−2) using pre-established calibration curves. Every sample was tested in triplicate, and mean values were reported to ensure data reliability.
3. Results and Discussion
3.1. FTIR Spectroscopy
Fourier transform infrared (FTIR) analysis was conducted to evaluate whether encapsulation in mesoporous silica (MS) induced chemical modifications in benzophenone-3 (BP-3) and octyl methoxycinnamate (OMC). Any chemical alteration might adversely affect the photoprotective properties of these UV filters or lead to the formation of potentially irritating or harmful substances.
Figure 1 displays the FTIR spectra of pure MS, pure BP-3, pure OMC, and the encapsulated composite S4M1B1.
The MS spectrum exhibited characteristic absorption bands typical of silica frameworks, including prominent Si–O–Si asymmetric stretching vibrations around 1080 cm−1, symmetric stretching at approximately 800 cm−1, and Si–OH stretching near 960 cm−1. Pure BP-3 revealed distinctive peaks at 3434 cm−1 (O–H stretching), 1599 cm−1 (C=O stretching), and aromatic C=C stretching vibrations at 1572, 1505, and 1443 cm−1. Additional signals at 1259 cm−1 (C–O in CH3–O) and 1204 cm−1 (phenolic C–O), as well as bending vibrations at 820, 698, and 597 cm−1 (C–H) were also clearly discernible. Pure OMC showed characteristic peaks at 2960 cm−1 (CH2 stretching), as well as pronounced C=O absorption at 1710 cm−1 and aromatic C=C bands similar to those of BP-3.
The composite S4M1B1 exhibited all characteristic absorption bands of BP-3 and OMC, along with the typical bands of MS, without any noticeable wavenumber shifts or emergence of new peaks. This absence of new absorption features indicates that no chemical interactions occurred between MS and the encapsulated UV filters. Thus, the FTIR results conclusively verify successful co-encapsulation of BP-3 and OMC within the MS pores, purely through physical entrapment.
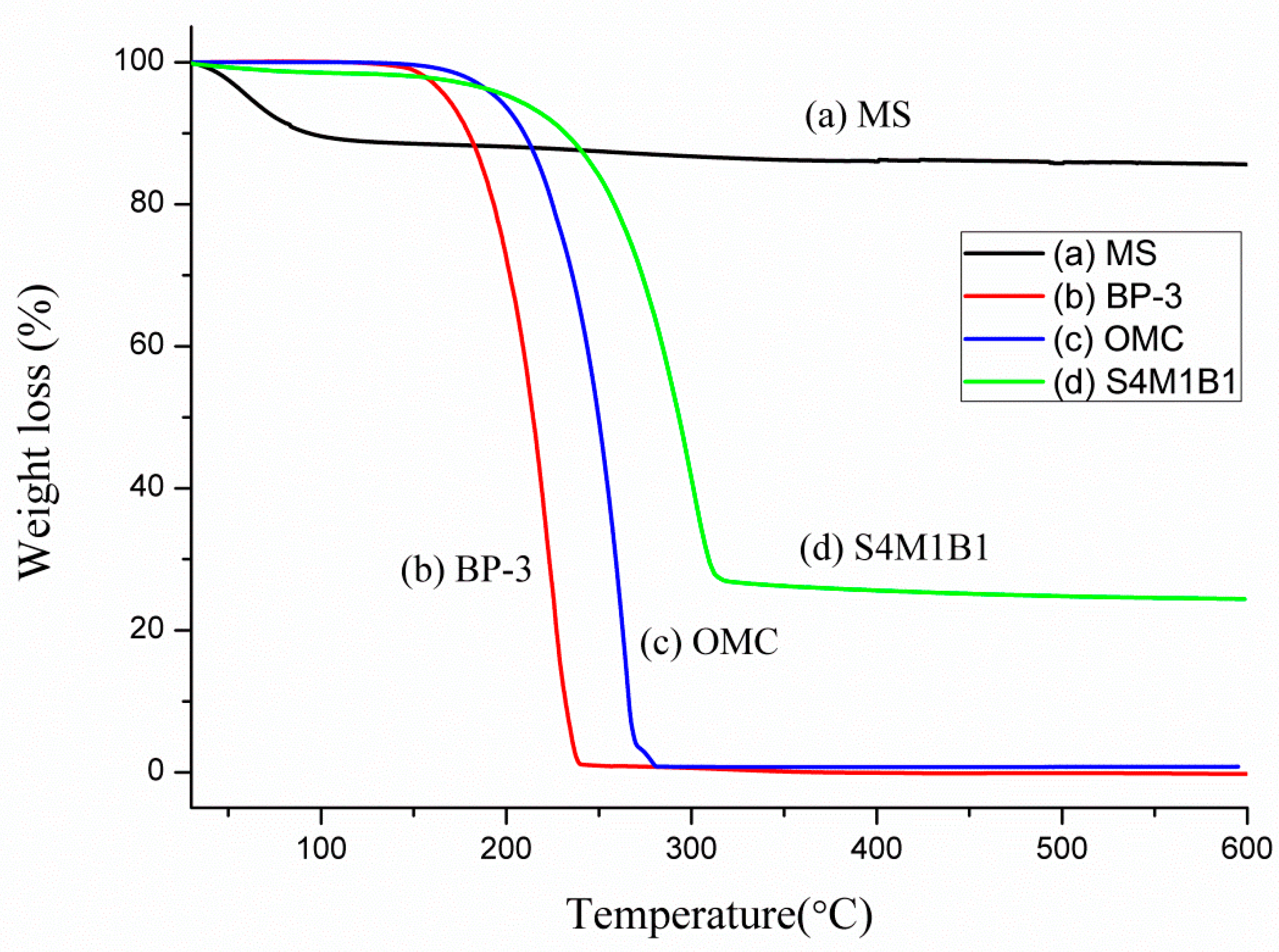
3.2. TGA Characterization and Analysis
