Mullite Synthesis Using Porous 3D Structures Consisting of Nanofibrils of Aluminum Oxyhydroxide Chemically Modified with Ethoxysilanes
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Influence of Surface Chemical Modification by Ethoxysilanes on Composition, Structure, and Phase State of PMOA Nanomaterials
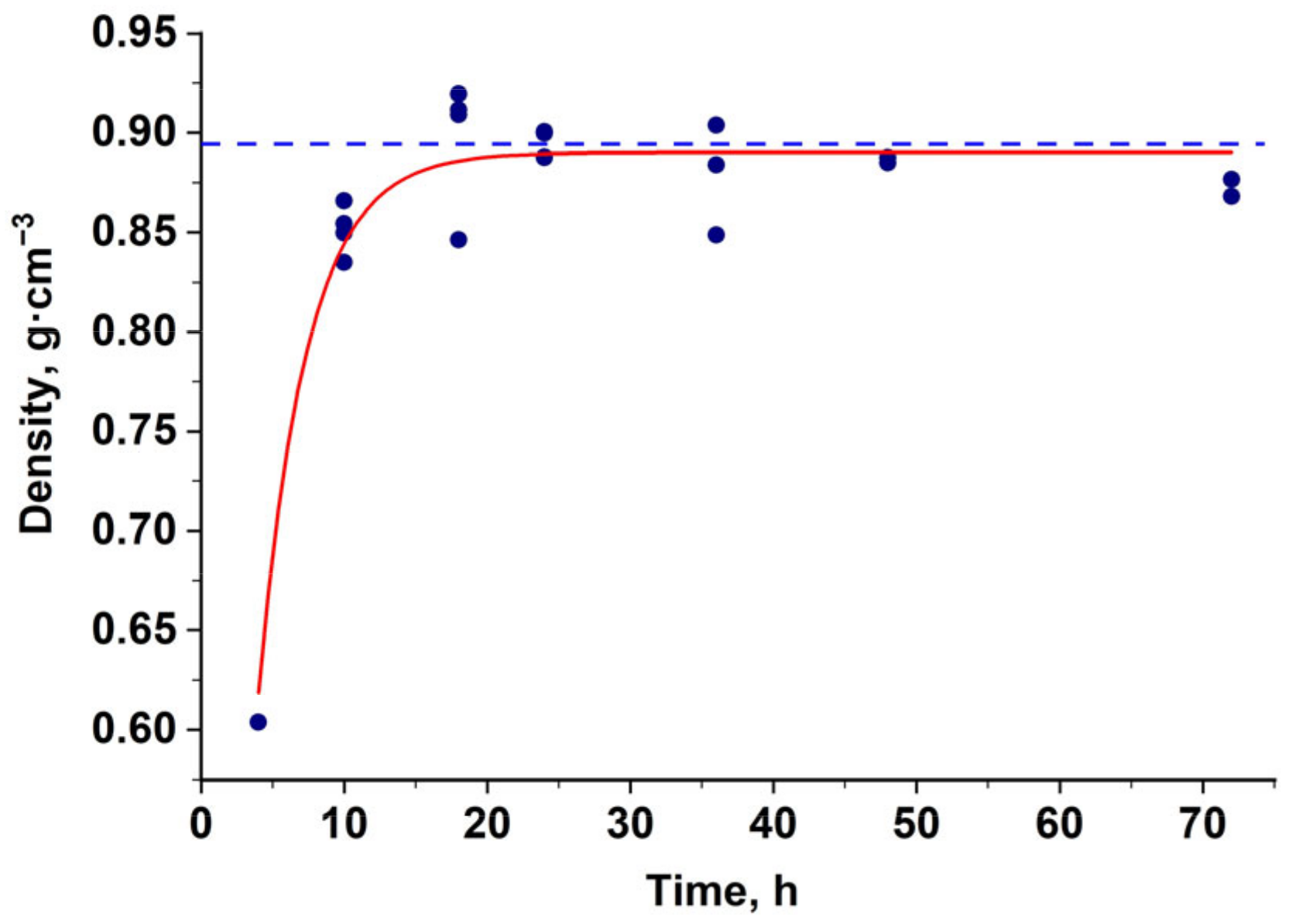
3.1.1. Preparation of Nanocomposites for Synthesis of Alumina–Silica Precursors
