Acid-Free Processing of Phosphorite Ore Fines into Composite Fertilizers Using the Mechanochemical Activation Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Origin and Characteristics of the Materials Used
- -
- phosphorite ore with a content of at least 24.5% P2O5, suitable for chemical processing for the extraction of phosphoric acid;
- -
- phosphorite ore with a content of at least 20% P2O5, suitable for electrothermal processing to obtain phosphorus and thermal phosphoric acid.
2.2. Physicochemical Methods of Analysis of Phosphorite Ore Fines Samples
2.3. Methods of Mechanical and Mechanochemical Activation
3. Results and Discussion
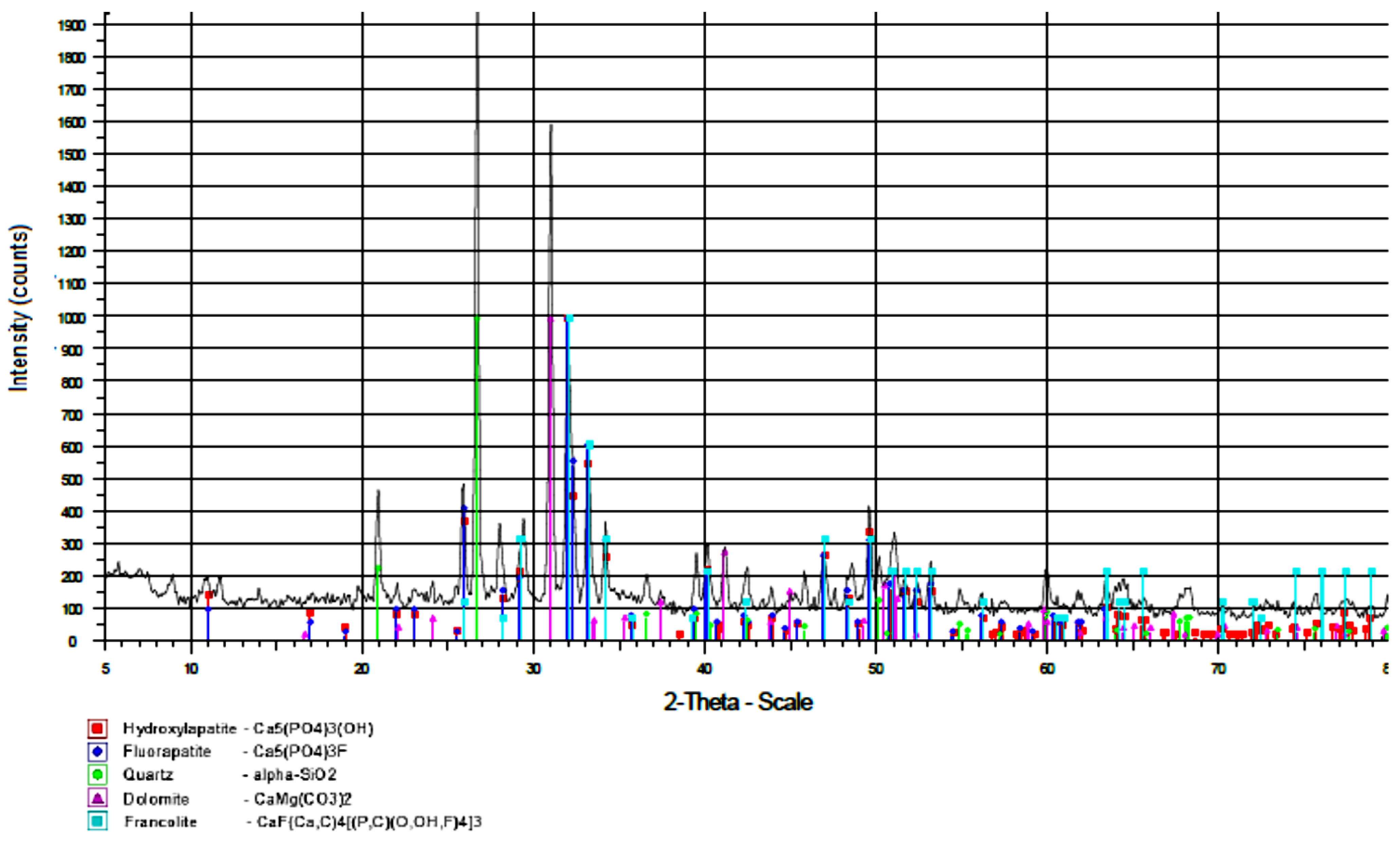
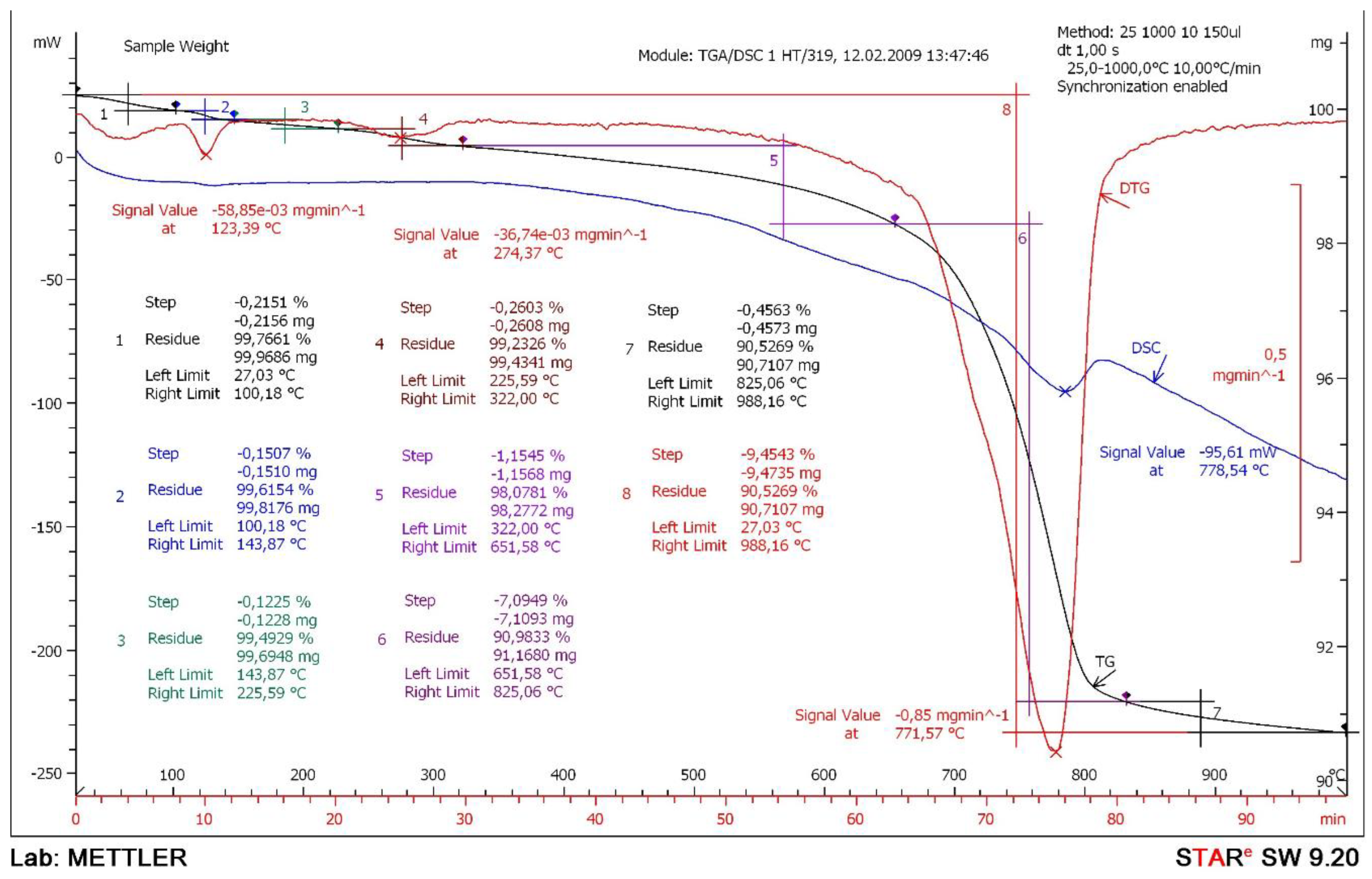
3.1. Results of Complex Physical and Chemical Studies
- -
- phosphorite flour is produced using a simple technology that excludes the formation of large-tonnage production waste—phosphogypsum and chemical effluents;
- -
- phosphorite flour is produced from available phosphate raw materials—phosphorites;
- -
- phosphorite flour, unlike water-soluble fertilizers, is a slowly soluble fertilizer with a long period of action.
3.2. Results of Experimental Studies
3.3. Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhantasov, K.T.; Dormeshkin, O.B.; Bazhirova, K.N. New types of phosphorus-containing complex mineral fertilizers and fertilizer mixtures. In Production Technologies and Agrochemical Efficiency; BSTU: Minsk, Russian, 2020; p. 325. [Google Scholar]