Thermogravimetric analysis (TGA) provided quantitative insights into the encapsulation efficiency and thermal stability of BP-3 and OMC when incorporated into mesoporous silica (MS). Since pure MS only exhibits a minimal weight loss below 600 °C, attributed solely to moisture desorption, any substantial mass loss detected at lower temperatures can confidently be attributed to the decomposition of encapsulated sunscreen agents.
Figure 2 presents TGA curves for bare MS, pure BP-3, pure OMC, and the composite S4M1B1.
As shown in
Figure 2a, pure MS exhibited negligible weight loss, confined to below 100 °C due to the evaporation of physically adsorbed water. Pure BP-3 (
Figure 2b) displayed its main decomposition step at approximately 226 °C, whereas pure OMC (
Figure 2c) decomposed at a slightly higher temperature around 262 °C, reflecting inherent differences in molecular structure and thermal stability.
For the encapsulated sample S4M1B1 (
Figure 2d), a single prominent weight-loss event occurred at around 285 °C, representing the simultaneous decomposition of co-encapsulated BP-3 and OMC. Notably, this event occurred at a significantly elevated temperature compared to the pure ingredients, indicating that encapsulation within the rigid mesoporous silica matrix enhanced the thermal stability of both sunscreen agents, likely due to confinement effects and limited molecular mobility.
The quantitative analysis of the TGA profile confirmed an exceptional sunscreen loading of approximately 72 wt%, significantly surpassing previously reported carrier systems. Such a high encapsulation efficiency is probably facilitated by the liquid-state OMC acting as a solvent, effectively dissolving solid BP-3 and promoting their uniform distribution and efficient entrapment within the mesoporous silica structure. This high loading capacity is beneficial, as it enables the formulation of high-SPF sunscreen products with reduced amounts of carrier materials, thereby preserving desirable sensory properties and enhancing consumer acceptance.
