Chemical and Thermal Analysis of Fly Ash-Reinforced Aluminum Matrix Composites (AMCs)
Abstract
1. Introduction
2. Methodology
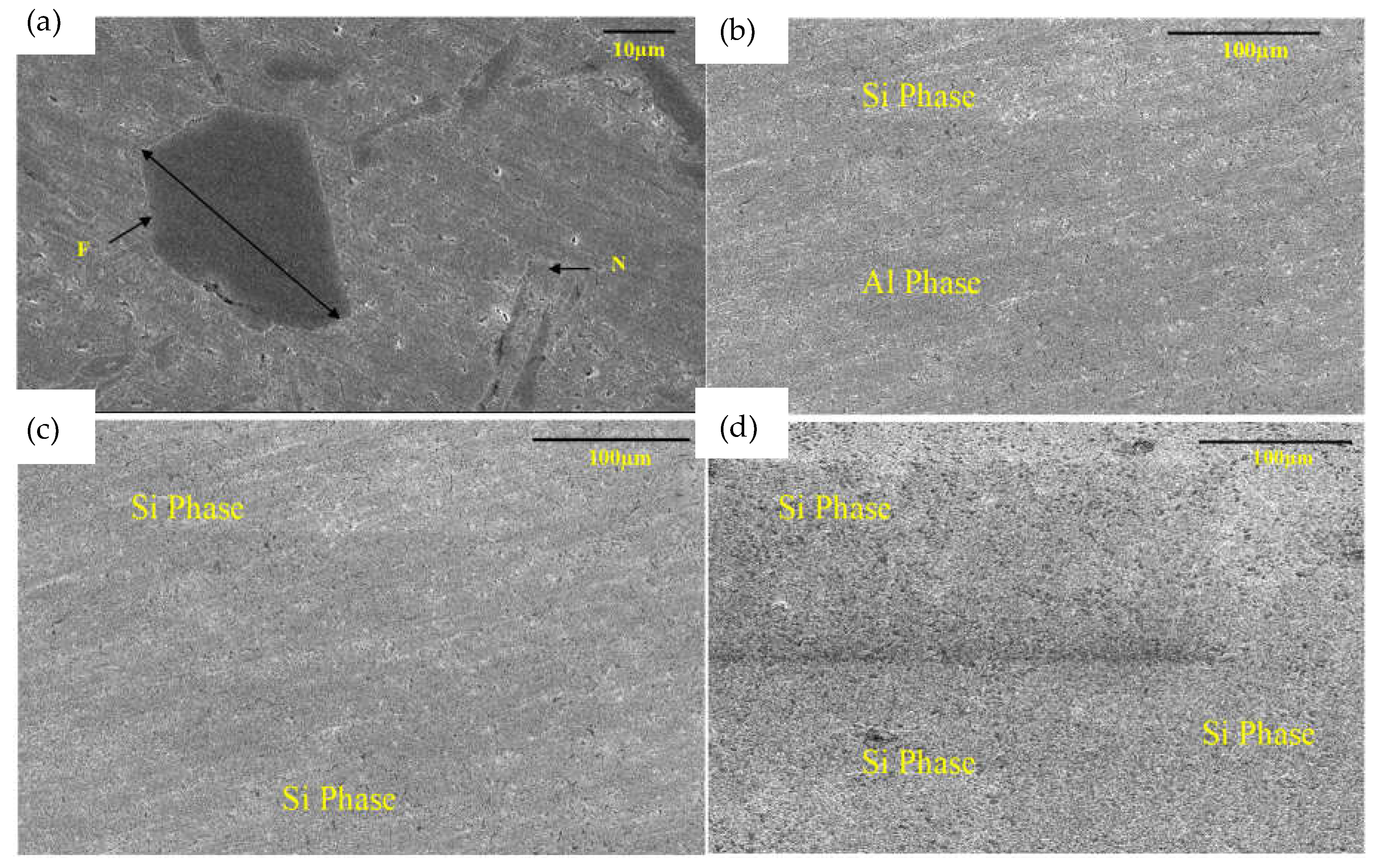
3. Results and Discussion
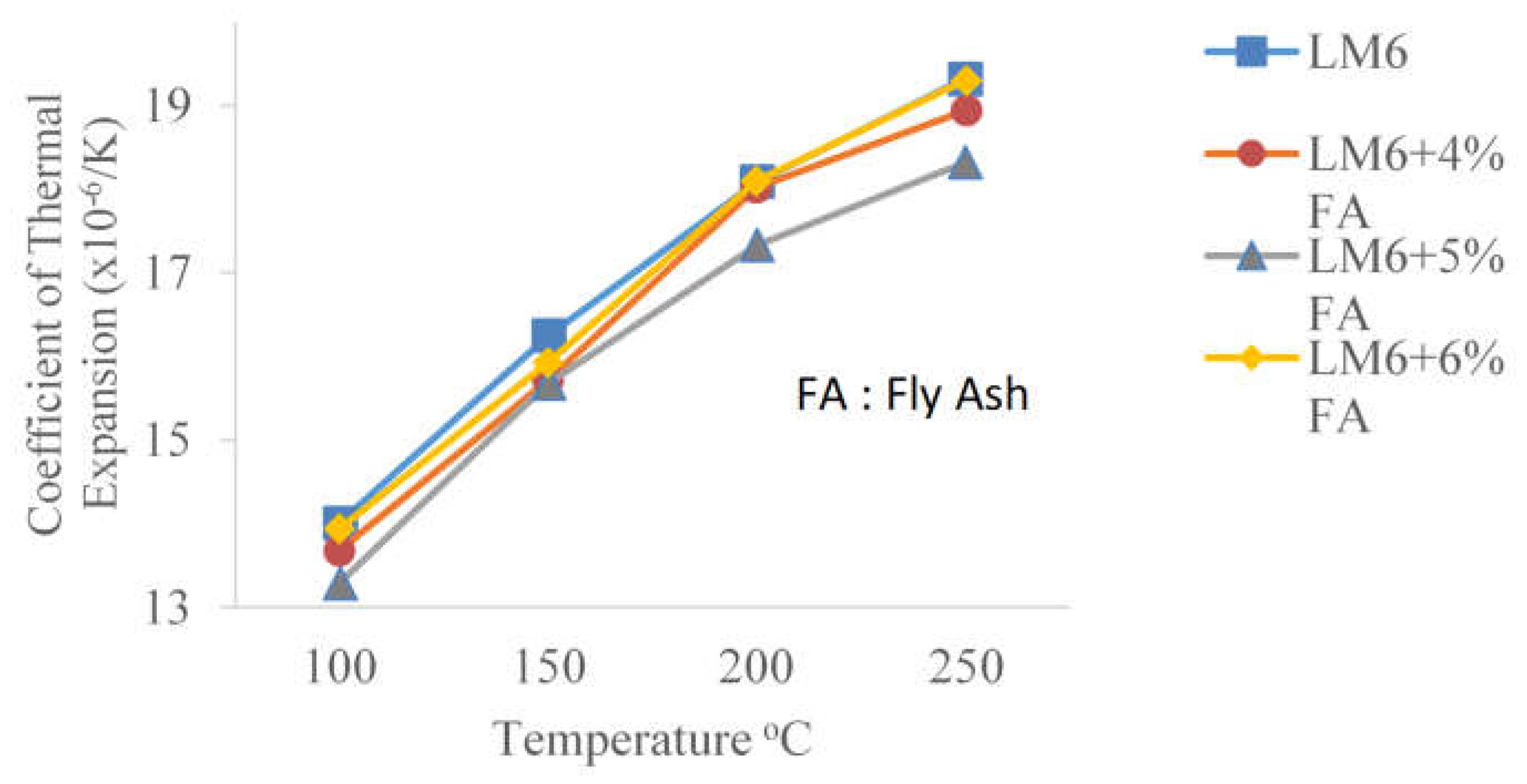