- Urszula, R.; Rusek, P.; Kołodyńska, D. Quality of Phosphate Rocks from Various Deposits Used in Wet Phosphoric Acid and P-Fertilizer Production. Materials 2023, 16, 793. [Google Scholar] [CrossRef]
- Duan, X.; Zou, C.; Jiang, Y.; Yu, X.; Ye, X. Effects of Reduced Phosphate Fertilizer and Increased Trichoderma Application on the Growth, Yield, and Quality of Pepper. Plants 2023, 12, 2998. [Google Scholar] [CrossRef] [PubMed]
- Ammar, M.; Ashraf, S.; Baltrusaitis, J. Nutrient-Doped Hydroxyapatite: Structure, Synthesis and Properties. Ceramics 2023, 6, 1799–1825. [Google Scholar] [CrossRef]
- Zhantasov, K.T.; Dormeshkin, O.B.; Bazhirova, K.N. Agronomical field testing of new multicomponent mineral fertilizers. Eurasian Chem.-Technol. J. 2015, 17, 79–86. [Google Scholar] [CrossRef]
- Notholt, A.; Sheldon, R.; Davidson, D. Phosphate Deposits of the World; Cambridge University Press: Cambridge, UK, 1989; Volume 2, p. 566. [Google Scholar]
- Edixhoven, J.D.; Gupta, J.; Savenije, H.H.G. Recent revisions of phosphate rock reserves and resources: A critique. Earth Syst. Dynam. 2014, 5, 491–507. [Google Scholar] [CrossRef]
- U.S. Geological Survey (USGS). Mineral Commodity Summaries for Phosphate Rock. 2021. Available online: https://pubs.usgs.gov/periodicals/mcs2021/mcs2021-phosphate.pdf (accessed on 1 January 2023).