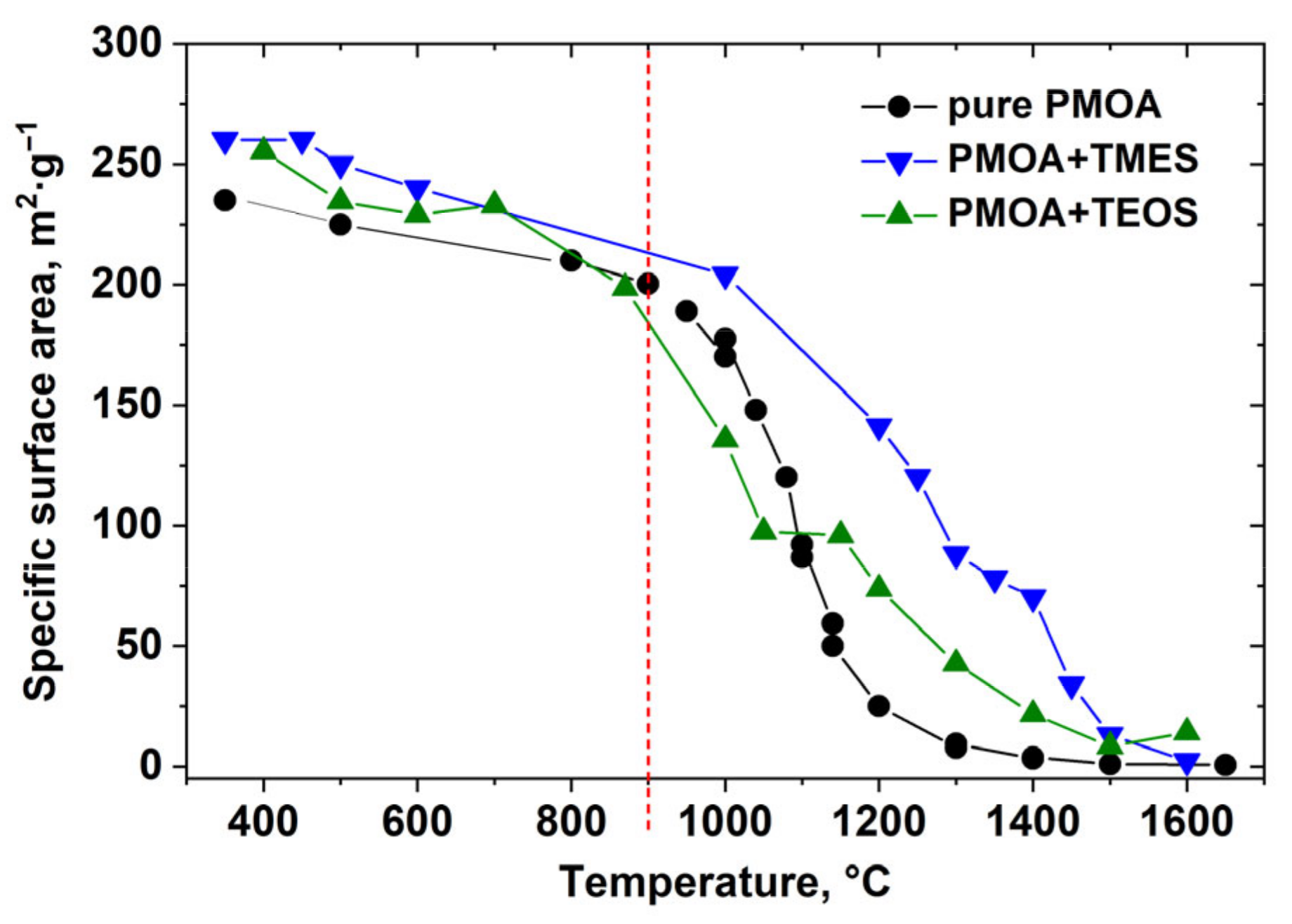
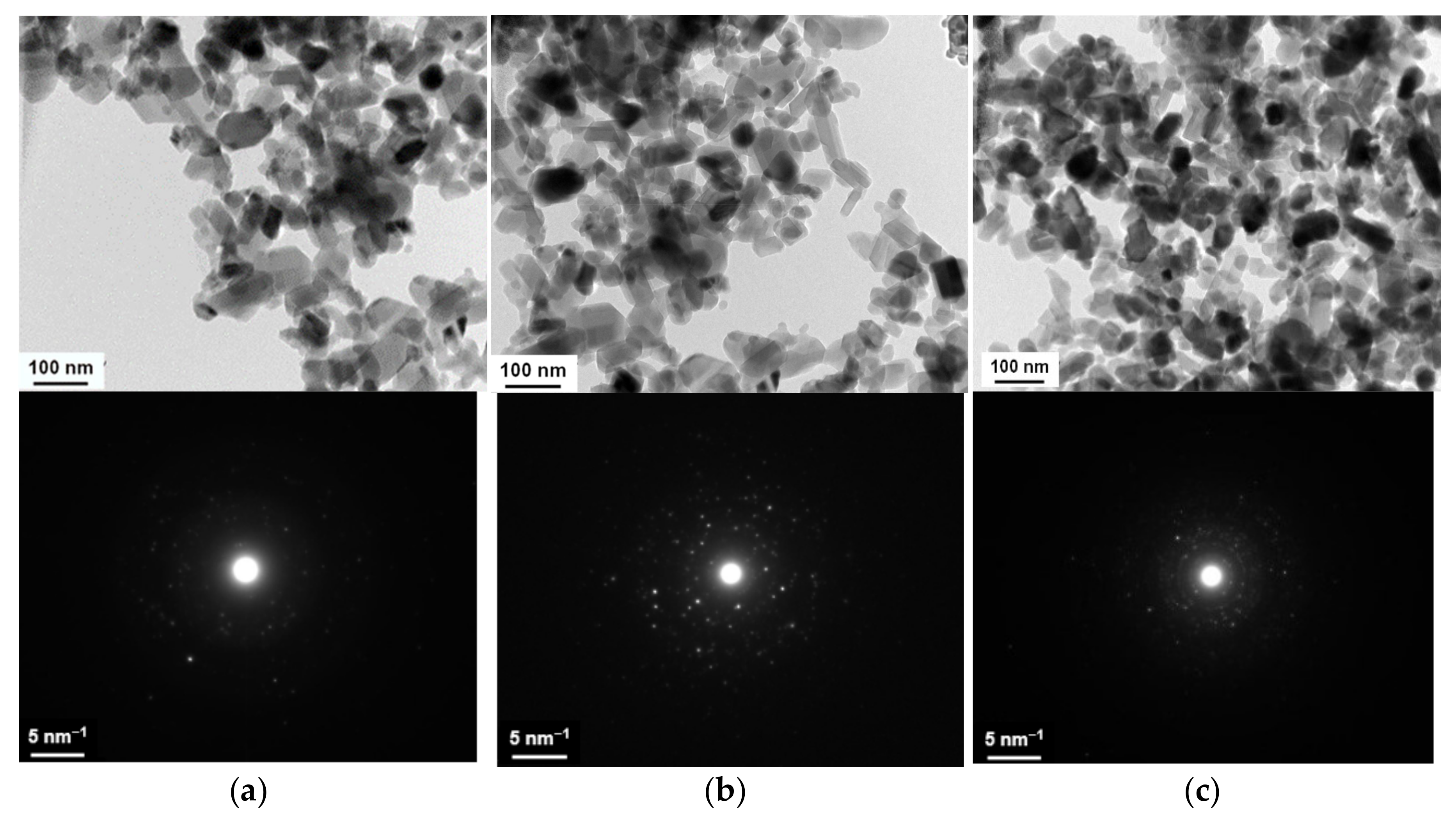
3.1.2. Thermal Treatment of Alumina–Silica Precursors
- Up to the annealing temperature of ~900 °C, the ethoxysilane layers’ effect on specific surface area changes in PMOA+TEOS nanocomposites is noticeable; however, the mass density increase relative to pure PMOA does not exceed 20 m2/g. We attribute this modest