3.1. Thermal Analysis of Pure LM6 and Fabricated Aluminum Matrix Composites (AMCs) Using the Coefficient of Thermal Expansion
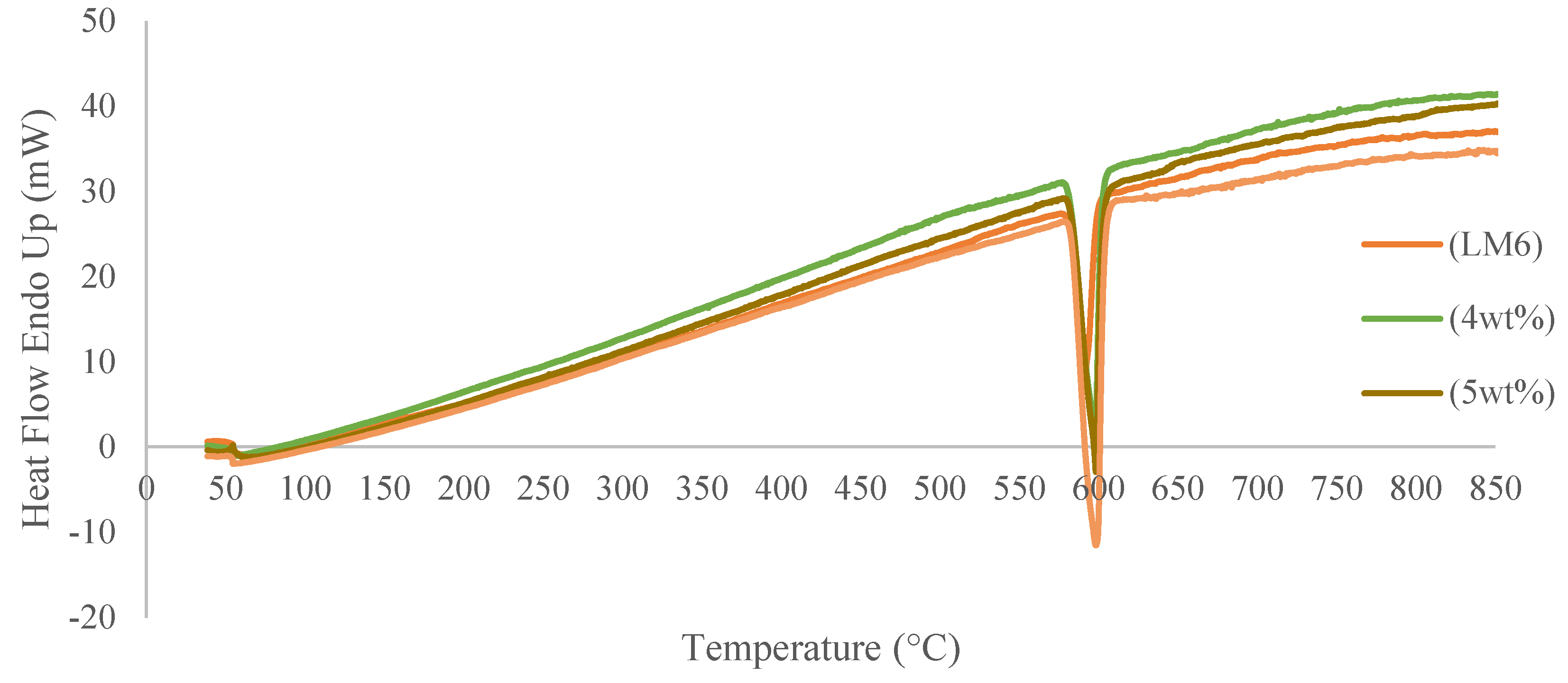
3.2. Thermal Analysis of Pure LM6 and Fabricated Aluminum Matrix Composites (AMCs) Using Differential Thermal Analysis (DTA)
4. Conclusions
- (1)
- The CTE values of Al-Si (LM6) fly ash composites decreased with the addition of fly ash particles. The lowest CTE value achieved by Al-Si (LM6) fly ash composites with 5 wt% of fly ash (18.32 × 10−6/K) is 5.18% lower than the CTE value of pure LM6.