- Bamiki, R.; Raji, O.; Ouabid, M.; Elghali, A.; Yazami, O.K.; Bodinier, J.-L. Phosphate Rocks: A Review of Sedimentary and Igneous Occurrences in Morocco. Minerals 2021, 11, 1137. [Google Scholar] [CrossRef]
- Messai, B.; Taieb, I.; Younes, S.B.; Lartiges, B.; Salem, E.B.; Ben, E.; Ellafi, A. Characterization of the Tunisian Phosphate Rock from Metlaoui-Gafsa Basin and Bio-Leaching Assays. Sustainability 2023, 15, 7204. [Google Scholar] [CrossRef]
- Serazetdinov, D.Z. Complex Processing of Phosphorites and Physical and Chemical Studies of Inorganic Materials; Gylym: Alma-Ata, Kazakhstan, 1991; p. 189. [Google Scholar]
- Bugenov, E.S.; Dzhusipbekov, U.Z. Physico-Chemical Bases of Phosphorus Production from Low-Grade Phosphorites; KSTU: Almaty, Kazakhstan, 2005; p. 384. [Google Scholar]
- Sadyrova, A.T.; Kalauova, A.S. Basic principles of creating non-waste/low-waste production in the processing of Karatau phosphorites. Chem. J. Kazakhstan 2012, 4, 174–190. [Google Scholar]
- Myrzaliev, B.; Toltebaeva, Z.; Bazhirova, K. The current state and prospects of the development of phosphoric industry of Kazakhstan. Actual Probl. Econ. 2015, 4, 131–135. [Google Scholar]
- Turgumbaeva, R.K. Study of physical and chemical properties of lithological differences of phosphorites. Bull. Al-Farabi Kazakh NU. Ser. Chem. 2004, 3, 306–313. [Google Scholar]
- Zhantasov, K.; Dormeshkin, O.; Bazhirova, K. The Current State of Obtaining a New Range of Mineral Fertilizers, Fertilizer Mixtures, with the Solution of Environmental Problems. Arch. Pet. Environ. Biotechnol. 2020, 5, 160. [Google Scholar] [CrossRef]
- El Afifi, E.M.; Hilal, M.A.; Attallah, M.F.; El-Reefy, S.A. Characterization of phosphogypsum wastes associated with phosphoric acid and fertilizer production. J. Environ. Radioact. 2009, 100, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lv, X.; Xiang, L. Review of the State of Impurity Occurrences and Impurity Removal Technology in Phosphogypsum. Materials 2023, 16, 5630. [Google Scholar] [CrossRef] [PubMed]
- Zhantasov, M.K.; Bimbetova, G.Z.; Kolesnikov, A.S.; Sadyrbayeva, A.S.; Orynbasarov, A.K.; Kutzhanova, A.N.; Turemuratov, R.S.; Botabaev, N.E.; Zhantasova, D. Examination of optimal parameters of oxy-ethylation of fatty acids with a view to obtaining demulsifiers for deliquefaction in the system of skimming and treatment of oil: A method to obtain demulsifier from fatty acids. Chem. Today 2016, 34, 72–77. [Google Scholar]
- Zhangabay, N.; Suleimenov, U.; Utelbayeva, A.; Buganova, S. Experimental research of the stress-strain state of prestressed cylindrical shells taking into account temperature effects. Case Stud. Constr. Mater. 2022, 18, e01776. [Google Scholar] [CrossRef]
- Volokitina, I.; Kurapov, G. Effect of Initial Structural State on Formation of Structure and Mechanical Properties of Steels Under ECAP. Met. Sci. Heat Treat. 2018, 59, 786–792. [Google Scholar] [CrossRef]
- Urakaev, F.K.; Khan, N.V.; Shalabaev, Z.S.; Tatykaev, B.B.; Nadirov, R.K.; Burkitbaev, M.M. Synthesis and photocatalytic properties of silver chloride/silver composite colloidal particles. Colloid J. 2020, 82, 76–80. [Google Scholar] [CrossRef]
- Zharmenov, A.; Yefremova, S.; Satbaev, B.; Shalabaev, N.; Satbaev, S.; Yermishin, S.; Kablanbekov, A. Production of Refractory Materials Using a Renewable Source of Silicon Dioxide. Minerals 2022, 12, 1010. [Google Scholar] [CrossRef]
- Shokirov, U.; Turabdjanov, S.M.; Kadirov, X.I.; Badriddinova, F.M.; Kedelbaev, B.S. Calculation and design of an industrial reactor for pyrroles synthesis/News of the National Academy of Sciences of the Republic of Kazakhstan. Ser. Geol. Tech. Sci. 2021, 445, 157–163. [Google Scholar]
- Satbaev, B.; Yefremova, S.; Zharmenov, A.; Kablanbekov, A.; Yermishin, S.; Shalabaev, N.; Satbaev, A.; Khen, V. Rice Husk Research: From Environmental Pollutant to a Promising Source of Organo-Mineral Raw Materials. Materials 2021, 14, 4119. [Google Scholar] [CrossRef] [PubMed]
- Otarbaev, N.S.; Kapustin, V.M.; Nadirov, K.S.; Bimbetova, G.Z.; Zhantasov, M.K.; Nadirov, R.K. New potential demulsifiers obtained by processing gossypol resin. Indones. J. Chem. 2019, 19, 959–966. [Google Scholar] [CrossRef]
- Zhangabay, N.; Suleimenov, U.; Utelbayeva, A.; Kolesnikov, A.; Baibolov, K.; Imanaliyev, K.; Moldagaliyev, A.; Karshyga, G.; Duissenbekov, B.; Fediuk, R.; et al. Analysis of a Stress-Strain State of a Cylindrical Tank Wall Vertical Field Joint Zone. Buildings 2022, 12, 1445. [Google Scholar] [CrossRef]
- Zhangabay, N.; Sapargaliyeva, B.; Suleimenov, U.; Abshenov, K.; Utelbayeva, A.; Kolesnikov, A.; Baibolov, K.; Fediuk, R.; Arinova, D.; Duissenbekov, B.; et al. Analysis of Stress-Strain State for a Cylindrical Tank Wall Defected Zone. Materials 2022, 15, 5732. [Google Scholar] [CrossRef] [PubMed]
- Volokitina, I. Evolution of the Microstructure and Mechanical Properties of Copper under ECAP with Intense Cooling. Met. Sci. Heat Treat. 2020, 62, 253–258. [Google Scholar] [CrossRef]
- Kolesnikov, A.S. Thermodynamic simulation of silicon and iron reduction and zinc and lead distillation in zincoligonite ore-carbon systems. Russ. J. Non-Ferr. Met. 2014, 55, 513–518. [Google Scholar] [CrossRef]
- Schipper, W.; Klapwijk, B.; Potjer, B.; Rulkens, W.; Temmink, H.; Kiestra, F.; Lijmbach, D. Phosphate recycling in the phosphorus industry. Environ. Technol. 2001, 22, 1337–1445. [Google Scholar] [CrossRef] [PubMed]
- Knubovets, R. Structural mineralogy and properties of natural phosphates. Rev. Chem. Eng. 1994, 9, 161–216. [Google Scholar] [CrossRef]
- Dorzhieva, S.G.; Bazarova, Z.G. The influence of lignin-containing additives on the solubility of mechanically activated phosphorites. Chem. Sustain. Dev. 2004, 12, 147–153. [Google Scholar]
- Chaikina, M.V.; Kryukova, G.N. Structural transformations of quartz and apatite during mechanical activation. J. Struct. Chem. 2004, 45, 122–127. [Google Scholar] [CrossRef]
- Lyakhov, N.Z.; Chaikina, M.V. Kinetics of dissolution of mechanically activated apatite. Sib. Chem. J. 1991, 5, 84–89. [Google Scholar]
- Boldyrev, V.V. Mechanochemistry and mechanical activation of solids. Proceedings of the Academy of Sciences of the Russian Federation. Chem. Ser. 1990, 10, 2228–2245. [Google Scholar]
- Chaikina, M.V. Mechanochemistry of Natural and Synthetic Apatites; GEO: Novosibirsk, Russia, 2002; p. 223.
