Biomimetic Hierarchical Nanocomposite Hydrogels: From Design to Biomedical Applications
Abstract
1. Introduction
2. The Functionality of Nanostructures in Biomimetic Hierarchical Nanocomposite Hydrogels
2.1. Topology Manipulation
2.2. Bioactive Reservoir
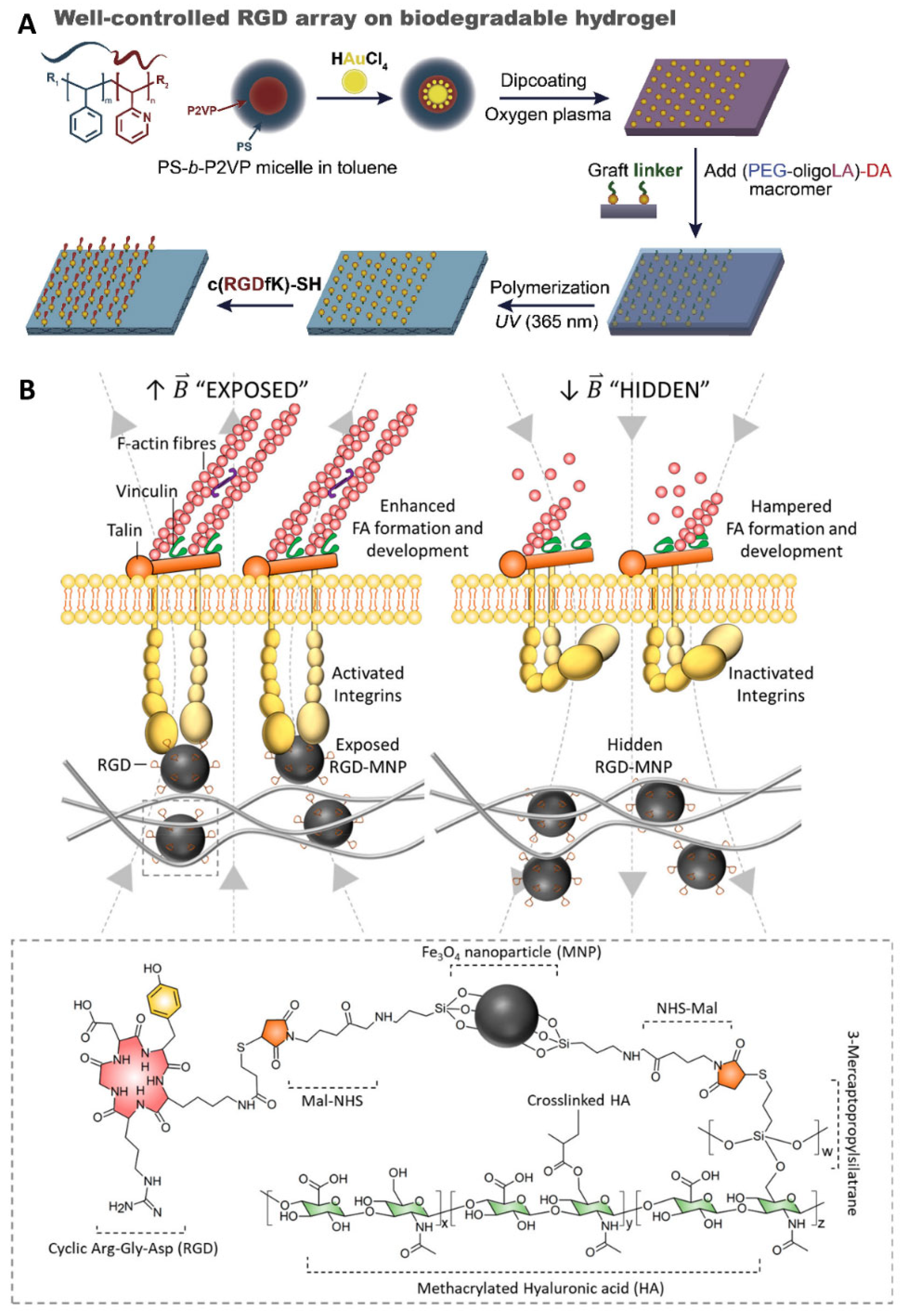
2.3. Ligand Presentation
3. The Biomedical Applications of Biomimetic Hierarchical Nanocomposite Hydrogels
3.1. Drug Delivery
3.2. Regenerative Medicine
3.2.1. Cartilage
3.2.2. Bone
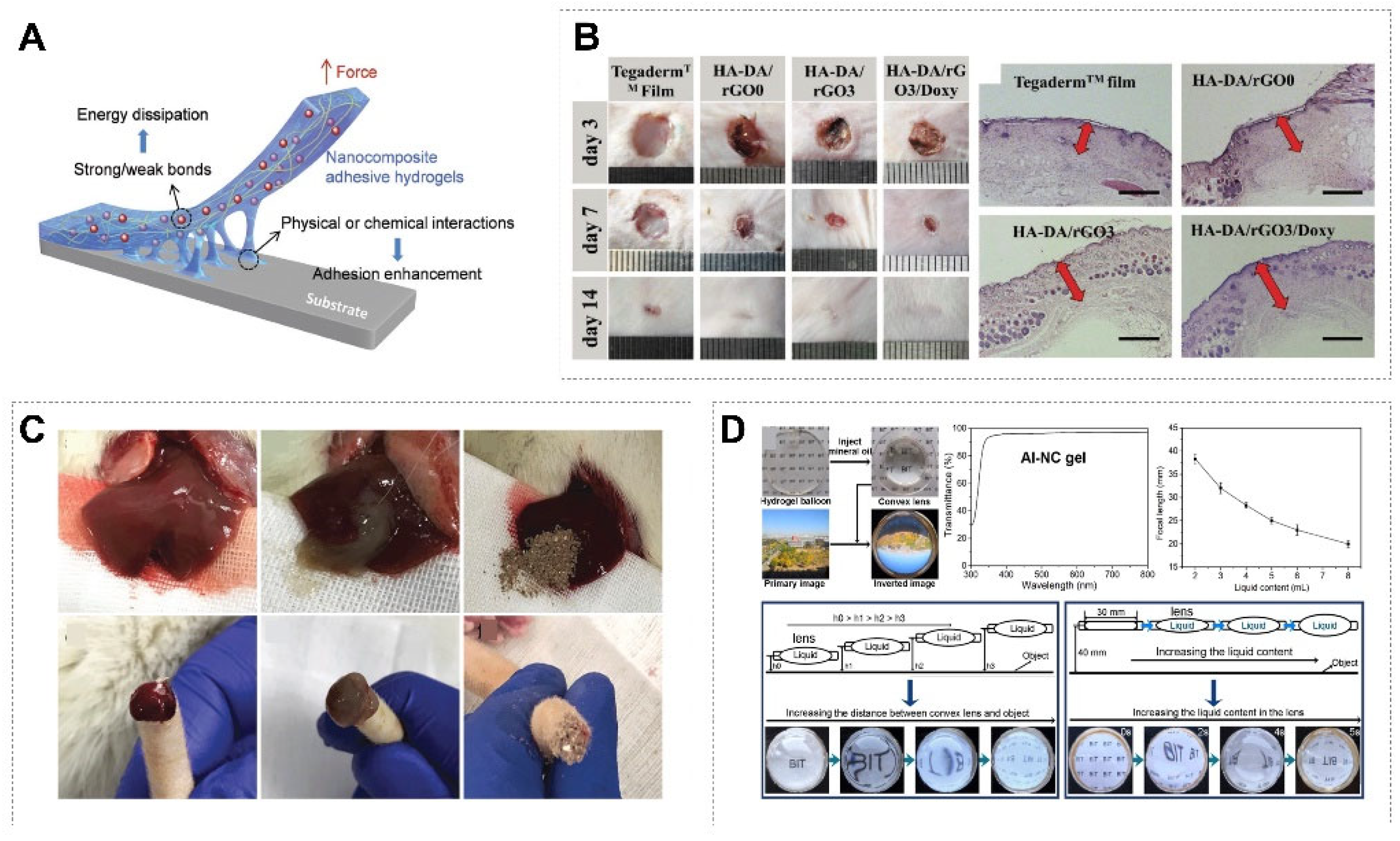
3.2.3. Skin
3.2.4. Nerve
3.3. Other Applications
4. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lutolf, M.P.; Hubbell, J.A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 2005, 23, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Klimek, K.; Ginalska, G. Proteins and Peptides as Important Modifiers of the Polymer Scaffolds for Tissue Engineering Applications—A Review. Polymers 2020, 12, 844. [Google Scholar] [CrossRef] [PubMed]
- Yoo, K.M.; Murphy, S.V.; Skardal, A. A Rapid Crosslinkable Maleimide-Modified Hyaluronic Acid and Gelatin Hydrogel Delivery System for Regenerative Applications. Gels 2021, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Yue, B. Biology of the extracellular matrix: An overview. J. Glaucoma 2014, 23, S20–S23. [Google Scholar] [CrossRef]
- Lu, P.; Takai, K.; Weaver, V.M.; Werb, Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb. Perspect. Biol. 2011, 3, a005058. [Google Scholar] [CrossRef]
- Geiger, B.; Yamada, K.M. Molecular architecture and function of matrix adhesions. Cold Spring Harb. Perspect. Biol. 2011, 3, a005033. [Google Scholar] [CrossRef]
- Trappmann, B.; Gautrot, J.E.; Connelly, J.T.; Strange, D.G.T.; Li, Y.; Oyen, M.L.; Stuart, M.A.C.; Boehm, H.; Li, B.J.; Vogel, V.; et al. Extracellular-matrix tethering regulates stem-cell fate. Nat. Mater. 2012, 11, 642–649. [Google Scholar] [CrossRef]
- Biondi, M.; Borzacchiello, A.; Mayol, L.; Ambrosio, L. Nanoparticle-Integrated Hydrogels as Multifunctional Composite Materials for Biomedical Applications. Gels 2015, 1, 162–178. [Google Scholar] [CrossRef]
- Pomari, A.A.D.; Montanheiro, T.L.D.; de Siqueira, C.P.; Silva, R.S.; Tada, D.B.; Lemes, A.P. Chitosan Hydrogels Crosslinked by Genipin and Reinforced with Cellulose Nanocrystals: Production and Characterization. J. Compos. Sci. 2019, 3, 84. [Google Scholar] [CrossRef]
