Abstract
In this study, PMMA/SiO2 composites were fabricated with monodispersed SiO2 and PMMA using four distinct methods—physical blending, in situ polymerization, random copolymerization, and block copolymerization—to investigate the composites’ thermal, mechanical, and optical properties. In the physical blending approach, SiO2 nanoparticles were dispersed in a PMMA solution, while during in situ polymerization, silica nanoparticles were incorporated during the synthesis of PMMA/SiO2 composites. 3-methacryloxypropyltrimethoxysilane (MPS) was modified on the SiO2 surface to introduce the reactive double bonds. The MPS@SiO2 was either random- or block-copolymerized with PMMA through RAFT polymerization. The PMMA/SiO2 composites prepared via these different methods were characterized using FTIR, TGA, and DSC to determine their chemical structures, thermal degradation temperatures, and glass transition temperatures, respectively. Scanning electron microscopy (SEM) was employed to observe the microstructures and dispersion of the composites. This comprehensive analysis revealed that the PMMA/SiO2 composites prepared via block copolymerization exhibited thermal stability at temperatures between 200 and 300 °C. Additionally, they demonstrated excellent transparency (86%) and scratch resistance (≥6H) while maintaining mechanical strength, suggesting their potential application in thermal insulation materials.
1. Introduction
Composite materials are formed by combining two or more materials through physical or chemical methods [1], typically involving a matrix material and one or more fillers [2]. Fillers play an important role in many fields, including thermal, electrical, tribological, and mechanical properties [2,3]. Among these properties, mechanical property enhancement received attention for polymeric composites since polymers are considered to lack mechanical tolerance. For example, carbon fiber fillers can effectively increase the strength and stiffness of composite materials [4] while also improving heat and corrosion resistance [5,6,7]. Glass fibers also offer high strength and stiffness, though they are more brittle and prone to fracture compared to carbon fibers [8,9]. Ceramics have extremely high hardness and wear resistance but are easily broken and not resistant to vibrations and thermal cycling [10,11]. The size and mixing method of the filler also influences the properties of composites [12,13,14,15]. Small particles possess a large surface energy, resulting in severe self-aggregation. Therefore, the dispersion of nanoparticles in the polymer matrix is difficult [16]. Excessive or uneven particle sizes can lead to anisotropy in the composite, impacting its mechanical properties. Thus, particles influence the strength of polymer composites in two distinct ways. One is the weakening effect caused by stress concentration, while the other is the strengthening effect, as these particles may act as barriers to crack propagation within the composites [17]. When the weakening effect predominates, the mechanical strength of the composite will be inferior to that of the matrix. Conversely, when the strengthening effect is more pronounced, the strength of composites is enhanced. Among the hard particles, silica particles have many advantages as filler candidates, including cost-effectiveness, good dispersibility, and mechanical strength [18,19,20,21]. In terms of thermal properties, silica also performs excellently as a reinforcing material, improving the thermal stability of composites and maintaining good stability in high-temperature environments. For example, Far et al. mixed the binary carbonate, Li2CO3-K2CO3, with SiO2 nanoparticles, and this was shown to increase the heat capacity by 19% [22].
The advantage of thermoplastics polymers lies in their malleability, enabling the creation of intricate shapes through processes such as thermoforming, injection molding, and 3D printing [23,24]. However, their heat and chemical resistance are generally inferior. The curing process of thermosetting materials is an irreversible chemical reaction, during which chemical bonds form between polymer molecules to create a 3D network structure [25]. This network structure gives thermosetting polymers excellent heat, chemical, and wear resistance. Thermoplastic materials, on the other hand, have reversible thermal deformation characteristics, allowing them to be reshaped multiple times through heating and cooling to produce complex shapes. However, their heat and chemical resistance are generally poor, necessitating the addition of fillers to enhance their properties [26]. Among the many thermoplastic materials, polymethyl methacrylate (PMMA) has high transparency and good optical transmittance, meaning it is commonly used to manufacture transparent products such as optical lenses and display panels. PMMA is also frequently used in the medical field, such as in dental materials and bone cements [27,28,29].
Therefore, the present study attempts to primarily use SiO2 nanoparticles and PMMA as the main materials to assess the preparation and properties of organic/inorganic composites. The Stöber method was first used to synthesize SiO2. 3-methacryloxypropyltrimethoxysilane (MPS) was then used to modify the surface with reactive unsaturated double bonds (MPS@SiO2), allowing it to copolymerize with methyl methacrylate monomers [30]. Four different methods—the physical blending of SiO2 and PMMA, the in situ polymerization of SiO2 with MMA, and the random and block copolymerization of MPS@SiO2 with MMA—were utilized to prepare blended PMMA/SiO2, in situ PMMA/SiO2, PMMA-co-SiO2, and PMMA-b-SiO2. FTIR, TGA, DSC, mechanical studies, scratch resistance, and transparency methods were adapted for analyzing the properties of PMMA/SiO2 composites. To the best of our knowledge, this is the first study that compares four different methods for preparing PMMA/SiO2 composites. It is believed that this study can benefit the synthesis of not only PMMA/SiO2 but also various other composites.