- (2)
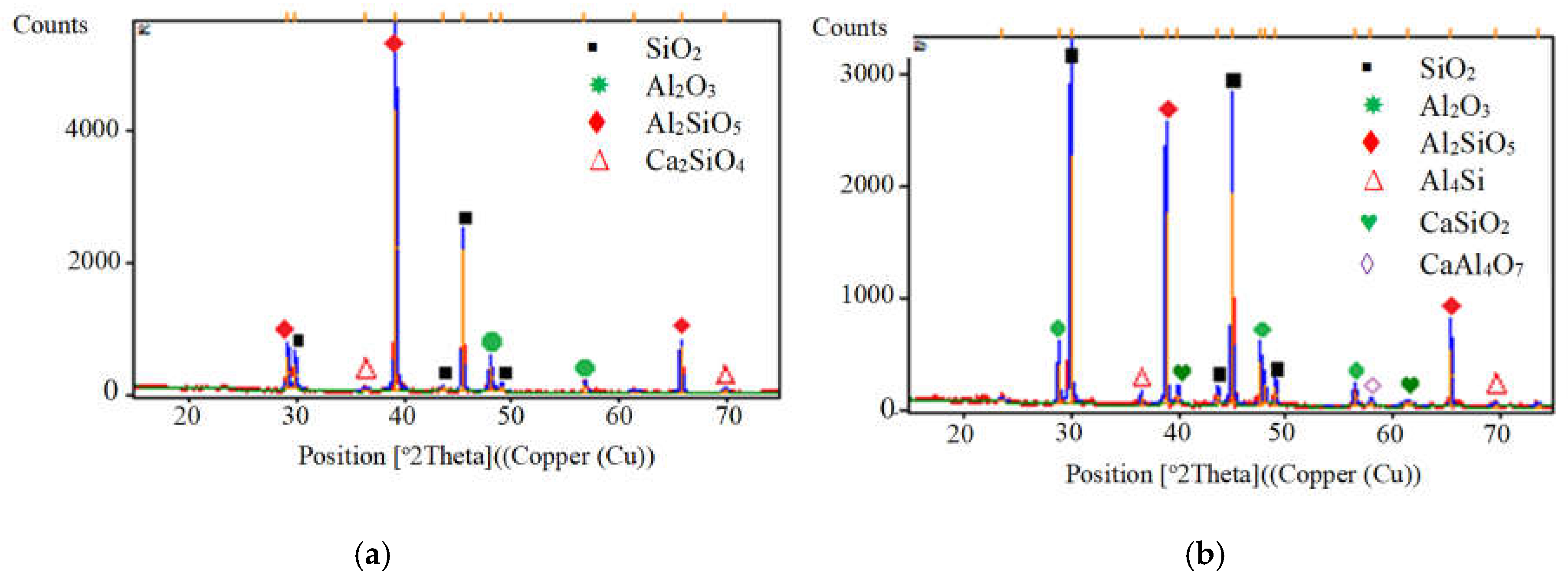
- The values of CTE obtained were parallel with the chemical reaction obtained in XRD analysis.
- (3)
- Based on DTA analysis, fly ash particles have no significant effect on the melting temperature of Al-Si (LM6) fly ash composites. However, the reaction that occurred among the particles was detected in the DTA graph.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jianbin, Z.; Hong, Y. Fabrication of an A356/fly-ash-mullite Interpenetration Composites and its Wear Properties. Ceram. Int. 2017, 43, 12996–13003. [Google Scholar]
- Liang, J.F.; Shueiwan, H.J. Reaction Effect of Fly Ash with Al-3Mg Melt on the Microstructure and Hardness of Aluminium Matrix Composites. Mater. Des. 2016, 89, 941–949. [Google Scholar]
- Blisset, R.S.; Rowson, N.A. A Review of the Multi-component Utilisation of Coal Fly Ash. Fuel 2012, 97, 1–23. [Google Scholar] [CrossRef]
- David, J.R.S.; Smart, D.S.R.; Dinaharan, I. Microstructure and Some Mechanical Properties of Fly Ash Particulate Reinforced AA6061 Aluminium Alloy Composites Prepared by Compocasting. Mater. Des. 2013, 49, 28–34. [Google Scholar] [CrossRef]
- Rohatgi, P.K. Low-cost, Fly Ash-containing Aluminium Matrix Composites. JOM 1994, 46, 55–59. [Google Scholar] [CrossRef]
- Shivananda, K.V.M.; Girish, D.P.; Keshavamurthy, R.; Varol, T.; Koppad, P.G. Mechanical and Thermal Properties of AA7075/TiO2/Fly Ash Hybrid Composites Obtained by Hot Forging. Prog. Nat. Sci. Mater. Int. 2017, 27, 474–481. [Google Scholar] [CrossRef]
- Wu, G.H.; Dou, Z.Y.; Jiang, L.T.; Cao, J.H. Damping Properties of Aluminium Matrix-Fly Ash Composites. Mater. Lett. 2006, 60, 2945–2948. [Google Scholar] [CrossRef]
- Kulkarni, S.G.; Meghnani, J.V.; Lal, A. Effect of Fly Ash Hybrid Reinforcement on Mechanical Property and Density of Aluminium 356 Alloy. Procedia Mater. Sci. 2014, 5, 746–754. [Google Scholar] [CrossRef]
- Rahimian, M.; Ehsani, N.; Parvin, N.; Baharvandi, H.R. The Effect of Particle Size, Sintering Temperature and Sintering Time on the Properties of Al-Al2O3 Composites, Made by Powder Metallurgy. J. Mater. Process Technol. 2009, 518, 100–107. [Google Scholar] [CrossRef]