modification to the diffusion-limited reactions at the interface between alumina and silica [21];
- The maximum bulk mass density difference between PMOA+TEMS and pure PMOA achieved at T ~1200 °C is 120 m2/g, while it does not exceed 50 m2/g in PMOA+TEOS. This increase is due to different initial conditions in the formation of 3D nanostructures in raw PMOA, PMOA+TEMS, and PMOA+TEOS;
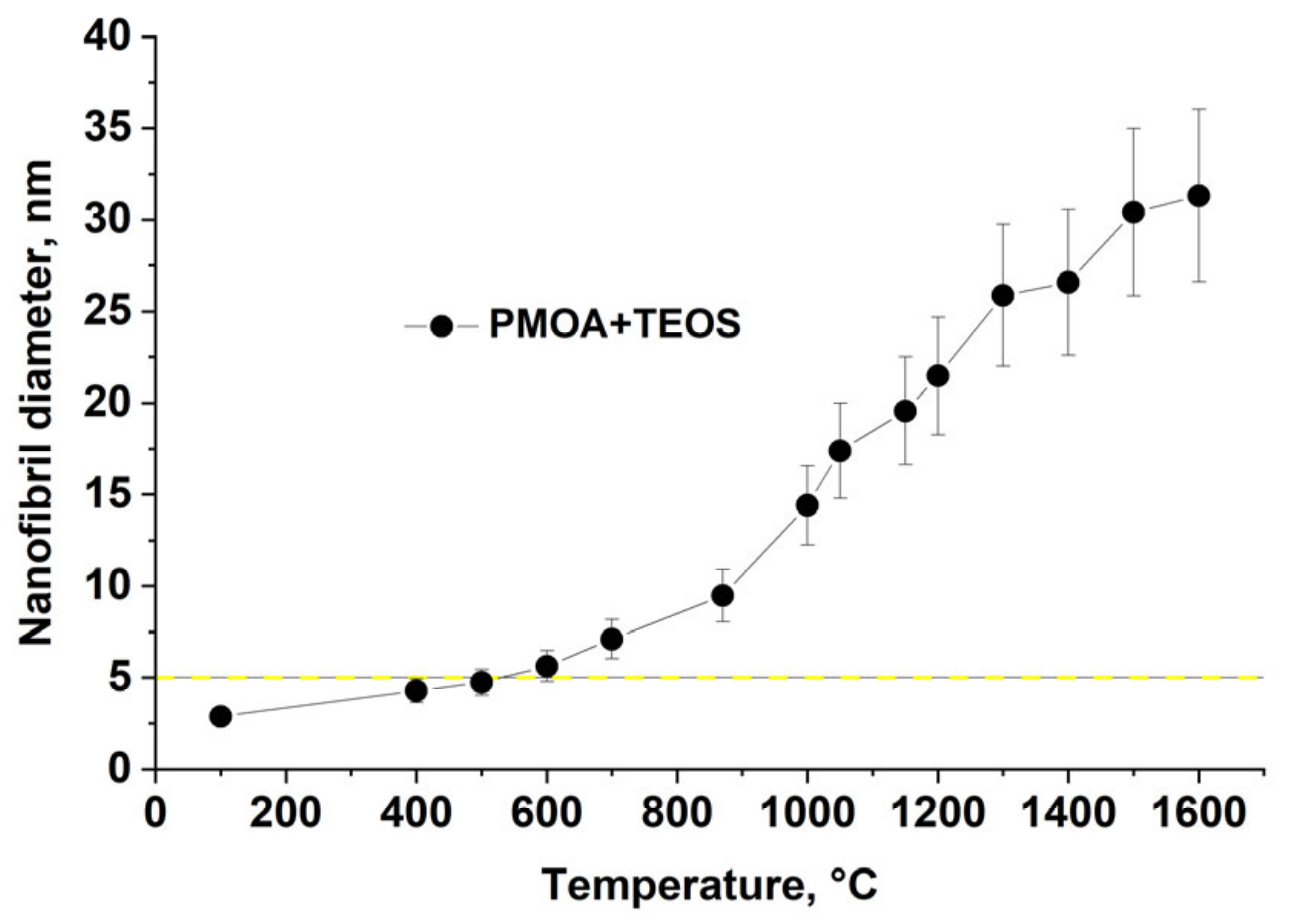
- Annealing at temperatures above 1450 °C leads to the stabilization of the 3D structure parameters: the fibrils acquire an ellipsoidal shape with the characteristic size of ≤ 2 μ for pure PMOA [20] and up to 70 nm for PMOA+TEOS samples (Figure 5). It is important to note that the solid-phase synthesis of mullite after 4 h annealing at temperatures T ≥ 1400 °C begins at the alumina–silica interface.