2. Experimental
2.1. Materials
Tetraethyl orthosilicate (TEOS) was purchased from Thermo Scientific. 3-methacryloxypropyl trimethoxysilane (MPS), methyl methacrylate (MMA), 2,2-Azobis(2-methylpropionitrile) (AIBN), and ammonium peroxydisulfate (APS) were purchased from Alfa, Linkou, Taiwan. The 35% Ammonia solution was purchased from Fisher, while the CTA, 4-cyano-4-(phenylcarbonothioylthio)pentanoic acid (CPDB) was purchased from Sigma Aldrich, Linkou, Taiwan. All chemical reagents are used directly as received without further purification.
2.2. Synthesis of the SiO2 and MPS@SiO2 Nanoparticles
In this experiment, 63.32 mL ethanol, 1.82 mL ammonia, 0.4 g deionized water, and 8.5 mL TEOS were stirred together and reacted at 40 °C for 50 min. The solution was then dried using a rotary evaporator to obtain solid particles as monodispersed SiO2. MPS@SiO2 was synthesized similarly to SiO2 with MPS added during the reaction.
2.3. Synthesis of Pristine PMMA, PMMA/SiO2 Composites with Different Methods
PMMA: In this step, 60 mL deionized water, 40 mL acetone, 8.6 mL MMA monomer, and 0.2 g APS initiator were added to a three-neck flask. The reaction was carried out at 75 °C for 24 h. The sample was purified using a rotary evaporator for later use.
The PMMA/SiO2 composite was synthesized using four different methods. The feeding amount of SiO2 to PMMA was set as 5 wt. % for the four methods.
Blending: The purified PMMA was dissolved in acetone. Then, 17.2 mL SiO2 solution was added and mixed uniformly, and it was dried overnight at 50 °C to obtain blended PMMA/SiO2 powder.
In situ polymerization: In this step, 60 mL deionized water, 40 mL acetone, 8.6 mL MMA monomer, and 17.2 mL SiO2 solution were added to a three-neck flask, and 0.2 g APS initiator was added. The reaction was carried out at 75 °C for 24 h. The sample was purified using a rotary evaporator to obtain in situ PMMA/SiO2 powder.
RAFT copolymerization: In this step, 100 mL ethanol, 0.48 g CPDB, 0.06 g AIBN, 17.2 mL MMA, and 32.4 mL MPS@SiO2 solution were added to a three-neck flask and mixed uniformly. The reaction was carried out at 70 °C under nitrogen for 40 h. The sample was purified using a rotary evaporator to obtain PMMA-co-SiO2.
RAFT block copolymerization: In this step, 100 mL ethanol, 0.48 g CPDB, 0.06 g AIBN and 17.2 mL MMA were added together and reacted at 70 °C under nitrogen for 24 h. Then, 32.4 mL MPS@SiO2 solution was added, and the reaction continued for 16 h under nitrogen. The sample was purified using a rotary evaporator to obtain PMMA-b-SiO2.
2.4. Structural Characterization of the PMMA/SiO2 Composites
A thermogravimetric analysis (TGA, Pyris 1 TGA) was used to determine the thermal decomposition of PMMA/SiO2 composites. The samples were heated from 50 °C to 700 °C with a heating rate of 20 °C/min, and the N2 flow rate was maintained at 20 mL/min. The glass transition temperatures (Tgs) of as-synthesized PMMA/SiO2 composites were determined using a differential scanning calorimeter (DSC, NETZSCH DSC 200F3) at a heating rate of 20 °C/min. The Tgs were then determined using the second heating curves. Fourier transform infrared spectroscopy (FTIR, HORIBA v FT-IR Spectrometer) absorption spectra were measured at frequencies ranging from 4000 to 400 cm−1 with a 4 cm−1 resolution. The samples were mixed with KBr and prepared as pellets.
2.5. Mechanical and Morphology of PMMA/SiO2 Composites
To analyze the mechanical properties, PMMA/SiO2 composites were first dispersed in acetone and then poured into a Teflon mold (ISO-37 dumbbell-shaped type 3). A universal material testing machine (YM-H3501-A02) was used to determine the Young’s modulus, yield strength, fracture strength, and maximum elongation of PMMA/SiO2 composites. The surface and structural morphologies of PMMA/SiO2 composites were studied using SEM (JEOL JSM-7610F) images. The scratch resistance (pencil hardness) of PMMA/SiO2 composite films was analyzed using a commercial pencil hardness tester (scratch hardness tester model 291, ERICHSEN test equipment). This test conforms to ASTM standard D3363, and involves different hardness grades (9B–9H) to evaluate the scratch resistance of PMMA/SiO2 composite films. The transparency of PMMA/SiO2 composite films was determined via a UV/Vis transmittance analysis in the range of 400–750 nm.