- Siddarth, P.; Rana, R.S.; Singh, S.K. Study on Mechanical Properties of Environment Friendly Aluminium E-Waste Composite with Fly Ash and E-Glass Fiber. Mater. Today Proc. 2017, 4, 3441–3450. [Google Scholar]
- Kingsly, J.A.G.; Sheriff, N.M.; Dinaharan, I.; Selvam, J.D. Production and Characterization of Rich Husk Ash Particulate Reinforced AA6061 Aluminium Alloy Composites by Compocasting. Trans. Nonferrous Met. Soc. China 2015, 25, 683–691. [Google Scholar]
- Jaswinder, S.; Amit, C. Characterization of Hybrid Aluminum Matrix Composites for Advanced Application-A Review. J. Mater. Res. Technol. 2016, 5, 159–169. [Google Scholar]
- Alaneme, K.K.; Sanusi, K.O. Microstructural Characteristics, Mechanical and Wear Behaviour of Aluminum Matrix Hybrid Composites Reinforced with Alumina, Rice husk Ash and Graphite. Eng. Sci. Technol. Int. J. 2015, 18, 416–422. [Google Scholar]
- Uju, W.A.; Oguocha, I.N.A. A Study of Thermal Expansion of Al-Mg Alloy Composites Containing Fly Ash. Mater. Des. 2012, 33, 503–509. [Google Scholar] [CrossRef]
- Aravindan, S.; Rao, P.V.; Ponappa, K. Evaluation of physical and mechanical properties of AZ91D/SiC composites by two step stir casting process. J. Magnes. Alloys 2015, 3, 52–62. [Google Scholar] [CrossRef]
- Guo, R.O.; Rohatgi, P.K. Chemical Reactions between Aluminium and Fly Ash during Synthesis and Reheating of Al-Fly Ash Composite. Metall. Mater. Trans. B 1998, 29, 519–525. [Google Scholar] [CrossRef]
- Zebarjad, S.M.; Sajjadi, S.A.; Karimi, E.Z.V. Influence of Nanosize Silicon Carbide on Dimensional Stability of Al/SiC Nanocomposite. Mater. Sci. 2008, 2008, 835746. [Google Scholar]
- Gupta, M.; Ling, S. Microstructure and Mechanical Properties of Hypo/Hyper-eutectic Al-Si Alloys Synthesized using a Near-net Shape Forming Technique. J. Alloys Compd. 1999, 287, 284–294. [Google Scholar] [CrossRef]
- Mohanavel, V.; Ravichandran, M.; Anandakrishnan, V.; Pramanik, A.; Meignanamoorthy, M.; Karthick, A.; Muhibbullah, M. Mechanical Properties of Titanium Diboride Particles Reinforced Aluminum Alloy Matrix Composites: A Comprehensive Review. Adv. Mater. Sci. Eng. 2021, 2021, 7602160. [Google Scholar] [CrossRef]