- Boldyrev, V.V.; Lyakhov, N.Z.; Chaikina, M.V. Mechanochemical technology for producing phosphorus fertilizers. Chem. Sustain. Dev. 1996, 4, 97–99. [Google Scholar]
- Kuanysheva, G.S.; Dalabaeva, N.S.; Balgysheva, B.D. Mechanochemical effect on the composition of Kazakhstan phosphorites in the presence of hydrosulfates and dihydrogen phosphates. News Natl. Acad. Sci. Repub. Kazakhstan. Chem. Ser. 2005, 5, 74–80. [Google Scholar]
- Zhantasov, K.T.; Dormeshkin, O.B.; Bazhirova, K.N. Energy and resource saving processing of low grade phosphorites. Theor. Found. Chem. Eng. 2015, 49, 277–279. [Google Scholar]
- Angelov, A.I.; Levin, B.V.; Klassen, P.V. World production and consumption of phosphate raw materials. Min. J. 2003, 4–5, 6–11. [Google Scholar]
- Bazhirov, T.S.; Protsenko, V.S.; Bazhirov, N.S.; Dauletiyarov, M.S.; Serikbayev, B.Y.; Bazhirova, K.N. Physicochemical investigations of vermiculite—Microporous component for heat-resistant materials. News Natl. Acad. Sci. Repub. Kazakhstan Ser. Chem. Technol. 2019, 5, 136–142. [Google Scholar] [CrossRef]
- Shubin, A.P. Phosphate Ores of Kazakhstan; Science: Alma-Ata, Kazakhstan, 1990; p. 320. [Google Scholar]
- Fang, N.; Shi, Y.; Chen, Z.; Sun, X.; Zhang, L.; Yi, Y. Effect of mechanochemical activation of natural phosphorite structure as well as phosphorus solubility. PLoS ONE 2019, 14, e0224423. [Google Scholar] [CrossRef] [PubMed]
- Veshnyakova, L.A.; Drozdyuk, T.A.; Aizenshtadt, A.M.; Frolova, M.A.; Tutygin, A.S. Surface activity of siliconcontaining rocks. Mater. Sci. 2016, 5, 45–48. [Google Scholar]
- Bazhirov, T.S.; Dauletiyarov, M.S.; Bazhirov, N.S.; Serikbayev, B.E.; Bazhirova, K.N. Study of physicochemical processes in mechanical activation of phosphorus slag. Voprosy Khimii I Khimicheskoi Tekhnologii 2017, 6, 57–62. [Google Scholar]
- Activator: Grinding, Activation, Synthesis. Available online: http://www.activator.ru/rus/equipment_mills.php (accessed on 1 January 2020).