3. Results and Discussion
3.1. Characterization of SiO2 and MPS@SiO2 Nanoparticles
The functional groups and particle sizes on the SiO2 surface before and after MPS modification were determined via FTIR and DLS. The absorption peaks at 466 cm−1 and 802 cm−1 correspond to the bending and symmetric stretching vibrations of the Si–O–Si bonds, as shown in Figure 1A. The peak at 1095 cm−1 represents the asymmetric stretching vibration of the Si–O–Si bond. A broad characteristic peak at 3463 cm−1 is attributed to the stretching vibration of hydroxyl groups on the SiO2 surface, which is responsible for reacting with the vinyl groups of the silane coupling agent MPS. After surface modification, new peaks appear at 1635, 1299, and 937 cm−1 for MPS@SiO2, representing the C=C stretching vibrations, C–O stretching vibrations, and Si–O–C stretching vibrations of MPS, respectively. Furthermore, the original hydroxyl peak at 3463 cm−1 was reduced, confirming the successful grafting of MPS onto the SiO2 surface, replacing the surface hydroxyl groups. Figure 1B,C depict the particle size distribution of SiO2 and MPS@SiO2. The results show that the average particle size of SiO2 is 60.75 nm, with a polydispersity index (PDI) of 0.095, indicating a uniform distribution. After modifying the SiO2 surface with MPS, the average particle size increased from 65 nm to 85 nm, and the polydispersity index slightly increased to 0.33.
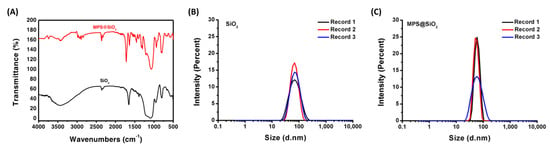
Figure 1.
(A) FTIR analysis of SiO2 and MPS@SiO2; DLS analysis of (B) SiO2 and (C) MPS@SiO2.
3.2. Characterization of the PMMA/SiO2 Composites
The results of FTIR analyses of PMMA, SiO2, and PMMA/SiO2 composites are shown in Figure 2A, which allows us to determine the interaction between the PMMA and SiO2 nanoparticles. The peak at 1731 cm−1 corresponds to the stretching of the carbonyl groups in PMMA. The peaks at 1454 cm−1 and 1392 cm−1 are attributed to the -CH stretching of PMMA, with 1454 cm−1 corresponding to the methyl group (-CH3) and 1392 cm−1 to the methylene group (-CH2-). After introducing SiO2 nanoparticles, all PMMA/SiO2 composites exhibit a characteristic peak at 1110 cm−1, belonging to the Si–O–Si bond, indicating the successful incorporation of SiO2. Additionally, the characteristic peak at 1643 cm−1 of PMMA-co-SiO2 and PMMA-b-SiO2 confirms the introduction of MPS@SiO2. On the other hand, blend PMMA/SiO2 leads to a stronger intensity in the characteristic spectral peaks than in situ PMMA/SiO2, PMMA-co-SiO2, and PMMA-b-SiO2. This indicates a sufficient SiO2 content in blend PMMA/SiO2 than in other PMMA/SiO2 composites. The glass transition temperatures (Tgs) of PMMA, blend PMMA/SiO2, in situ PMMA/SiO2, PMMA-co-SiO2, and PMMA-b-SiO2 are 101 °C, 102 °C, 109 °C, 131 °C, and 126 °C, as shown in the DSC analysis (Figure 2B). The physical introduction of SiO2 does not significantly affect the Tgs of PMMA/SiO2 composites due to the absence of chemical bonding. However, SiO2 is integrated into the polymer backbone through polymerization, effectively enhancing the Tgs of PMMA/SiO2 composites. Figure 2C shows a comparison of the thermal weight losses of PMMA and PMMA/SiO2 composites at different temperatures. It is obvious that there are three distinct thermal decomposition stages for PMMA (Figure 2C). The first stage is observed between 165 °C and 250 °C with 6.38 wt. % weight loss attributed to the depolymerization initiated from head-to-head linkage within the polymer chain [31,32]. The second stage occurs between 250 °C and 300 °C with 26.41 wt. % weight loss resulting from the thermal decomposition of the unsaturated chain ends. Above 300 °C, breaking and decomposition among the PMMA backbone occurs, leading to the most significant weight loss. The thermal decomposition of blende PMMA/SiO2 is relatively lower in the range of 200 °C to 300 °C (Figure 2D), indicating that the unsaturated chain ends do not affect the weight loss resulting from the SiO2 protection layer, thereby enhancing the thermal