- Deng, J.; Yuk, H.; Wu, J.J.; Varela, C.E.; Chen, X.Y.; Roche, E.T.; Guo, C.F.; Zhao, X.H. Electrical bioadhesive interface for bioelectronics. Nat. Mater. 2021, 20, 229–236. [Google Scholar] [CrossRef]
- Courtine, G.; Sofroniew, M.V. Spinal cord repair: Advances in biology and technology. Nat. Med. 2019, 25, 898–908. [Google Scholar] [CrossRef]
- Ham, T.R.; Leipzig, N.D. Biomaterial strategies for limiting the impact of secondary events following spinal cord injury. Biomed. Mater. 2018, 13, 024105. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.T.A.; Kim, Y.M.; Park, H.H.; Hwang, D.H.; Cui, Y.; Lee, E.M.; Yahn, S.; Lee, J.K.; Song, S.C.; Kim, B.G. An injectable hydrogel enhances tissue repair after spinal cord injury by promoting extracellular matrix remodeling. Nat. Commun. 2017, 8, 533. [Google Scholar] [CrossRef] [PubMed]
- Haggerty, A.E.; Maldonado-Lasunción, I.; Oudega, M. Biomaterials for revascularization and immunomodulation after spinal cord injury. Biomed. Mater. 2018, 13, 044105. [Google Scholar] [CrossRef] [PubMed]
- Azarfam, M.Y.; Nasirinezhad, M.; Naeim, H.; Zarrintaj, P.; Saeb, M. A Green Composite Based on Gelatin/Agarose/Zeolite as a Potential Scaffold for Tissue Engineering Applications. J. Compos. Sci. 2021, 5, 125. [Google Scholar] [CrossRef]
- Kakarla, A.B.; Kong, I.; Nukala, S.G.; Kong, W. Mechanical Behaviour Evaluation of Porous Scaffold for Tissue-Engineering Applications Using Finite Element Analysis. J. Compos. Sci. 2022, 6, 46. [Google Scholar] [CrossRef]
- Yin, B.H.; Yang, H.R.; Yang, M. Integrating Soft Hydrogel with Nanostructures Reinforces Stem Cell Adhesion and Differentiation. J. Compos. Sci. 2022, 6, 19. [Google Scholar] [CrossRef]
- Chaudhuri, O.; Cooper-White, J.; Janmey, P.A.; Mooney, D.J.; Shenoy, V.B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 2020, 584, 535–546. [Google Scholar] [CrossRef]
- Guimaraes, C.F.; Gasperini, L.; Marques, A.P.; Reis, R.L. The stiffness of living tissues and its implications for tissue engineering. Nat. Rev. Mater. 2020, 5, 351–370. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef]
- Kubow, K.E.; Conrad, S.K.; Horwitz, A.R. Matrix Microarchitecture and Myosin II Determine Adhesion in 3D Matrices. Curr. Biol. 2013, 23, 1607–1619. [Google Scholar] [CrossRef] [PubMed]