- Avvakumov, E.G.; Gusev, A.A. Mechanical Activation Methods in the Processing of Natural and Man-Made Raw Materials; Geo: Novosibirsk, Russia, 2009; p. 155. [Google Scholar]
- Boldyrev, V.V. Mechanochemistry and mechanical activation of solid substances. Adv. Chem. 2006, 75, 203–216. [Google Scholar]
- Bochenek, D.; Bartkowska, J.A.; Kozielski, L. Mechanochemical Activation and Spark Plasma Sintering of the Lead-Free Ba(Fe1/2Nb1/2)O3 Ceramics. Materials 2021, 14, 2254. [Google Scholar] [CrossRef]
- Kaia, T.; Kaljuvee, T.; Petkova, V. Impact of mechanical activation on physical and chemical properties of phosphorite concentrates. Int. J. Miner. Process. 2011, 100, 104–109. [Google Scholar]
- Chaykina, M.V. Prospects for Mechanochemical Technology for Obtaining Phosphate Fertilizers. Moscow, Russia, 2007; p. 261. [Google Scholar]
- Naghadeh, H.; Abdollahy, M.; Darban, A.; Pourghahramani, P. Mechanical activation of phosphate concentrates to enhance dissolution efficiency of rare earth elements from a kinetic viewpoint. J. Min. Environ. 2019, 10, 373–388. [Google Scholar]
- Fang, N.; Liang, S.; Dai, H.; Xiao, H.; Han, X.; Liu, G. The Improved Phosphorus Solubility of Mechanochemically Activated Phosphate Rock and Its Effect on Soil-Available Phosphorus in Weakly Acidic Soil. Sustainability 2022, 14, 7869. [Google Scholar] [CrossRef]
- Drozdyuk, T.A.; Frolova, M.A.; Aizenshtadt, A.M.; Calay, K.R.; Jhatial, A.A. Preliminary Study on the Mechanical Activation and High-Temperature Treatment of Saponite-Containing Tailings Generated during Kimberlite Ore Dressing. Appl. Sci. 2022, 12, 4957. [Google Scholar] [CrossRef]
- Butyagin, P.Y. Problems and prospects for the development of mechanochemistry. Adv. Chem. 1994, 63, 1031–1043. [Google Scholar]
- Lu, L.; Lai, M.O. Mechanical Alloy; Kluwer Academic Publishers: Boston, MA, USA; London, UK, 1998; p. 292. [Google Scholar]
- Koch, C.C. Material synthesis by mechanical alloying. Annu. Rev. Mater. Sci. 1989, 19, 121–143. [Google Scholar] [CrossRef]