stability [33]. The in situ PMMA/SiO2 followed the same trend as PMMA, but the two thermal decomposition peaks were delayed from 250 °C and 300 °C to 280 °C and 330 °C, respectively (based on the differential spectrum, unshown). This delay can be attributed to the spatial barrier formed by PMMA-encapsulated SiO2, which acts against the heat transfer path and slows heat transfer [34,35]. A 3 wt. % mass residue can be observed at temperatures above 500 °C, confirming the presence of SiO2 in the blend and in situ PMMA/SiO2 composites. However, from the thermal decomposition (Figure 2C) and differential plots of PMMA-co-SiO2 and PMMA-b-SiO2, it can be found that the weight loss that originally occurred before the main chain decomposition was not present (165–250 °C). At the same time, the weight loss caused by the thermal decomposition of the unsaturated chain ends was significantly reduced from 28.6 wt. % to below 5 wt. %. This reduction is due to the controlled nature of RAFT polymerization, in which the stable intermediate prevents the termination and avoids the formation of unsaturated chain ends. Thus, the maximum weight loss in PMMA-co-SiO2 and PMMA-b-SiO2 occurred above 335 °C. Meanwhile, the remaining weights of PMMA-co-SiO2 and PMMA-b-SiO2 were 7.69% and 12.61% higher than the feeding ratio (5 wt. %) due to the introduction of SiO2 by RAFT polymerization. In short, we can conclude that the thermal stability improves between 200 °C and 300 °C regardless of the method of SiO2 introduction by comparing the relationship between mass loss and temperature, as shown in Figure 2D. The thermal degradation of the PMMA main chain results in a significant weight loss for PMMA, blend PMMA/SiO2, and in situ PMMA/SiO2 beyond 300 °C. However, RAFT polymerization effectively maintains the thermal stability of PMMA/SiO2 composites until 350 °C owing to the better dispersion and chemical bonding, which shows the potential in thermal insulation applications.
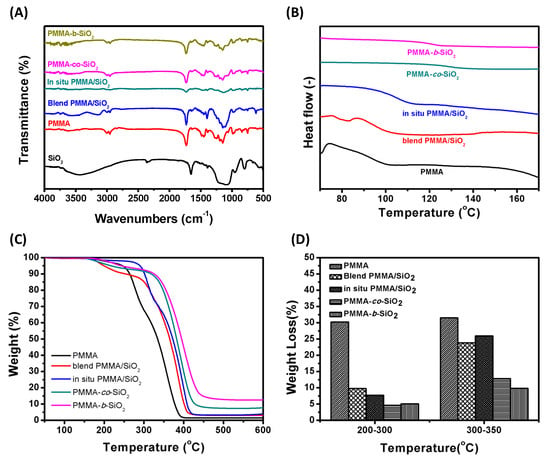
Figure 2.
Characterization of PMMA and PMMA/SiO2 composites: (A) FTIR spectrum, (B) DSC analysis, (C) TGA analysis, and (D) weight loss within specific temperature regions of PMMA, and PMMA/SiO2 composites.
3.3. Mechanical Properties of the PMMA/SiO2 Composites
Effective stress transfer is the most crucial factor in strengthening two-phase composites. Different blend methods lead to different dispersions of silica in the PMMA/SiO2 composites, therefore influencing the results of universal testing. For poorly bonded particles, stress transfer at the particle/polymer interface is inefficient. However, in composites containing well-bonded particles, the incorporation of particles into the polymer results in increased strength. Figure 3 illustrates the stress–strain relationship of PMMA/SiO2 composites, in which the blend PMMA/SiO2 and in situ PMMA/SiO2 are shown to enhance the Young’s modulus and yield strength of PMMA/SiO2 composites, while the PMMA-co-SiO2 and PMMA-b-SiO2 exhibit less pronounced improvements in their strength, as shown in Table 1. It is expected that the introduction of SiO2 nanoparticles can improve the mechanical properties of PMMA/SiO2 composites because of the bridging effect. The preparation of PMMA/SiO2 composites via RAFT copolymerization was expected to effectively bond SiO2 to PMMA and enhance mechanical strength. However, the opposite was observed, as PMMA/SiO2 composites prepared by physical methods, i.e., blend and in situ PMMA/SiO2, exhibited higher strength. It is speculated that the RAFT copolymerization process may have introduced the agglomeration of SiO2 nanoparticles or disrupted the orderly long-chain structure of PMMA, weakening the overall mechanical properties [36]. It is noted that PMMA-b-SiO2 shows more strain than other PMMA/SiO2 composites. Since PMMA is inherently a brittle acrylic-based polymer, the combination of a tolerance of higher strain and lower stiffness may also result from the decreased molecular weight of the PMMA matrix.