- Doyle, A.D.; Carvajal, N.; Jin, A.; Matsumoto, K.; Yamada, K.M. Local 3D matrix microenvironment regulates cell migration through spatiotemporal dynamics of contractility-dependent adhesions. Nat. Commun. 2015, 6, 8720. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.H.; Wang, H.X.; Fang, C.; Yang, Y.K.; Xia, X.Y.; Yang, B.G.; Lin, Y.; Li, G.; Bian, L.M. Microscopic local stiffening in a supramolecular hydrogel network expedites stem cell mechanosensing in 3D and bone regeneration. Mater. Horiz. 2021, 8, 1722–1734. [Google Scholar] [CrossRef] [PubMed]
- Rose, J.C.; Camara-Torres, M.; Rahimi, K.; Kohler, J.; Moller, M.; De Laporte, L. Nerve Cells Decide to Orient inside an Injectable Hydrogel with Minimal Structural Guidance. Nano Lett. 2017, 17, 3782–3791. [Google Scholar] [CrossRef]
- Yao, Z.; Yuan, W.H.; Xu, J.K.; Tong, W.X.; Mi, J.; Ho, P.C.; Chow, D.H.K.; Li, Y.; Yao, H.; Li, X.; et al. Magnesium-Encapsulated Injectable Hydrogel and 3D-Engineered Polycaprolactone Conduit Facilitate Peripheral Nerve Regeneration. Adv. Sci. 2022, 9, 2202102. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.M.; Kim, M.; Feng, Q.; Lin, S.; Wei, K.C.; Li, R.; Choi, C.J.; Kim, T.H.; Li, G.; Oh, J.M.; et al. Nanolayered hybrid mediates synergistic co-delivery of ligand and ligation activator for inducing stem cell differentiation and tissue healing. Biomaterials 2017, 149, 12–28. [Google Scholar] [CrossRef]
- He, J.H.; Shi, M.T.; Liang, Y.P.; Guo, B.L. Conductive adhesive self-healing nanocomposite hydrogel wound dressing for photothermal therapy of infected full-thickness skin wounds. Chem. Eng. J. 2020, 394, 124888. [Google Scholar] [CrossRef]
- Phuong, P.T.M.; Jhon, H.; In, I.; Park, S.Y. Photothermal-modulated reversible volume transition of wireless hydrogels embedded with redox-responsive carbon dots. Biomater. Sci. 2019, 7, 4800–4812. [Google Scholar] [CrossRef]
- Qin, J.; Asempah, I.; Laurent, S.; Fornara, A.; Muller, R.N.; Muhammed, M. Injectable Superparamagnetic Ferrogels for Controlled Release of Hydrophobic Drugs. Adv. Mater. 2009, 21, 1354–1357. [Google Scholar] [CrossRef]
- Han, L.; Zhang, Y.N.; Lu, X.; Wang, K.F.; Wang, Z.M.; Zhang, H.P. Polydopamine Nanoparticles Modulating Stimuli-Responsive PNIPAM Hydrogels with Cell/Tissue Adhesiveness. ACS Appl. Mater. Interfaces 2016, 8, 29088–29100. [Google Scholar] [CrossRef]