- Heinicke, G. Tribochemistry; Akademie-Verlag: Berlin, Germany, 1984; p. 495. [Google Scholar]
- Heinicke, G. Tribochemistry; Mir: Moscow, Russia, 1987; p. 582. [Google Scholar]
- Pelovski, Y.; Petkova, V.; Dombalov, I. Thermotribochemicaltreatment of low grade natural phosphates. J. Therm. Anal. Calorim. 2007, 88, 207–212. [Google Scholar] [CrossRef]
- Avvakumov, E.M.; Senna, N.; Kosova, N. Soft Mechanochemical Synthesis: A Basis for New Chemical Technologies; Springer: New York, NY, USA; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001; p. 200. [Google Scholar]
- Pelovski, Y.; Petkova, V.; Dombalov, I. Thermal analysis of mechanoactivated mixtures of tunisia phosphorite and ammonium sulfate. J. Therm. Anal. Calorim. 2003, 72, 967–980. [Google Scholar] [CrossRef]
- Yaneva, V.; Petkova, V.; Dombalov, I. Structural transformations of Syrian phosphorite during mechanochemical activation. Chem. Sustain. Dev. 2001, 2, 351–358. [Google Scholar]
- Chaikina, M.V.; Bulina, N.V.; Vinokurova, O.B. Possibilities of Mechanochemical Synthesis of Apatites with Different Ca/P Ratios. Ceramics 2022, 5, 404–422. [Google Scholar] [CrossRef]
- Ibrahim, S.S.; El-Midany, A.A.; Boulos, T.R. Economic preferences of mechanical activation over mineral benefficiation for phosphate rock direct applications. Physicochem. Probl. Miner. Process. 2010, 44, 63–78. [Google Scholar]
- Kalymbetov, G.Y.; Kedelbayev, B.S.; Yelemanova, Z.R.; Sapargaliyeva, B. Effects of Different Biostimulants on Seed Germination of Sorghum Plants. J. Ecol. Eng. 2023, 24, 134–142. [Google Scholar]
- Yaneva, V.; Petrov, O.; Petkova, V. Structural and spectroscopic studies of mechanochemically activated nanosized apatite from Syria. Mater. Res. Bull. 2009, 44, 693–699. [Google Scholar] [CrossRef]
- Petkova, V.; Koleva, V.; Kostova, B.; Sarov, S. Structural and thermal transformations on high energy milling of natural apatite. J. Therm. Anal. Calorim. 2015, 121, 217–225. [Google Scholar] [CrossRef]