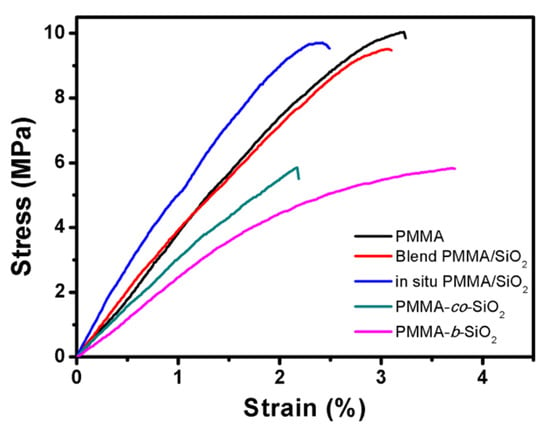
Figure 3.
Tensile stress–strain curves of the PMMA/SiO2 composites prepared by different methods.

Table 1.
The mechanical properties of the PMMA/SiO2 composites.
3.4. Morphology of the PMMA/SiO2 Composites
To investigate the relationship between the mechanical properties and morphology of PMMA/SiO2 composites prepared by different methods, thin films of PMMA/SiO2 were fabricated using the solution casting method, and we analyzed the surface and cross-section morphology using SEM images. From the SEM images of PMMA (Figure 4A,F), it can be observed that the PMMA surface was very smooth, while the surface and cross-section structure became uneven with particle aggregation after introducing SiO2 via physical blending or in situ polymerization (Figure 4B,C,G,H). The surface structures of PMMA-co-SiO2 and PMMA-b-SiO2 showed slight aggregation but remained relatively smooth (Figure 4D,E). Meanwhile, the cross-section images of PMMA-co-SiO2 and PMMA-b-SiO2 exhibit a uniform layered structure without aggregation, which suggests that RAFT polymerization provides better thermal stability and more effective dispersion to the PMMA/SiO2 composites compared to physical blending and in situ polymerization.
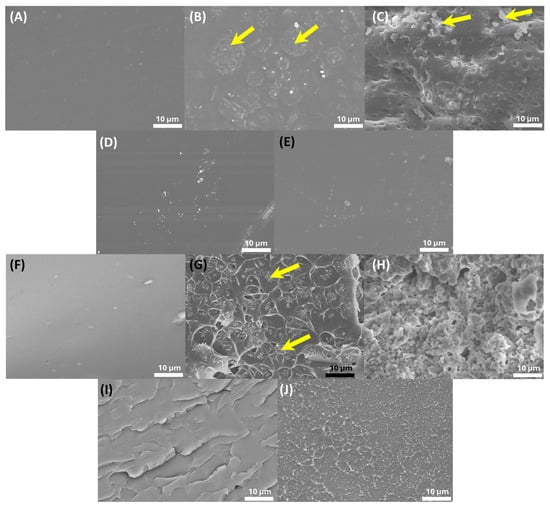
Figure 4.
SEM images of the PMMA/SiO2 composites. Surface-area view: (A) PMMA, (B) blend PMMA/SiO2, (C) in situ PMMA/SiO2, (D) PMMA-co-SiO2, and (E) PMMA-b-SiO2. Cross-section area view: (F) PMMA, (G) blend PMMA/SiO2, (H) in situ PMMA/SiO2, (I) PMMA-co-SiO2, and (J) PMMA-b-SiO2.
The scratch resistance of PMMA/SiO2 composites was measured using a pencil hardness tester, and the results are shown in Table 2. It has been found that pristine PMMA has a scratch resistance of 2H under a 500 g load. Both in situ PMMA/SiO2 and PMMA-co-SiO2 showed only minor improvements in scratch resistance, increasing by just one grade to 3H. However, the scratch resistance of blend PMMA/SiO2 was significantly enhanced, reaching a grade of 5H, clearly due to the influence of SiO2. PMMA-b-SiO2 demonstrated moderate scratch resistance at 4H, which may be attributed to the RAFT-polymerized composite maintaining structural uniformity. This uniformity, while improving thermal stability, also helps preserve scratch resistance.

Table 2.
The scratch resistance of PMMA/SiO2 composites.
PMMA is a polymer with high hardness and transparency; the purpose of our research is to improve its thermal stability but maintain these intrinsic properties after introducing SiO2 nanoparticles. Figure 5 shows the transmittance of different PMMA/SiO2 composite films prepared via the solution casting method at visible light wavelengths. The maximum transmittance of PMMA is 85.6%, while blend PMMA/SiO2, in situ PMMA/SiO2, PMMA-co-SiO2, and PMMA-b-SiO2 have maximum transmittances of 71.4%, 66.6%, 65.8%, and 86.5%, respectively. It is obvious that after introducing SiO2 nanoparticles, the transmittance of the blend PMMA/SiO2, in situ PMMA/SiO2, and PMMA-co-SiO2 composites significantly decreased to lower than 70%. Notably, PMMA-b-SiO2 had a minimal impact on the transmittance of PMMA because of the block copolymer structure. The block conformation tends to create the PMMA-rich domain and silica domain. The PMMA-rich domain is dominant, which is similar to pure PMMA, so the transmittance remains.