- Peng, Y.M.; Liu, Q.J.; He, T.L.; Ye, K.; Yao, X.; Ding, J.D. Degradation rate affords a dynamic cue to regulate stem cells beyond varied matrix stiffness. Biomaterials 2018, 178, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.H.D.; Wong, W.K.R.; Lai, C.H.N.; Oh, J.; Li, Z.; Chen, X.Y.; Yuan, W.H.; Bian, L.M. Soft Polymeric Matrix as a Macroscopic Cage for Magnetically Modulating Reversible Nanoscale Ligand Presentation. Nano Lett. 2020, 20, 3207–3216. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Pouraghaei Sevari, S.; Chen, C.; Sarrion, P.; Moshaverinia, A. RGD-Modified Alginate-GelMA Hydrogel Sheet Containing Gingival Mesenchymal Stem Cells: A Unique Platform for Wound Healing and Soft Tissue Regeneration. ACS Biomater. Sci. Eng. 2021, 7, 3774–3782. [Google Scholar] [CrossRef]
- Yuan, W.; Li, Z.; Xie, X.; Zhang, Z.Y.; Bian, L. Bisphosphonate-based nanocomposite hydrogels for biomedical applications. Bioact. Mater. 2020, 5, 819–831. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Hou, K.; Ren, Q.; Chen, G.; Wei, P.; Zhu, M. Nanoparticle–Polymer Synergies in Nanocomposite Hydrogels: From Design to Application. Macromol. Rapid Commun. 2018, 39, e1800337. [Google Scholar] [CrossRef] [PubMed]
- Vashist, A.; Kaushik, A.; Ghosal, A.; Bala, J.; Nikkhah-Moshaie, R.; W, A.W.; Manickam, P.; Nair, M. Nanocomposite Hydrogels: Advances in Nanofillers Used for Nanomedicine. Gels 2018, 4, 75. [Google Scholar] [CrossRef]
- Gaharwar, A.K.P.N.A.; Khademhosseini, A. Nanocomposite hydrogels for biomedical applications. Biotechnology and bioengineering. Biotechnol. Bioeng. 2014, 111, 441–453. [Google Scholar] [CrossRef]
- Zhang, K.; Lin, S.; Feng, Q.; Dong, C.; Yang, Y.; Li, G.; Bian, L. Nanocomposite hydrogels stabilized by self-assembled multivalent bisphosphonate-magnesium nanoparticles mediate sustained release of magnesium ion and promote in-situ bone regeneration. Acta Biomater. 2017, 64, 389–400. [Google Scholar] [CrossRef]
- Shi, L.; Han, Y.; Hilborn, J.; Ossipov, D. “Smart” drug loaded nanoparticle delivery from a self-healing hydrogel enabled by dynamic magnesium-biopolymer chemistry. Chem. Commun. 2016, 52, 11151–11154. [Google Scholar] [CrossRef]