- Usmanov, K.; Chernyakova, R.; Dzhusipbekov, U. Influence of modifying additives on the properties of dispersed phosphorites. Perspect. Innov. Econ. Business. Int. Cross-Ind. J. 2010, 6, 131–133. [Google Scholar] [CrossRef]
- Jin, L.; Sun, L.; Wang, L.; Shi, Y. Studies on the mechanical activation of Huangmailing phosphorite. Res. J. Chem. Environ. 2013, 17, 156–162. [Google Scholar]
- Gapparov, J.; Syrlybekkyzy, S.; Filin, A.; Kolesnikov, A.; Zhatkanbayev, Y. Overview of techniques and methods of processing the waste of stale clinkers of zinc production. MIAB Min. Informational Anal. Bull. 2024, 4, 44–55. [Google Scholar]


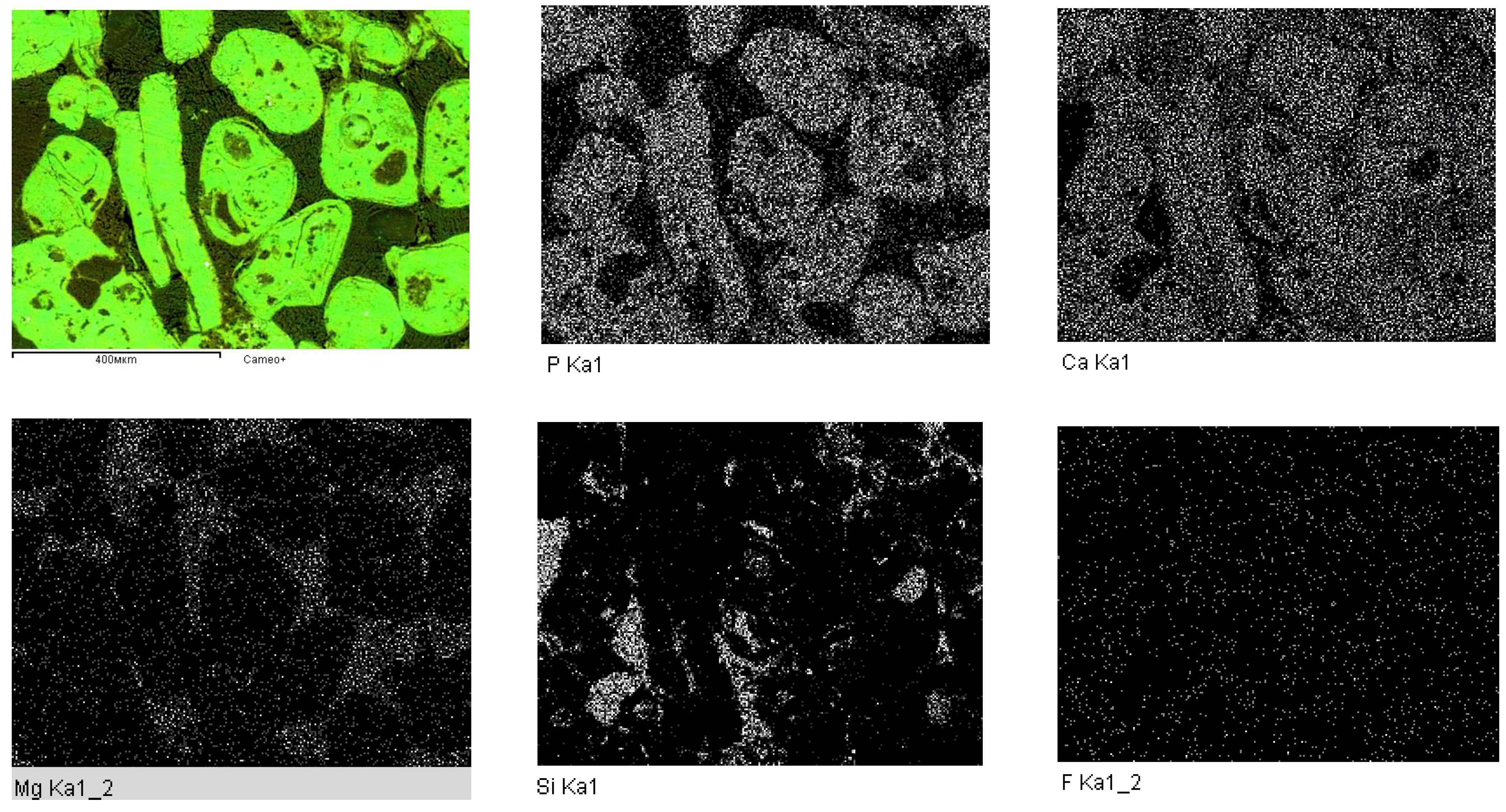

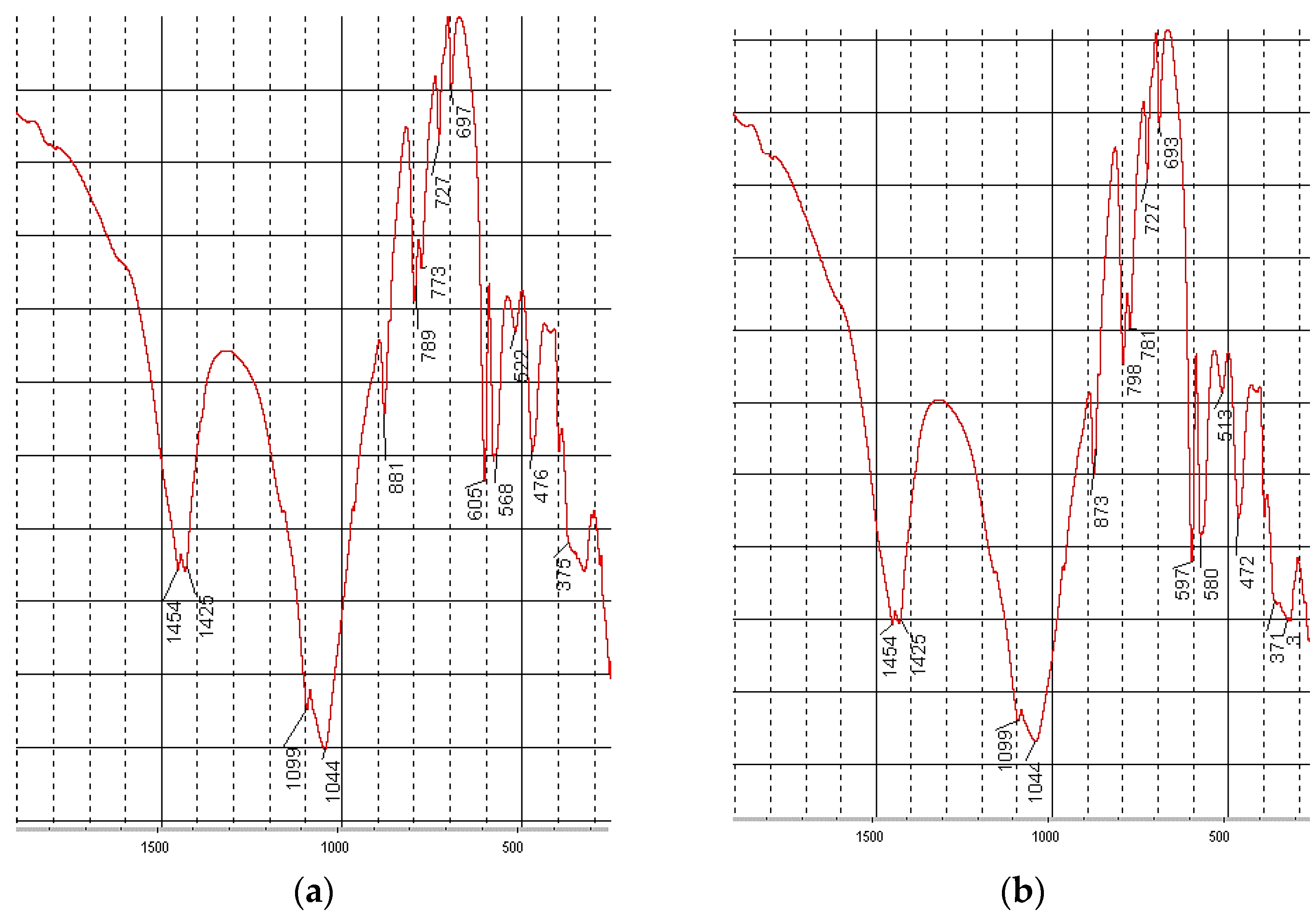



| Types of Phosphoritic Ore | Content of Main Components in Ore, % | |||||
|---|---|---|---|---|---|---|
| P2O5 | CaO | MgO | SiO2 | Al2O3 | CO2 | |
| rich (phosphorite) ore | 29–32 | 43–47 | 2–3 | 10–15 | 0.6–0.8 | 4–6 |
| carbonate ore | 28–30 | 43–48 | 1–2 | 10–15 | 1–1 | 6–8 |
| carbonate-siliceous ore | 21–23 | 37–40 | 3–4 | 19–25 | 1–2 | 2–6 |
| siliceous ore | 21–23 | 36–39 | - | 30–32 | - | - |
| poor (off-balance sheet) ore | 19–21 | 32–40 | 3–4 | 26–28 | 1–2 | 5–7 |
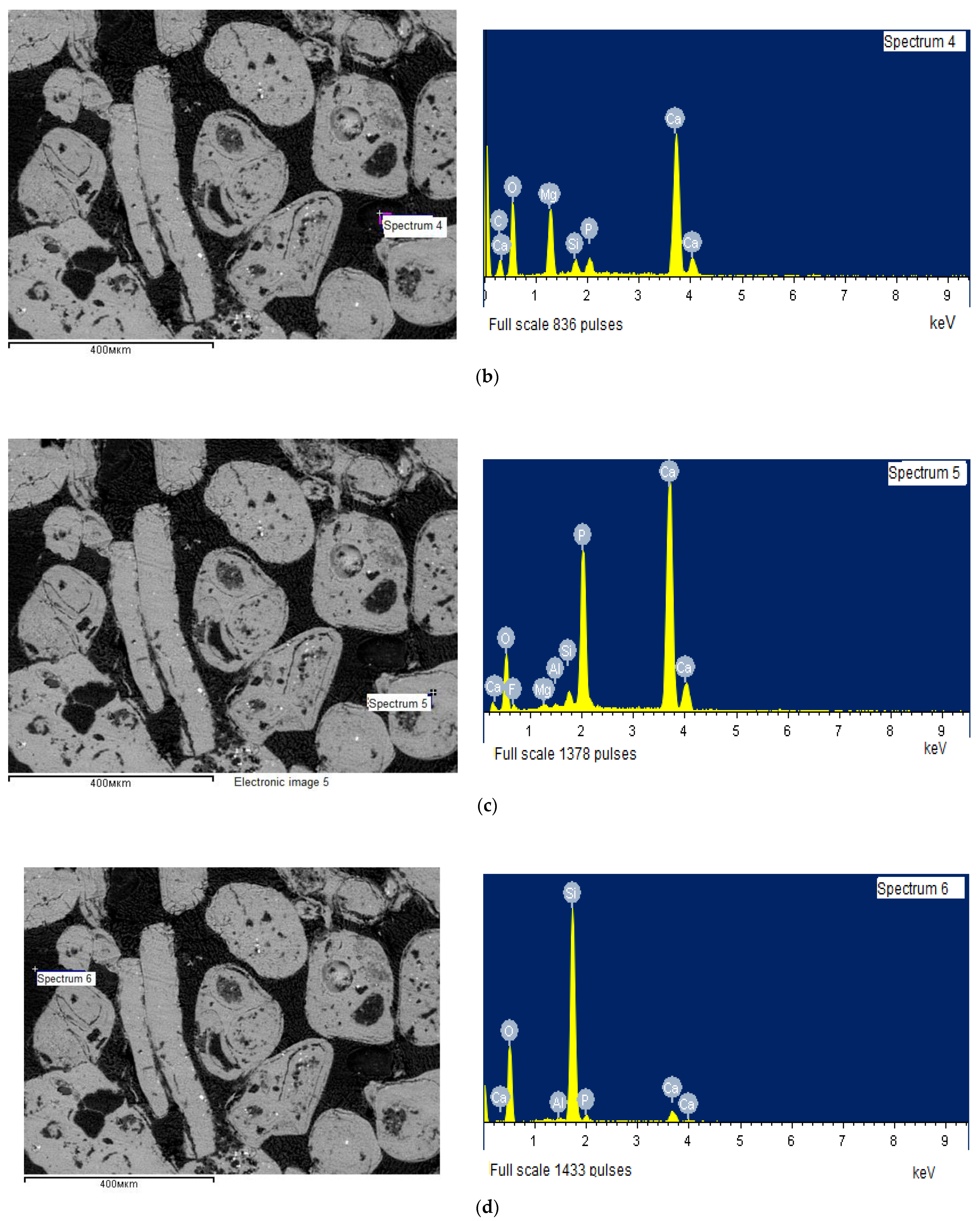
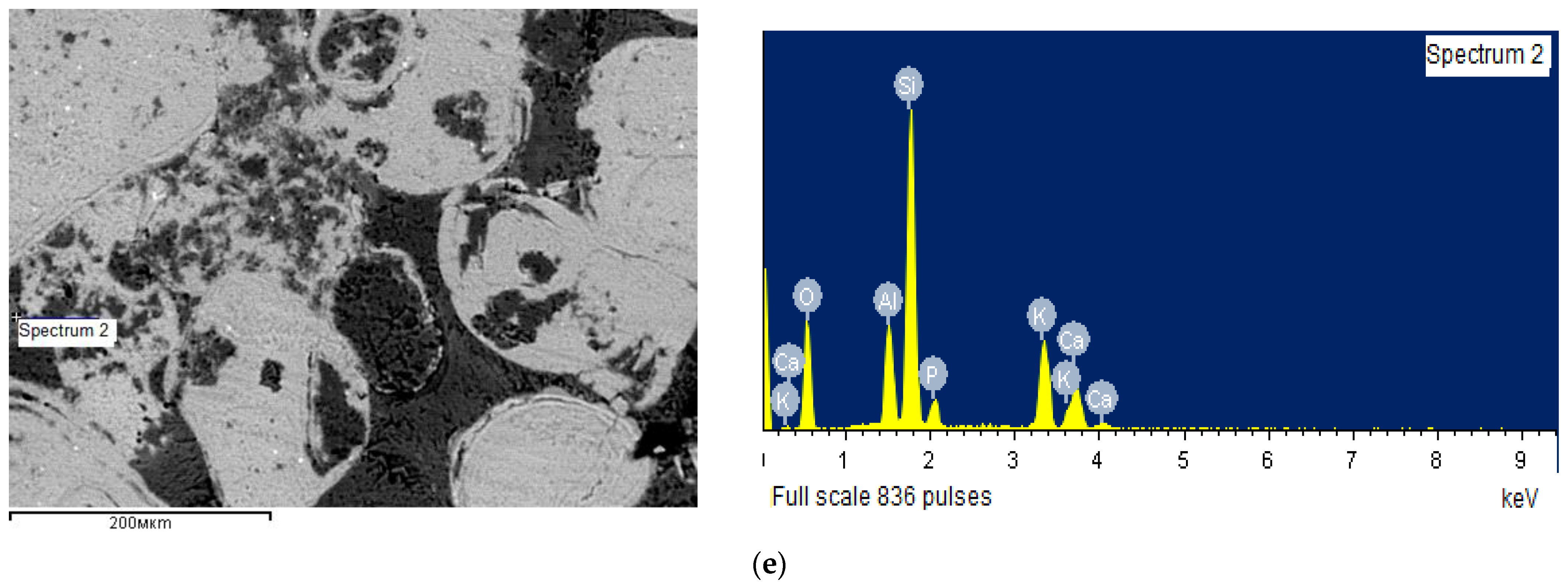
| Spectrum No. | Si | Al | Ca | Mg | Mn | Na | K | P | Fe | Ti | S | F |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spectrum 1 | 10.64 | 2.05 | 25.36 | 1.57 | 0.16 | 0.21 | 1.13 | 9.82 | 1.92 | 0.03 | 0.34 | 2.25 |
| Spectrum 2 | 25.76 | 7.48 | 6.11 | - | - | - | 11.74 | 3.52 | - | - | - | - |
| Spectrum 4 | 1.52 | 0.25 | 33.43 | 15.01 | - | - | - | 2.62 | - | - | - | - |
| Spectrum 5 | 1.17 | 0.27 | 37.75 | 0.35 | - | - | - | 17.30 | - | - | - | 3.82 |
| Spectrum 6 | 41.06 | 0.51 | 4.50 | 0.33 | - | - | - | 2.14 | - | - | - | - |
| Activated Material | Activation Time, min | Total Content of P2O5 in the Phosphorite Ore Fines, % | Content of the Citrate Soluble form P2O5, % | |