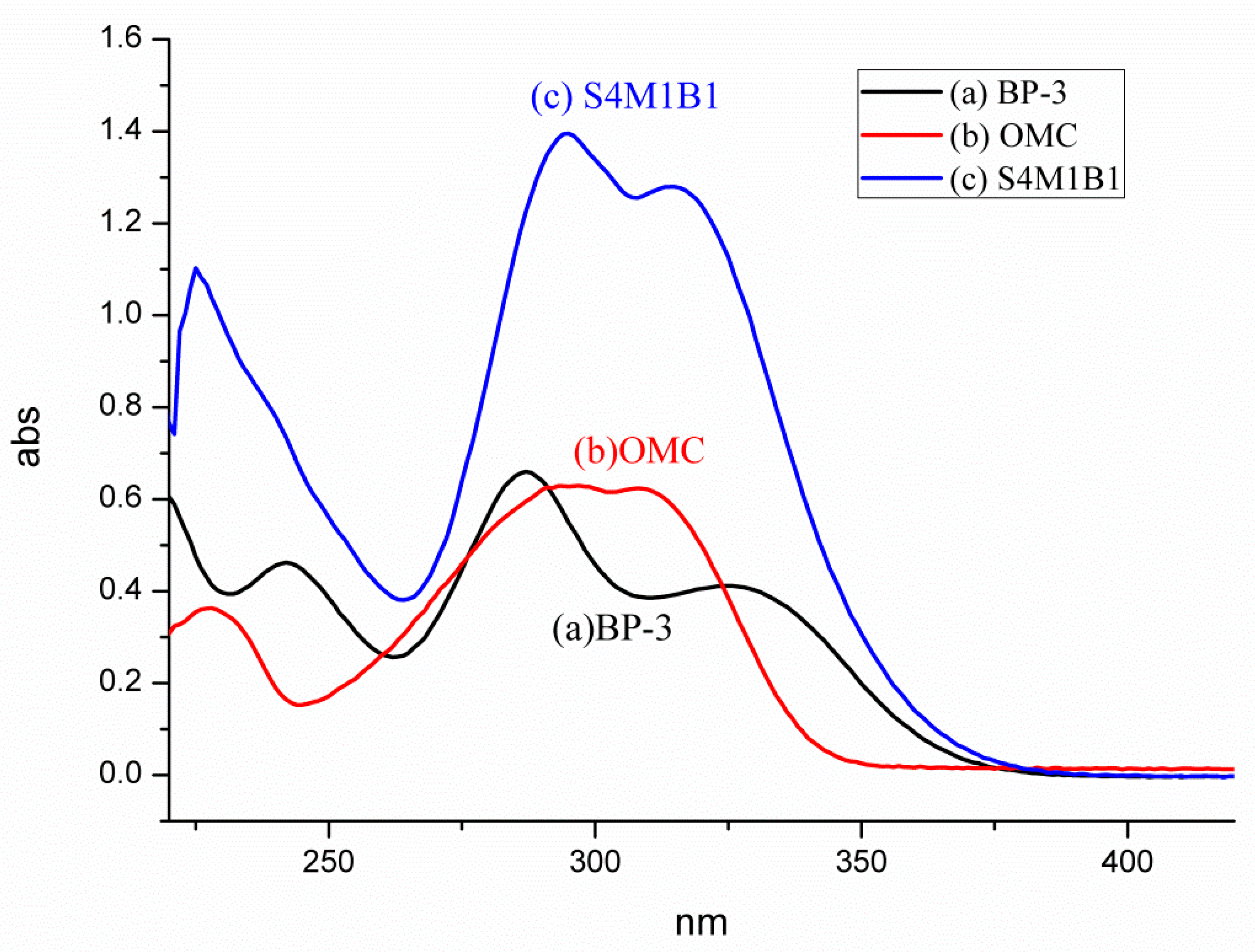
3.3. UV–Vis Spectroscopy
UV–vis spectroscopy provided further evidence, confirming the successful encapsulation of BP-3 and OMC within the mesoporous silica (MS) [
21]. Following established methods, the sample S4M1B1 was dispersed in isopropanol, sonicated, centrifuged, and filtered, with the resulting solution analyzed via UV–vis spectroscopy (
Figure 3).
Individual spectra (
Figure 3a,b) revealed distinctive absorption patterns: BP-3 exhibited strong absorption bands spanning both UVB (290–320 nm) and UVA (320–400 nm) regions, whereas OMC predominantly absorbed within the UVB region. Importantly, the UV spectrum of S4M1B1 (
Figure 3c) displayed clear absorption peaks corresponding to both UV filters, confirming their simultaneous presence in the composite. Slight deviations or minor shifts in wavelength position, observed for some absorption bands, may indicate subtle additive or intermolecular interactions within the confined environment of the silica pores.