- Amini-Fazl, M.S.; Mohammadi, R.; Kheiri, K. 5Fluorouracil loaded chitosan/polyacrylic acid/Fe3O4 magnetic nanocomposite hydrogel as a potential anticancer drug delivery system. Int. J. Biol. Macromol. 2019, 132, 506–513. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, H.; Gao, F.; Xu, Z.; Dai, F.; Liu, W. An Injectable Supramolecular Polymer Nanocomposite Hydrogel for Prevention of Breast Cancer Recurrence with Theranostic and Mammoplastic Functions. Adv. Funct. Mater. 2018, 28, 1801000. [Google Scholar] [CrossRef]
- Javanmardi, S.; Tamaddon, A.M.; Aghamaali, M.R.; Ghahramani, L.; Abolmaali, S.S. Redox-sensitive, PEG-shielded carboxymethyl PEI nanogels silencing MicroRNA-21, sensitizes resistant ovarian cancer cells to cisplatin. Asian J. Pharm. Sci. 2020, 15, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Xi, Y.; Xue, Y.; Wang, M.; Liu, Y.; Guo, Y.; Lei, B. Injectable Self-Healing Antibacterial Bioactive Polypeptide-Based Hybrid Nanosystems for Efficiently Treating Multidrug Resistant Infection, Skin-Tumor Therapy, and Enhancing Wound Healing. Adv. Funct. Mater. 2019, 29, 1806883. [Google Scholar] [CrossRef]
- Ohta, S.; Hiramoto, S.; Amano, Y.; Sato, M.; Suzuki, Y.; Shinohara, M.; Emoto, S.; Yamaguchi, H.; Ishigami, H.; Sakai, Y.; et al. Production of Cisplatin-Incorporating Hyaluronan Nanogels via Chelating Ligand-Metal Coordination. Bioconjug. Chem. 2016, 27, 504–508. [Google Scholar] [CrossRef]
- Pardo, A.; Gómez-Florit, M.; Barbosa, S.; Taboada, P.; Domingues, R.M.A.; Gomes, M.E. Magnetic Nanocomposite Hydrogels for Tissue Engineering: Design Concepts and Remote Actuation Strategies to Control Cell Fate. ACS Nano 2021, 15, 175–209. [Google Scholar] [CrossRef]
- Merino, S.; Martín, C.; Kostarelos, K.; Prato, M.; Vázquez, E. Nanocomposite hydrogels: 3D polymer-nanoparticle synergies for on-demand drug delivery. ACS Nano 2015, 9, 4686–4697. [Google Scholar] [CrossRef]
- Siegel, R.A.; Gu, Y.; Lei, M.; Baldi, A.; Nuxoll, E.E.; Ziaie, B. Hard and soft micro- and nanofabrication: An integrated approach to hydrogel-based biosensing and drug delivery. J. Control. Release 2010, 141, 303–313. [Google Scholar] [CrossRef]
- Du, W.Z.; Zong, Q.D.; Guo, R.R.; Ling, G.X.; Zhang, P. Injectable Nanocomposite Hydrogels for Cancer Therapy. Macromol. Biosci. 2021, 21, 2100186. [Google Scholar] [CrossRef]
- Xia, B.; Zhang, W.W.; Shi, J.S.; Li, J.C.; Chen, Z.Y.; Zhang, Q. NIR light-triggered gelling in situ of porous silicon nanoparticles/PEGDA hybrid hydrogels for localized combinatorial therapy of cancer cells. J. Appl. Polym. Sci. 2019, 136, 47443. [Google Scholar] [CrossRef]