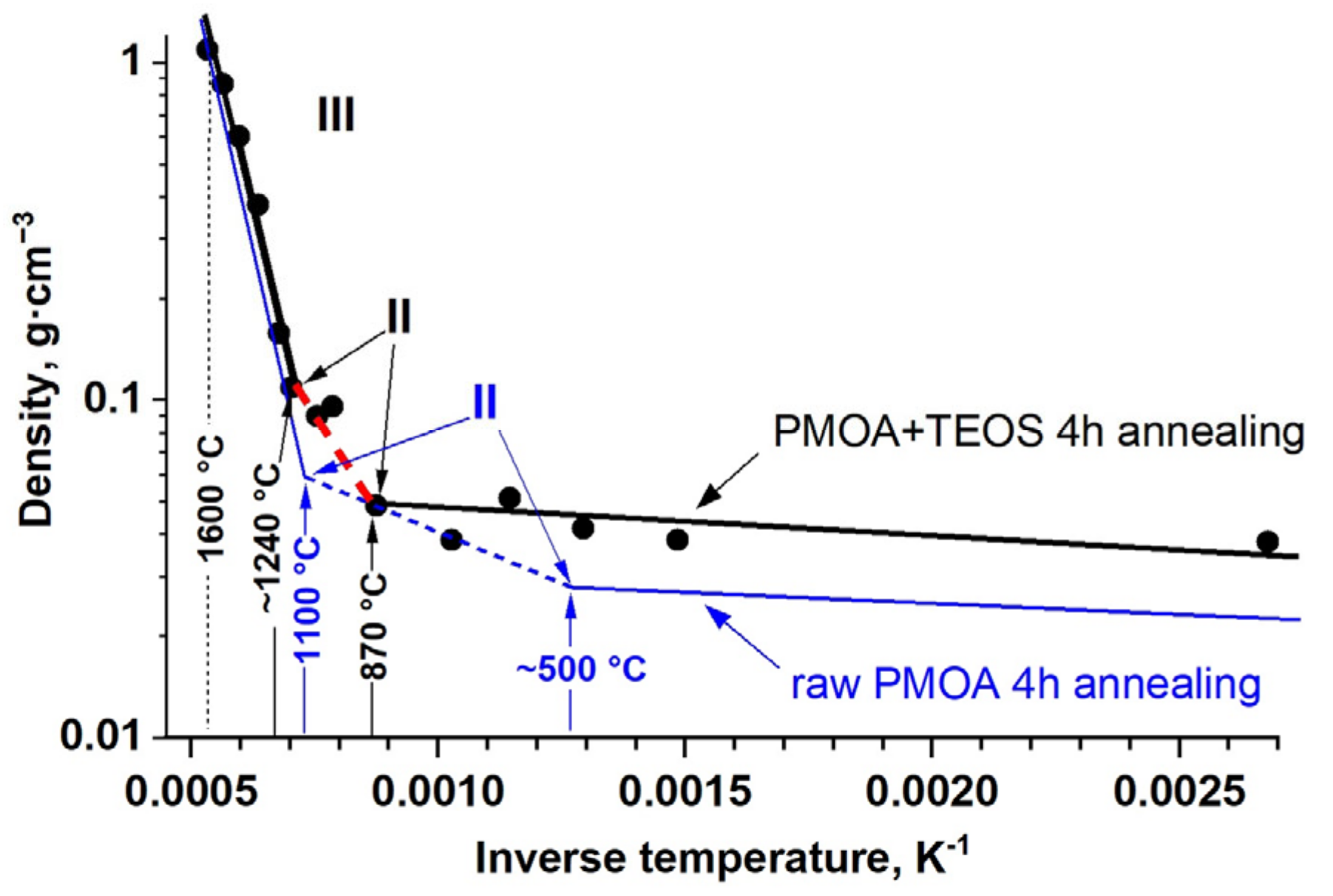
3.2. Nanomullite Synthesis from Aluminosilicate Nanocomposites

- -
- T ≤ 870 °C. Dehydration of aluminum oxyhydroxide. The presence of an amorphous SiO2 layer on the nanofibril surface limits water desorption and the formation of pre-boehmite structural elements;
- -
- T ≤ 1000 °C. Changes in nanofibril morphology and decreases in specific surface area due to the activation of diffusional processes inside the fibrils (Figure 3). The surface diffusion in the nanofibrils is significantly limited by the presence of an amorphous SiO2 layer. The crystallization begins, resulting in the formation of the γ-Al2O3 phase;
- -
- T ≤ 1300 °C. Beginning of the structural phase transformation γ-Al2O3 → θ-Al2O3;
- -
- T ~ 1350 °C. Beginning of the structural phase transformation θ-Al2O3 → α-Al2O3;
- -
- T ≥ 1400 °C. Beginning of mullite synthesis at the α-Al2O3 fibril interfaces with amorphous SiO2.
3.3. Nanomullite Synthesis from the Aluminosilicate Nanocomposites
| Physical Processes and the Model Parameters | Raw PMOA | PMOA + MTMS | PMOA + TEOS |
|---|---|---|---|
| Stages I–III: Dehydration, Diffusion, Crystallization. The nanofibril density is ≈ 2.25 g/cm3 The dominant process is diffusion at the surfaces and interfaces: | |||
| Pre-exponential factor D0, m2/s Activation energy of diffusion ED, kJ/mol | 6.0·10−18 28 | 3.5·10−17 68.5 | 2.33∙10−18 61 |
| Stages IV–VIII: Crystallization, Sintering, Mullite synthesis. The nanofibril density is ≈ 3.1 g/cm3. The dominant process is sintering: | |||
| Free volume constant (shrinkage rate) B0, s−1 Activation energy of the sintering Eb, kJ/mol | 2.3·10−2 58 | 1.23·10−1 87.5 | 1.2·10−2 92.1 |
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bowen, N.L.; Greig, J.W. The System: Al2O3·SiO2. J. Am. Ceram. Soc. 1924, 7, 238–254. [Google Scholar] [CrossRef]
- Aramaki, S.; Roy, R. Revised Phase Diagram for the System Al2O3−SiO2. J. Am. Ceram. Soc. 1962, 45, 229–242. [Google Scholar] [CrossRef]
- Fischer, R.X.; Schneider, H.; Schmücker, M. Crystal structure of Al-rich mullite. Am. Mineral. 1994, 79, 983–990. [Google Scholar]
- Lazic, B.; Krüger, H.; Kaindl, R.; Perfler, L.; Kremenovic, A.; Cvetkovic, V.; Withers, R.L. Superstructure of Mullite-type KAl9O14. Chem. Mater. 2013, 25, 496–502. [Google Scholar] [CrossRef]