Quantitative analysis based on UV–vis data confirmed the total sunscreen loading as 72 wt%, comprising 46 wt% BP-3, and 26 wt% OMC. The relatively lower encapsulation ratio of OMC likely results from its liquid nature, which may facilitate partial leaching or incomplete encapsulation during the preparation and washing steps.
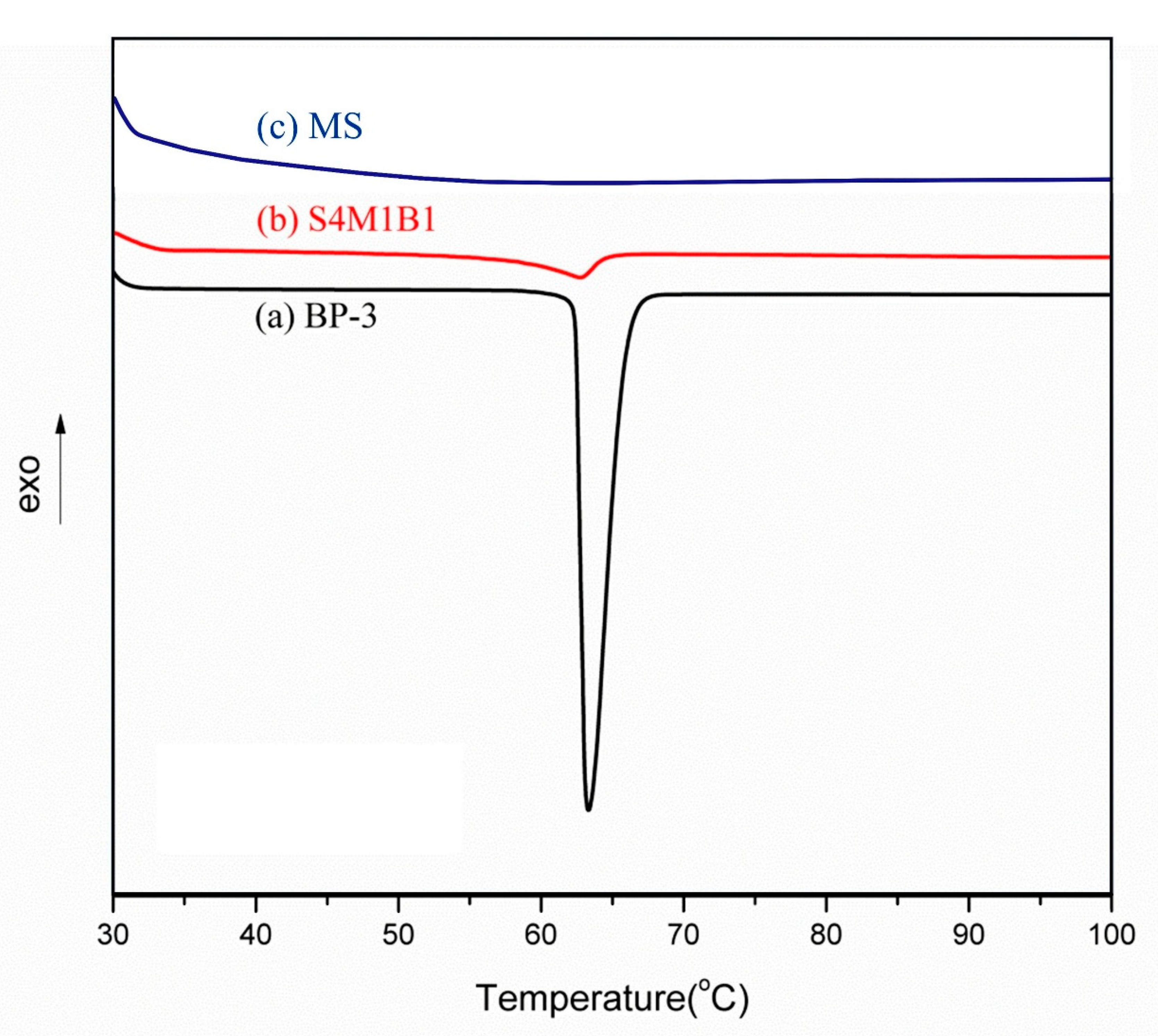
3.4. DSC Characterization and Analysis
Differential scanning calorimetry (DSC) was employed to evaluate changes in crystallinity and thermal behavior of BP-3 after encapsulation into mesoporous silica (MS). DSC thermograms for pure BP-3 and the encapsulated S4M1B1 are illustrated in
Figure 4.