|---|---|---|---|---|
| Absolute | Relative | |||
| Phosphorite ore fines | - | 20.92 | 4.53 | 17.63 |
| 5 | 20.91 | 9.78 | 46.65 | |
| 10 | 20.92 | 11.03 | 52.66 | |
| 15 | 20.92 | 11.09 | 52.95 | |
| 20 | 20.91 | 11.13 | 53.18 | |
| Activated Mixture | The Proportion of Ammonium Sulfate in the Mixture, % | Total Content of P2O5 in Fine of Phosphorite Ore, % | The Content in the Mixture of Citrate-Soluble form of Phosphorus, % | |
|---|---|---|---|---|
| Absolute | Relative | |||
| Phosphorite ore fines and ammonium sulfate | 10 | 20.91 | 10.09 | 53.61 |
| 20 | 20.92 | 10.01 | 59.83 | |
| 30 | 20.92 | 9.72 | 66.39 | |
| 40 | 20.91 | 8.83 | 70.41 | |
| 50 | 20.92 | 7.36 | 70.36 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazhirova, K.; Zhantasov, K.; Bazhirov, T.; Kolesnikov, A.; Toltebaeva, Z.; Bazhirov, N. Acid-Free Processing of Phosphorite Ore Fines into Composite Fertilizers Using the Mechanochemical Activation Method. J. Compos. Sci. 2024, 8, 165. https://doi.org/10.3390/jcs8050165
Bazhirova K, Zhantasov K, Bazhirov T, Kolesnikov A, Toltebaeva Z, Bazhirov N. Acid-Free Processing of Phosphorite Ore Fines into Composite Fertilizers Using the Mechanochemical Activation Method. Journal of Composites Science. 2024; 8(5):165. https://doi.org/10.3390/jcs8050165
Chicago/Turabian StyleBazhirova, Kamshat, Kurmanbek Zhantasov, Tynlybek Bazhirov, Alexandr Kolesnikov, Zarina Toltebaeva, and Nurlybek Bazhirov. 2024. "Acid-Free Processing of Phosphorite Ore Fines into Composite Fertilizers Using the Mechanochemical Activation Method" Journal of Composites Science 8, no. 5: 165. https://doi.org/10.3390/jcs8050165
APA StyleBazhirova, K., Zhantasov, K., Bazhirov, T., Kolesnikov, A., Toltebaeva, Z., & Bazhirov, N. (2024). Acid-Free Processing of Phosphorite Ore Fines into Composite Fertilizers Using the Mechanochemical Activation Method. Journal of Composites Science, 8(5), 165. https://doi.org/10.3390/jcs8050165