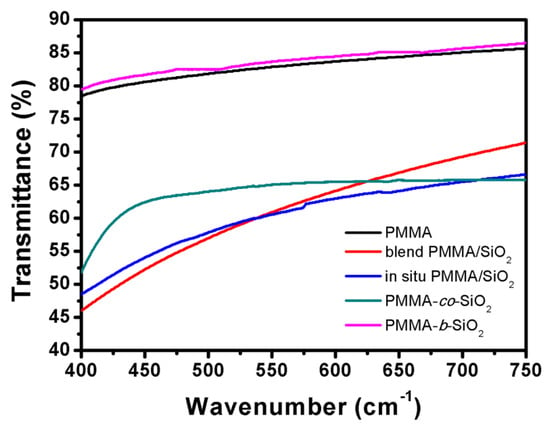
Figure 5.
The visible-light transparency of PMMA/SiO2 composites.
4. Conclusions
We prepared organic/inorganic composite materials (PMMA/SiO2 composites) by incorporating SiO2 nanoparticles as fillers into PMMA through various blending methods and conducted a series of investigations into their thermal, mechanical, and optical properties. A TGA analysis showed that each blending method effectively improved the thermal properties of PMMA. Physical blending (blend PMMA/SiO2) retained the polymer molecular weight, while in situ PMMA/SiO2 delayed the thermal decomposition temperature by 30 °C due to the steric hindrance provided by SiO2 nanoparticles. Random (PMMA-co-SiO2) and block copolymerization (PMMA-b-SiO2) reduced the unsaturated chain segments of PMMA, resulting in better thermal stability than physical blending. A DSC analysis confirmed the glass transition temperatures (Tgs) of different samples, verifying that incorporating SiO2 through copolymerization effectively delayed the Tgs. The results from the universal testing indicate that all blending methods effectively improved the mechanical properties of PMMA/SiO2 composites. The results of UV/Vis scanning and scratching tests reveal that PMMA-b-SiO2 provides moderate scratch resistance (4H) and transmittance (85.6%) compared to PMMA. In conclusion, the block copolymerization method for preparing PMMA-b-SiO2 preserved the high hardness and transmittance of the original PMMA while maintaining its mechanical strength and improving its thermal stability with only a slight sacrifice in maximum tensile strength. We believe this simple and controllable method can provide a new perspective regarding various polymers and fillers, making it suitable for applications in thermal insulation materials.
Author Contributions
C.-K.L.: Investigation, Methodology, Data curation; J.-W.X.: Investigation, Methodology, Writing—original draft; P.-J.T.: Writing—original draft, Funding acquisition; H.-Y.W.: Data curation, Writing—original draft; Z.-W.L.: Writing—original draft; T.-Y.L.: Investigation, Conceptualization; C.-Y.K.: Conceptualization, Funding acquisition, Supervision, Project administration, Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Science and Technology Council, Taiwan grant number NSTC 111-222-E-027-022-MY3; National Taipei University of Technology and Chang Gung Memorial Hospital Joint Research Program NTUT-CGMH-111-07; Chang Gung Memorial Hospital CORPG3M0171.
Data Availability Statement
Dataset available on request from the authors.
Acknowledgments
The authors thank Ren-Jie Chung for providing research suggestions and the analysis instruments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Boz, Z. Packaging. In Encyclopedia of Food Safety, 2nd ed.; Smithers, G.W., Ed.; Academic Press: Oxford, UK, 2024; pp. 631–647. [Google Scholar] [CrossRef]
- Yadav, R.; Singh, M.; Shekhawat, D.; Lee, S.-Y.; Park, S.-J. The role of fillers to enhance the mechanical, thermal, and wear characteristics of polymer composite materials: A review. Compos. Part A Appl. Sci. Manuf. 2023, 175, 107775. [Google Scholar] [CrossRef]
- Bommegowda, K.B.; Renukappa, N.M.; Rajan, J.S. Role of Fillers in Controlling the Properties of Polymer Composites: A Review; Springer International Publishing: Cham, Switzerland, 2021; pp. 637–648. [Google Scholar]
- Van de Werken, N.; Tekinalp, H.; Khanbolouki, P.; Ozcan, S.; Williams, A.; Tehrani, M. Additively manufactured carbon fiber-reinforced composites: State of the art and perspective. Addit. Manuf. 2020, 31, 100962. [Google Scholar] [CrossRef]
- Zheng, X.; Ryeon Kim, B.; Joo Hong, S.; Lee, J.-G.; Woo Park, C. Heat transfer analysis of carbon fiber-reinforced corrugated polymer plate heat exchangers. Appl. Therm. Eng. 2024, 244, 122684. [Google Scholar] [CrossRef]