- Zhao, P.T.; Wang, L.D.; Du, Z.M.; Xu, S.C.; Jin, P.P.; Fei, W.D. Thermal Expansion Behaviour of Aluminium Matrix Composite Reinforced by SnO2-coated Al18B4O33 Whisker. Mater. Chem. Phys. 2013, 138, 856–861. [Google Scholar] [CrossRef]
- Wright, A.F.; Fitch, A.N.; Wright, A.C. The Preparation and Structure of the α- and β-Quartz Polymorphs of Beryllium Fluoride. J. Solid State Chem. 1988, 73, 298–304. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Sudo, H.; Kuzuu, N. Thermal Expansion of Vitreous Silica: Correspondence between Dilatation Curve and Phase Transitions in Crystalline Silica. J. Appl. Phys. 1997, 82, 4121–4123. [Google Scholar] [CrossRef]
- Guo, R.Q.; Venugopalan, D.; Rohatgi, P.K. Differential Thermal Analysis to Establish the Stability of Aluminum-Fly Ash Composites during Synthesis and Reheating. Mater. Sci. Eng. A 1998, 241, 1840190. [Google Scholar] [CrossRef]
- Singla, M.; Dwivedi, D.D.; Singh, L.; Chawla, V. Development of Aluminium Based Silicon Carbide Particulate Metal Matrix Composite. J. Miner. Mater. Charact. Eng. 2009, 8, 455–467. [Google Scholar] [CrossRef]
- Wu, S.Q.; Wei, Z.S.; Tjong, S.C. The mechanical and Thermal Expansion Behaviour of An Al-Si Alloy Composite Reinforced with Potassium Titanate Whisker. Compos. Sci. Technol. 2000, 60, 2873–2880. [Google Scholar] [CrossRef]
- Maria, L.A.G.; Vieira, M.D.; Mota, D.A.P.; Cerqueira, W.V.; Teixeira, A.M.R.F. Differential thermal analysis of a zeolite Y crystalline structure in a catalyst. J. Therm. Anal. Calorim. 2010, 101, 965–971. [Google Scholar]
- Balaraman, S.; Baskaran, I.; Sivakumar, K. Phase Transition Behavior of Nanocrystalline Al2O3 Powders. Soft Nanosci. Lett. 2013, 3, 69–74. [Google Scholar]
- Pavlovic, Z.A.; Terzic, A.; Milicic, L.; Radojevic, Z.; Pavlovic, L. Sustainable Solutions for Managing Environmentally Hazardous Waste Materials: Reapplication of Fly Ash. In Proceedings of the 2nd International Symposium on Environmental and Material Flow Management, Zenica, Bosnia and Herzegovina, 7–9 June 2012. [Google Scholar]
- Terzic, A.; Pavlovic, L.j.; Obradovic, N.; Pavlovic, V.; Stojanovic, J.; Milicic, L.j.; Radojevic, Z.; Ristic, M.M. Synthesis and sintering of high-temperature composites based on mechanically activated fly ash. Sci. Sinter. 2012, 44, 135–146. [Google Scholar] [CrossRef]