Pure BP-3 (
Figure 4a) exhibited a sharp, clearly defined endothermic melting peak at approximately 63.3 °C, reflecting its high crystalline purity. Upon encapsulation within the mesoporous silica (sample S4M1B1,
Figure 4b), the melting peak appeared at nearly the identical temperature, indicating no significant alterations to the intrinsic melting characteristics of BP-3, further confirming that encapsulation did not chemically alter BP-3.
However, a notable decrease in the enthalpy of fusion was observed in the composite sample S4M1B1, implying significantly reduced crystallinity. This reduction in crystallinity strongly suggests that BP-3 molecules are uniformly dispersed and spatially confined within silica pores, thereby preventing extensive recrystallization. Such enhanced dispersion not only improves homogeneity but also promotes improved and consistent UV-filtering performance in practical sunscreen formulations.
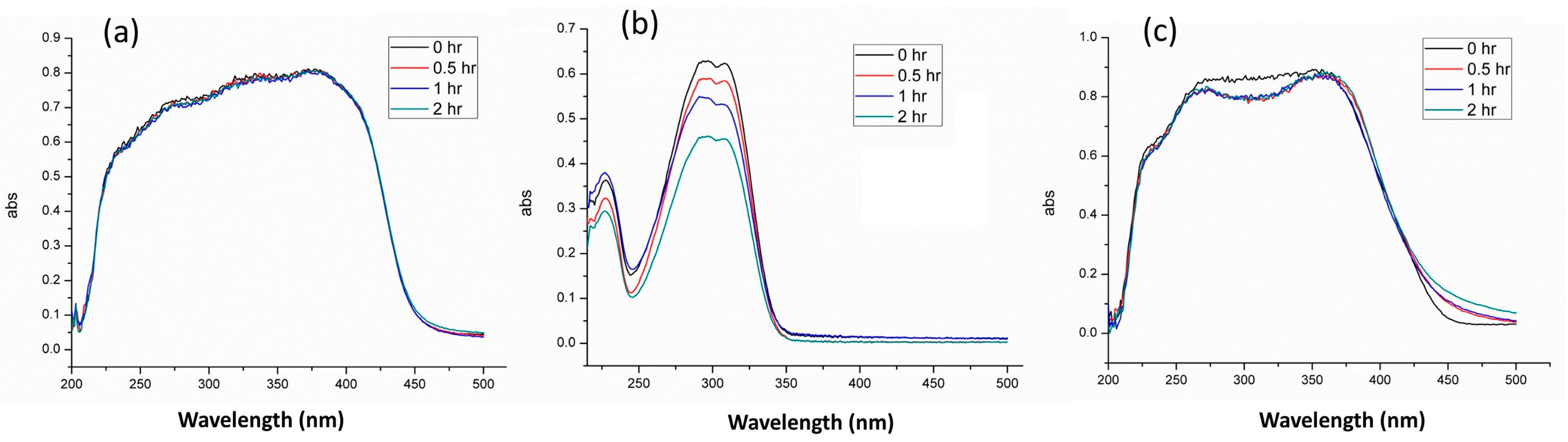
3.5. Photostability Evaluation
Photostability assessments were performed to evaluate the long-term effectiveness and safety of encapsulated UV filters under prolonged UV irradiation. Many organic sunscreens, notably octyl methoxycinnamate (OMC), are known to degrade upon UV exposure, diminishing their protective efficiency and potentially forming harmful reactive intermediates.