- Schneider, H.; Fischer, R.X.; Schreuer, J. Mullite: Crystal Structure and Related Properties. J. Am. Ceram. Soc. 2015, 98, 2948–2967. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, T.; Yi, Z.; Yan, L.; Liu, S.; Yao, X.; Guo, A.; Liu, J.; Hou, F. Multiscale mullite fiber/whisker reinforced silica aerogel nanocomposites with enhanced compressive strength and thermal insulation performance. Ceram. Int. 2020, 46, 28561–28568. [Google Scholar] [CrossRef]
- Epicier, T.; O’Keefe, M.A.; Thomas, G. Atomic imaging of 3:2 mullite. Acta Cryst. 1990, A46, 948–962. [Google Scholar] [CrossRef]
- Aryal, S.; Rulis, P.; Ching, W.Y. Mechanical Properties and Electronic Structure of Mullite Phases Using First-Principles Modeling. J. Am. Ceram. Soc. 2012, 95, 2075–2088. [Google Scholar] [CrossRef]
- Aksay, I.A.; Dabbs, D.M.; Sarikaya, M. Mullite for Structural, Electronic, and Optical Applications. J. Am. Ceram. Soc. 1991, 74, 2343–2358. [Google Scholar] [CrossRef]
- Klug, F.J.; Prochazka, S.; Doremus, R.H. Alumina-Silica Phase Diagram in the Mullite Region. J. Am. Ceram. Soc. 1987, 70, 750–759. [Google Scholar] [CrossRef]
- Cui, K.; Zhang, Y.; Fu, T.; Wang, J.; Zhang, X. Toughening Mechanism of Mullite Matrix Composites: A Review. Coatings 2020, 10, 672. [Google Scholar] [CrossRef]
- Cameron, W.E. Mullite: A Substituted Alumina. Am. Mineral. 1977, 62, 747–755. [Google Scholar]
- Lenz, S.; Schneider, H.; Fischer, R.X. Mullite-type Na0.67Al6O9.33 and a discussion of iota-alumina. J. Eur. Ceram. Soc. 2020, 40, 4276–4280. [Google Scholar] [CrossRef]
- Saito, Y.; Takei, T.; Hayashi, S.; Yasumori, A.; Okada, K. Effects of Amorphous and Crystalline SiO2 Additives on γ-Al2O3-to-α-Al2O3 Phase Transitions. J. Am. Ceram. Soc. 1998, 81, 2197–2200. [Google Scholar] [CrossRef]
- Horiuchi, T.; Sugiyama, T.; Mori, T. Factors for maintenance of a high surface area of silica-coated α-alumina after heating > 1573 K. J. Mater. Chem. 1993, 3, 861–865. [Google Scholar] [CrossRef]
- Mizushima, Y.; Hori, M. Preparation of heat-resistant alumina aerogels. J. Mater. Res. 1993, 8, 2993–2999. [Google Scholar] [CrossRef]
- Mazerolles, L.; Michel, D.; di Costanzo, T.; Vignes, J.L. New Developments in Nanometric Porous Mullite, Spinel, and Aluminas. In Innovative Processing and Synthesis of Ceramics, Glasses, and Composites VI; Bansal, N.P., Singh, J.P., Eds.; Ceramic Transactions; The American Ceramic Society: Columbus, OH, USA, 2002; Volume 135, pp. 227–235. [Google Scholar]