- Liu, J.; Huang, X.; Ren, Y.; Wong, L.M.; Liu, H.; Wang, S. Galvanic corrosion protection of Al-alloy in contact with carbon fibre reinforced polymer through plasma electrolytic oxidation treatment. Sci. Rep. 2022, 12, 4532. [Google Scholar] [CrossRef]
- Luo, G.-M.; Liang, C.-H. Strength Verification of a Carbon-fibre-reinforced Plastic Patch Used to Repair a Cracked Aluminium Alloy Plate. Appl. Compos. Mater. 2024, 31, 265–289. [Google Scholar] [CrossRef]
- Pandiaraj, V.; Ramkumar, P.; Tharik, J.M.; Vikram, R.; Logasubramani, S.; Vivek, C.M. Chemical resistance and mechanical characteristic evaluation of glass fiber reinforced plastic (GFRP) and hybrid natural composites. Interactions 2024, 245, 173. [Google Scholar] [CrossRef]
- Beck, R.; Prewitz, M. Experimental investigation of tensile properties of glass capillary hybridized carbon fiber reinforced plastic (GCRP) for structurally integrated hydrogen storage. Int. J. Hydrogen Energy 2024, 62, 321–330. [Google Scholar] [CrossRef]
- Panpho, P.; Charoonsuk, T.; Vittayakorn, N.; Bongkarn, T.; Sumang, R. Flexible hybrid piezo/triboelectric energy harvester based on a lead-free BNT-BT-KNN ceramic-polymer composite film. Ceram. Int. 2024. [Google Scholar] [CrossRef]
- Monia, T.; Ridha, B.C. Polymer-ceramic composites for bone challenging applications: Materials and manufacturing processes. J. Thermoplast. Compos. Mater. 2024, 37, 1540–1557. [Google Scholar] [CrossRef]
- Pavlyuchkova, E.A.; Malkin, A.Y.; Kornev, Y.V.; Simonov-Emel’yanov, I.D. Distribution of Filler in Polymer Composites. Role of Particle Size and Concentration. Polym. Sci. Ser. A 2024, 66, 113–120. [Google Scholar] [CrossRef]
- Akhyar; Gani, A.; Ibrahim, M.; Ulmi, F.; Farhan, A. The influence of different fiber sizes on the flexural strength of natural fiber-reinforced polymer composites. Results Mater. 2024, 21, 100534. [Google Scholar] [CrossRef]
- Albdiry, M. Effect of melt blending processing on mechanical properties of polymer nanocomposites: A review. Polym. Bull. 2024, 81, 5793–5821. [Google Scholar] [CrossRef]
- Ali, Z.; Yaqoob, S.; Yu, J.; D’Amore, A. Critical review on the characterization, preparation, and enhanced mechanical, thermal, and electrical properties of carbon nanotubes and their hybrid filler polymer composites for various applications. Compos. Part C Open Access 2024, 13, 100434. [Google Scholar] [CrossRef]
- Liu, T.; Zhong, J. Effect of Dispersion of Nano-inorganic Particles on the Properties of Polymer Nanocomposites. IOP Conf. Ser. Mater. Sci. Eng. 2019, 563, 022026. [Google Scholar] [CrossRef]
- Fu, S.-Y.; Feng, X.-Q.; Lauke, B.; Mai, Y.-W. Effects of particle size, particle/matrix interface adhesion and particle loading on mechanical properties of particulate–polymer composites. Compos. Part B Eng. 2008, 39, 933–961. [Google Scholar] [CrossRef]
- Bahramnia, H.; Semnani, H.M.; Habibolahzadeh, A.; Abdoos, H. Epoxy/polyurethane hybrid nanocomposite coatings reinforced with MWCNTs and SiO2 nanoparticles: Processing, mechanical properties and wear behavior. Surf. Coat. Technol. 2021, 415, 127121. [Google Scholar] [CrossRef]
- Tao, J.; Dong, L.; Wu, Y.; Liu, X.; Xie, J.; Wu, H.; Ran, Q. Fabrication of room temperature self-healing, robust superhydrophobic coatings via spraying dual cross-linking supramolecular silicone polymer/SiO2 composite. Compos. Part B Eng. 2024, 273, 111245. [Google Scholar] [CrossRef]
- Made Joni, I.; Vanitha, M.; Panatarani, C.; Faizal, F. Dispersion of amorphous silica nanoparticles via beads milling process and their particle size analysis, hydrophobicity and anti-bacterial activity. Adv. Powder Technol. 2020, 31, 370–380. [Google Scholar] [CrossRef]
- Zhao, Z.; Liang, N.; Shimizu, T.; Shingubara, S.; Ito, T. Antifouling performance against SiO2 particulate matter adhesion of Cyclo Olefin Polymer nanopillar surfaces. Environ. Sci. Nano 2024. [Google Scholar] [CrossRef]