Figure 5 compares the photostability profiles of pure BP-3, pure OMC, and the encapsulated formulation (S4M1B1) after two hours of simulated sunlight exposure. The pure BP-3 (
Figure 5a) exhibited stable UV absorbance throughout the entire irradiation period, confirming its inherent photostability. Conversely, the pure OMC (
Figure 5b) showed a progressive decline in absorbance intensity, clearly demonstrating intrinsic photolability and the potential formation of degradation products.
Encouragingly, S4M1B1 (
Figure 5c) initially showed only a minor decrease in UV absorbance within the first 30 min, likely due to minimal amounts of surface-adsorbed OMC. After this brief initial drop, the absorbance stabilized and remained nearly constant throughout the remaining irradiation period. This result highlights the protective role played by mesoporous silica encapsulation, effectively isolating the OMC molecules and significantly reducing their susceptibility to photodegradation. Such enhanced photostability ensures sustained UV-protective performance, improving both the safety profile and durability of sunscreen products.
3.6. Sun Protection Factor
This study evaluated the sun protection efficacy of the samples using a modified in vitro testing method based on the protocol proposed by Diffey and Robson [
22]. A 3M Transpore™ tape was used as an artificial skin substrate due to its surface roughness, which effectively simulates the condition of real skin after sunscreen application. This method is cost-effective, easy to use, and utilizes readily available materials [
22].
Table 1 summarizes the SPF and UVA-PF values for the base emulsion (MS-E), the physical mixture emulsion (S4M1B1-Mix-E), and the encapsulated composite emulsion (S4M1B1-E).
The results show that the base emulsion without UV filters (MS-E) provided negligible UV protection. In contrast, the emulsion containing the S4M1B1 composite significantly outperformed the physical mixture, with SPF and UVA-PF values approximately 40% higher. This enhancement is primarily attributed to the improved dispersion and stability of the UV filters—benzophenone-3 (BP-3) and octyl methoxycinnamate (OMC)—after encapsulation within the mesoporous silica carrier, leading to more uniform distribution and enhanced protective performance. These findings reaffirm the effectiveness of mesoporous silica in simultaneously encapsulating multiple UV filters, addressing the demand for high-performance broad-spectrum sunscreens.
As demonstrated in our previous studies, mesoporous silica loaded solely with OMC exhibited a significantly higher SPF value; however, because OMC primarily absorbs UVB radiation, its UVA protection factor (UVA-PF) was relatively low [
23]. Conversely, mesoporous silica loaded solely with BP-3 provided a much higher UVA-PF but a relatively low SPF, due to BP-3’s broader but less intense UVB absorption [
24]. Therefore, co-encapsulating OMC and BP-3 within the same mesoporous silica carrier combines the strengths of both UV filters, delivering complementary UVB and UVA protection and markedly enhancing overall photoprotective performance.
In addition, the higher SPF of S4M1B1-E arises from improved dispersion of UV filters after co-encapsulation in mesoporous silica, which prevents BP-3 recrystallization (as shown in DSC) and maintains it in a finely dispersed state. This enables effective synergy with OMC for enhanced broad-spectrum protection, whereas unencapsulated BP-3 tends to recrystallize, reducing dispersion and diminishing synergistic effects [
24].
3.7. In Vitro Skin Permeation Analysis
Many organic UV filters can pose potential risks such as skin irritation, photosensitivity, or systemic toxicity if they penetrate the skin [
25]. To evaluate the safety of UV filters encapsulated within mesoporous silica (MS), this study employed a Franz diffusion cell system for skin-permeation testing. An “infinite dose” method was used, where 500 mg of each sample was applied to the donor chamber to maintain a constant release rate of active ingredients throughout the test [
26].
Figure 6 illustrates the cumulative permeation profiles of BP-3 and OMC from both the physical mixture emulsion (S4M1B1-Mix-E) and the encapsulated composite emulsion (S4M1B1-E) over 24 h. Encapsulation significantly reduced skin permeation: BP-3 and OMC in the MS-based emulsion exhibited permeation values of only 87.9 ± 3.6 µg/cm
2 and 51.6 ± 2.3 µg/cm
2, respectively—both over 55% lower than those in the physical mixture (192.6 ± 5.6 µg/cm
2 for BP-3 and 114.1 ± 3.9 µg/cm
2 for OMC). This notable reduction suggests that mesoporous silica effectively restricts the molecular diffusion of UV filters, thereby reducing the risk of systemic exposure. The slightly higher permeation of BP-3 compared to OMC is likely due to its higher loading ratio in the composite.