- Beguin, B.; Garbowski, E.; Primet, M. Stabilization of alumina toward thermal sintering by silicon addition. J. Catal. 1991, 127, 595–604. [Google Scholar] [CrossRef]
- Katada, N.; Ishiguro, H.; Muto, K.; Niwa, M. A heat-resisting acid catalyst: Thermal stability and acidity of a thin silica layer on alumina calcined at 1493 K. Chem. Vap. Depos. 1995, 1, 54–60. [Google Scholar] [CrossRef]
- Khodan, A.; Nguyen, T.H.N.; Esaulkov, M.; Kiselev, M.R.; Amamra, M.; Vignes, J.-L.; Kanaev, A. Porous monoliths consisting of aluminum oxyhydroxide nanofibrils: 3D structure, chemical composition, and phase transformations in the temperature range 25–1700 °C. J. Nanopart. Res. 2018, 20, 194. [Google Scholar] [CrossRef]
- Khodan, A.; Kanaev, A.; Esaulkov, M.; Kiselev, M.; Nadtochenko, V. Effects of Surface Chemical Modification by Ethoxysilanes on the Evolution of 3D Structure and Composition of Porous Monoliths Consisting of Alumina Hydroxide Nanofibrils in the Temperature Range 25–1700 °C. Nanomaterials 2022, 12, 3591. [Google Scholar] [CrossRef]
- Khatim, O.; Nguyen, T.H.N.; Amamra, M.; Museur, L.; Khodan, A.; Kanaev, A. Synthesis and photoluminescence properties of nanostructured mullite/-Al2O3. Acta Mater. 2014, 71, 108–116. [Google Scholar] [CrossRef]
- Seong, H.K.; Kim, U.; Kim, M.H.; Choi, H.J.; Lee, Y.; Seo, W.S. Synthesis and optical properties of mullite nanowires. J. Am. Ceram. Soc. 2007, 90, 1937–1939. [Google Scholar] [CrossRef]
- Vignes, J.-L.; Mazerolles, L.; Michel, D. A novel method for preparing porous alumina objects. Key Eng. Mater. 1997, 132–136, 432–435. [Google Scholar] [CrossRef]
- Di Costanzo, T.; Fomkin, A.A.; Frappart, C.; Khodan, A.N.; Kuznetsov, D.G.; Mazerolles, L.; Michel, D.; Minaev, A.A.; Sinitsin, V.A.; Vignes, J.-L. New Method of Porous Oxide Synthesis: Alumina and Alumina Based Compounds. Mater. Sci. Forum. 2004, 453–454, 315–322. [Google Scholar] [CrossRef]
- Vignes, J.-L.; Frappart, C.; di Costanzo, T.; Rouchaud, J.-C.; Mazerolles, L.; Michel, D. Ultraporous monoliths of alumina prepared at room temperature by aluminium oxidation. J. Mater. Sci. 2008, 43, 1234–1240. [Google Scholar] [CrossRef]