- El Far, B.; Rizvi, S.M.M.; Nayfeh, Y.; Shin, D. Investigation of heat capacity and viscosity enhancements of binary carbonate salt mixture with SiO2 nanoparticles. Int. J. Heat Mass Transf. 2020, 156, 119789. [Google Scholar] [CrossRef]
- Jeon, J.G.; So, B.J.; Choi, Y.; Han, Y.; Kim, T.; Shin, G.; Lee, J.H.; Kim, H.J.; Kim, J.H.; Farhangdoust, S.; et al. Thermo-mechanical properties of shape-recoverable structural composites via vacuum-assisted resin transfer molding process and in-situ polymerization of poly (tert-butyl acrylate-co-acrylic acid) copolymer. Compos. Part A Appl. Sci. Manuf. 2024, 185, 108360. [Google Scholar] [CrossRef]
- Formon, G.J.M.; Jayaratnam, J.; Guibert, C.; Van Zee, N.J.; Nicolaÿ, R. Cross-Linking Vitrimers after Melt Processing Using Supramolecularly Masked Dynamic Cross-Linkers. Macromolecules 2024, 57, 8277–8286. [Google Scholar] [CrossRef]
- Feng, T.; Xu, P.; Wang, Y.; Gao, Y.; Wang, H.; Dong, J.; Peng, H.-X.; Qin, F. Magnetic fiber enabled curing electrogram: Real-time process monitoring for thermosetting polymer materials. Compos. Sci. Technol. 2022, 227, 109598. [Google Scholar] [CrossRef]
- Alghamdi, M.N. Thermoplastic composite system using polymer blend and fillers. J. King Saud Univ. Eng. Sci. 2022, 34, 361–365. [Google Scholar] [CrossRef]
- Tavakoli, M.; Bakhtiari, S.S.E.; Karbasi, S. Incorporation of chitosan/graphene oxide nanocomposite in to the PMMA bone cement: Physical, mechanical and biological evaluation. Int. J. Biol. Macromol. 2020, 149, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Merajikhah, A.; Soleimani, M. Bone Cement and Occupational Hazards for Healthcare Providers and Patients: A Narrative Review. Curr. Surg. Rep. 2024, 12, 252–259. [Google Scholar] [CrossRef]
- Wan, Z.; Gao, Y.; Wang, Y.; Zhang, X.; Gao, X.; Zhou, T.; Zhang, Z.; Li, Z.; Lin, Y.; Wang, B.; et al. High-purity butoxydibutylborane catalysts enable the low-exothermic polymerization of PMMA bone cement with enhanced biocompatibility and osseointegration. J. Mater. Chem. B 2024, 2, 8911–8918. [Google Scholar] [CrossRef]
- Wang, X.-D.; Shen, Z.-X.; Sang, T.; Cheng, X.-B.; Li, M.-F.; Chen, L.-Y.; Wang, Z.-S. Preparation of spherical silica particles by Stöber process with high concentration of tetra-ethyl-orthosilicate. J. Colloid Interface Sci. 2010, 341, 23–29. [Google Scholar] [CrossRef]
- Young, J.B.; Hughes, R.W.; Tamura, A.M.; Bailey, L.S.; Stewart, K.A.; Sumerlin, B.S. Bulk depolymerization of poly(methyl methacrylate) via chain-end initiation for catalyst-free reversion to monomer. Chem 2023, 9, 2669–2682. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Inaba, A.; Brown, J.E.; Hatada, K.; Kitayama, T.; Masuda, E. Effects of weak linkages on the thermal and oxidative degradation of poly(methyl methacrylates). Macromolecules 1986, 19, 2160–2168. [Google Scholar] [CrossRef]
- Zhan, Y.; Nan, B.; Liu, Y.; Jiao, E.; Shi, J.; Lu, M.; Wu, K. Multifunctional cellulose-based fireproof thermal conductive nanocomposite films assembled by in-situ grown SiO2 nanoparticle onto MXene. Chem. Eng. J. 2021, 421, 129733. [Google Scholar] [CrossRef]
- Bee, S.-L.; Abdullah, M.A.A.; Mamat, M.; Bee, S.-T.; Sin, L.T.; Hui, D.; Rahmat, A.R. Characterization of silylated modified clay nanoparticles and its functionality in PMMA. Compos. Part B Eng. 2017, 110, 83–95. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, X.; Li, D.; Zuo, X.; Yang, H. Multifunctional composite phase change materials: Preparation, enhanced properties and applications. Compos. Part A Appl. Sci. Manuf. 2024, 185, 108331. [Google Scholar] [CrossRef]
- Yuan, M.; Huang, D.; Zhao, Y. Development of Synthesis and Application of High Molecular Weight Poly(Methyl Methacrylate). Polymers 2022, 14, 2632. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).