- Klancnik, G.; Medved, J.; Mrvar, P. Differential Thermal Analysis (DTA) and Differential Scanning Calorimetry (DSC) as a Method of Material Investigation. Mater. Geoenvironment 2010, 57, 127–142. [Google Scholar]
- Ropp, R.C. Encyclopedia of the Alkaline Earth Compounds; Elsevier: Amsterdam, The Netherlands, 2012; p. 109. ISBN 9780444595508. [Google Scholar]



| Element | Cu | Fe | Mg | Mn | Ni | Pb | Si | Al |
|---|---|---|---|---|---|---|---|---|
| Percentage (%) | 0.10 | 0.60 | 0.10 | 0.50 | 0.10 | 0.10 | 10.00–1300 | Remainder |
| Compound | Na2O | MgO | Al2O3 | SiO2 | CaO | K2O | Fe2O3 | TiO2 | LOI |
|---|---|---|---|---|---|---|---|---|---|
| Percentage (%) | 0.481 | 1.102 | 15.939 | 65.61 | 2.098 | 0.684 | 4.517 | 0.813 | 1.87 |
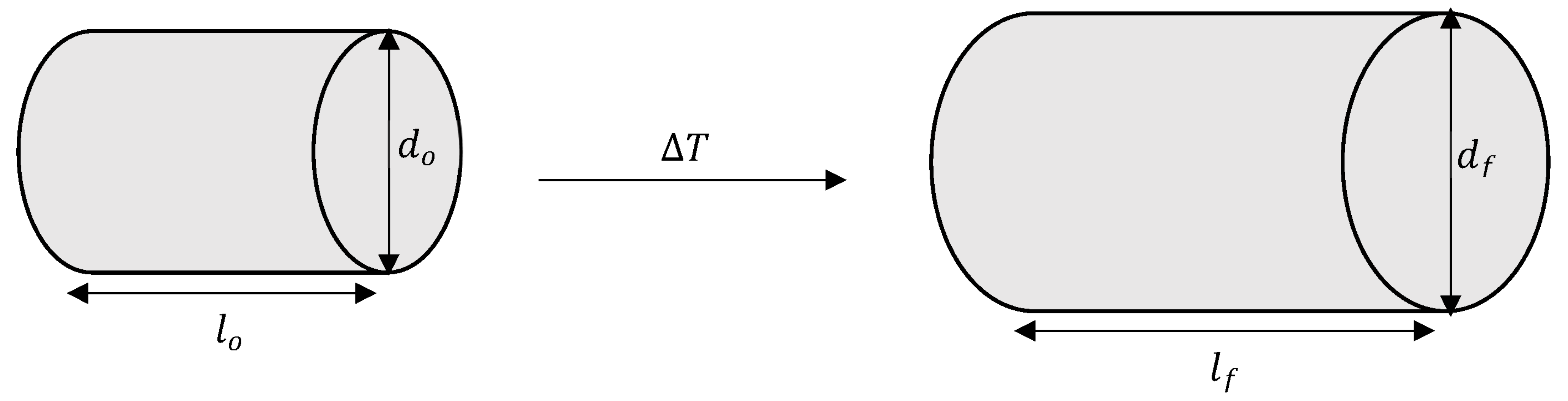
| Sample | Before | After | Percentage of Difference (%) (Length) | Percentage of Difference (%) (Diameter) | ||
|---|---|---|---|---|---|---|
| Length (mm) | Diameter (mm) | Length (mm) | Diameter (mm) | |||
| LM6 | 13.29 | 7.34 | 13.31 | 7.37 | 0.15 | 0.41 |
| LM6 + 4%FA | 11.90 | 7.31 | 11.91 | 7.33 | 0.08 | 0.27 |
| LM6 + 5%FA | 12.09 | 6.35 | 12.10 | 6.36 | 0.08 | 0.17 |
| LM6 + 6%FA | 10.41 | 6.46 | 10.41 | 6.47 | 0 | 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nordin, S.S.; Mhd Noor, E.E.; Chockalingam, P. Chemical and Thermal Analysis of Fly Ash-Reinforced Aluminum Matrix Composites (AMCs). J. Compos. Sci. 2024, 8, 170. https://doi.org/10.3390/jcs8050170
Nordin SS, Mhd Noor EE, Chockalingam P. Chemical and Thermal Analysis of Fly Ash-Reinforced Aluminum Matrix Composites (AMCs). Journal of Composites Science. 2024; 8(5):170. https://doi.org/10.3390/jcs8050170
Chicago/Turabian StyleNordin, Siti Syazwani, Ervina Efzan Mhd Noor, and Palanisamy Chockalingam. 2024. "Chemical and Thermal Analysis of Fly Ash-Reinforced Aluminum Matrix Composites (AMCs)" Journal of Composites Science 8, no. 5: 170. https://doi.org/10.3390/jcs8050170
APA StyleNordin, S. S., Mhd Noor, E. E., & Chockalingam, P. (2024). Chemical and Thermal Analysis of Fly Ash-Reinforced Aluminum Matrix Composites (AMCs). Journal of Composites Science, 8(5), 170. https://doi.org/10.3390/jcs8050170