- Khodan, A.N.; Bykov, A.V.; Kiselev, M.R. The Effect of Chemical Modification of the Surface by Oxysilanes on Changes in the Structural and Phase States of Highly Porous Aluminum Oxyhydroxides at Annealing up to 1200 °C. Prot. Met. Phys. Chem. Surf. 2023, 59, 145–150. [Google Scholar] [CrossRef]
- Geguzin, Y.E. Diffusion porosity in metals and alloys. Phys.-Uspekhi Adv. Phys. Sci. 1957, LXI, 217–247. [Google Scholar]
- Ivensen, V.A. Use of a mathematical model for pore volume shrinkage over a wide temperature range. Powder Metall. Met. Ceram. 1996, 34, 528–533. [Google Scholar] [CrossRef]





| Stage | Structural and Phase Transformations | Transition Temperature | ||
|---|---|---|---|---|
| PMOA | PMOA + TEOS | Shift, ΔT | ||
| I–II | Polymolecular polynuclear state → Amorphous phase | ≤100 | ≤120 | ~20 |
| II–III | Dehydration: Amorphous phase → Amorphous phase with the elements of pre-boehmite structures | ≤460 | ≤870 | ~400 |
| III–IV | Crystallization: Amorphous pre-boehmite structures → γ-Al2O3 | ≤700 | ≥900 | ~200 |
| IV–V | Crystallization + Sintering: γ-Al2O3 → θ-Al2O3 | ≤1050 | ≤1300 | ~250 |
| V–VI | θ-Al2O3 → α-Al2O3 | ≤1100 | ≥1350 | ~250 |
| VII | Mullite synthesis | - | ≥1400 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khodan, A.; Nguyen, T.H.N.; Kanaev, A. Mullite Synthesis Using Porous 3D Structures Consisting of Nanofibrils of Aluminum Oxyhydroxide Chemically Modified with Ethoxysilanes. J. Compos. Sci. 2024, 8, 469. https://doi.org/10.3390/jcs8110469
Khodan A, Nguyen THN, Kanaev A. Mullite Synthesis Using Porous 3D Structures Consisting of Nanofibrils of Aluminum Oxyhydroxide Chemically Modified with Ethoxysilanes. Journal of Composites Science. 2024; 8(11):469. https://doi.org/10.3390/jcs8110469
Chicago/Turabian StyleKhodan, Anatole, Thi Hang Nga Nguyen, and Andrei Kanaev. 2024. "Mullite Synthesis Using Porous 3D Structures Consisting of Nanofibrils of Aluminum Oxyhydroxide Chemically Modified with Ethoxysilanes" Journal of Composites Science 8, no. 11: 469. https://doi.org/10.3390/jcs8110469
APA StyleKhodan, A., Nguyen, T. H. N., & Kanaev, A. (2024). Mullite Synthesis Using Porous 3D Structures Consisting of Nanofibrils of Aluminum Oxyhydroxide Chemically Modified with Ethoxysilanes. Journal of Composites Science, 8(11), 469. https://doi.org/10.3390/jcs8110469