3.8. Characterization of Surface Area, Particle Size, and Morphology
Nitrogen adsorption–desorption isotherm analysis revealed that the pristine mesoporous silica (MS) exhibited a high specific surface area of 523 m
2 g
−1, an average pore diameter of 9.8 nm, and a total pore volume of 1.4 cm
3 g
−1 (
Figure 7). The isotherm profile displayed a type IV pattern with a pronounced H1 hysteresis loop at relative pressures (P/P
0) between 0.8 and 1.0, characteristic of well-defined mesoporous materials with uniform cylindrical pores. The corresponding Barrett–Joyner–Halenda (BJH) pore size distribution confirmed a narrow pore size range centered at approximately 10 nm.
Previous studies have consistently reported that encapsulation of organic UV filters within MS frameworks leads to a reduction in specific surface area and pore volume, accompanied by a modest increase in particle size, due to partial occupation of the mesopores and surface coverage by the encapsulated molecules. This structural modification is considered beneficial for stabilizing the active agents while maintaining sufficient porosity for controlled release applications.
To verify the encapsulation efficiency of BP-3 and OMC within mesoporous silica (MS), scanning electron microscopy (SEM) was employed to examine the surface morphology of the S4M1B1 composite, both before and after washing with the solvent M-318. This comparison helps determine whether the UV filters reside primarily within the silica pores or on the surface.
As shown in
Figure 8a, the unwashed S4M1B1 sample exhibits the typical porous structure of MS with minimal surface residues, indicating only slight surface adsorption of UV filters. After solvent washing and drying (
Figure 8b), surface residues were notably removed, revealing a clean and intact mesoporous structure with no visible filter aggregates. These observations confirm that both BP-3 and OMC were predominantly encapsulated within the internal pores of MS rather than being surface bound. This finding aligns with the reduced permeation results from the Franz diffusion test, further validating that the mesoporous silica carrier effectively minimizes direct skin contact with the UV filters, thereby enhancing the safety and comfort of sunscreen formulations.
4. Conclusions
This study successfully demonstrated the simultaneous encapsulation of two organic UV filters—benzophenone-3 (BP-3) and octyl methoxycinnamate (OMC)—within mesoporous silica (MS). FTIR spectra of the S4M1B1 composite retained all characteristic absorption bands of BP-3 and OMC, with no emergence of new peaks or significant wavenumber shifts, confirming the absence of chemical bond formation. Likewise, the UV–vis spectra displayed maximum absorption peaks identical to those of the pure components, indicating that their optical properties remained intact. SPF measurements further supported that BP-3 and OMC preserved their independent molecular structures upon encapsulation. Collectively, these findings verify that co-encapsulation in mesoporous silica proceeds without chemical or structural alterations, thereby maintaining the intrinsic UV-absorbing properties of the active ingredients and ensuring their effectiveness in sunscreen formulations. Moreover, TGA analysis indicated an exceptionally high encapsulation efficiency of up to 72 wt%, significantly superior to other carrier systems. Sunscreen formulations containing the MS-encapsulated agents (S4M1B1-E) exhibited SPF and UVA-PF values nearly 40% higher compared to physical mixtures, and markedly enhanced the photostability of OMC.
Additionally, Franz diffusion cell assays demonstrated that MS encapsulation effectively reduced skin permeation of BP-3 and OMC to less than half that observed in physical mixtures, thereby significantly minimizing the risk of skin irritation and allergic reactions. DSC and SEM analyses further confirmed that encapsulation promotes uniform dispersion within the silica carrier, reducing recrystallization and enhancing sunscreen stability and efficacy. Collectively, these findings indicate that mesoporous silica encapsulation represents a promising and commercially viable strategy for producing safer, more effective, and broad-spectrum sunscreen formulations.