- Li, W.; Deng, Y.; Chu, Q.; Zhang, P. Gut microbiome and cancer immunotherapy. Cancer Lett. 2019, 447, 41–47. [Google Scholar] [CrossRef]
- Wu, M.; Chen, J.S.; Huang, W.J.; Yan, B.; Peng, Q.Y.; Liu, J.F.; Chen, L.Y.; Zeng, H.B. Injectable and Self-Healing Nanocomposite Hydrogels with Ultrasensitive pH-Responsiveness and Tunable Mechanical Properties: Implications for Controlled Drug Delivery. Biomacromolecules 2020, 21, 2409–2420. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, P.; Li, P.; Xue, A.B.; Zhang, X.K.; Zhang, H.Y.; Jin, X.B. A magnetic chitosan hydrogel for sustained and prolonged delivery of Bacillus Calmette-Guerin in the treatment of bladder cancer. Biomaterials 2013, 34, 10258–10266. [Google Scholar] [CrossRef]
- Xu, X.Y.; Huang, Z.Y.; Huang, Z.Q.; Zhang, X.F.; He, S.Y.; Sun, X.Q.; Shen, Y.F.; Yan, M.N.; Zhao, C.S. Injectable, NIR/pH-Responsive Nanocomposite Hydrogel as Long-Acting Implant for Chemophotothermal Synergistic Cancer Therapy. ACS Appl. Mater. Inter. 2017, 9, 20361–20375. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Yan, L.W.; Qiu, S.; He, F.L.; Gu, F.B.; Liu, X.L.; Qi, J.; Zhu, Q.T. Customized Scaffold Design Based on Natural Peripheral Nerve Fascicle Characteristics for Biofabrication in Tissue Regeneration. BioMed Res. Int. 2019, 2019, 3845780. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Vacanti, J. Advances in tissue engineering. J. Pediatr. Surg. 2016, 51, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Guo, L.; Wei, G. Recent Advances in the Fabrication and Environmental Science Applications of Cellulose Nanofibril-Based Functional Materials. Materials 2021, 14, 5390. [Google Scholar] [CrossRef]
- Lin, K.T.; Wang, A.; Nguyen, A.B.; Iyer, J.; Tran, S.D. Recent Advances in Hydrogels: Ophthalmic Applications in Cell Delivery, Vitreous Substitutes, and Ocular Adhesives. Biomedicines 2021, 9, 1203. [Google Scholar] [CrossRef]
- Olate-Moya, F.; Arens, L.; Wilhelm, M.; Mateos-Timoneda, M.A.; Engel, E.; Palza, H. Chondroinductive Alginate-Based Hydrogels Having Graphene Oxide for 3D Printed Scaffold Fabrication. ACS Appl. Mater. Inter. 2020, 12, 4343–4357. [Google Scholar] [CrossRef]
- Zhang, K.Y.; Jia, Z.F.; Yang, B.G.; Feng, Q.; Xu, X.; Yuan, W.H.; Li, X.F.; Chen, X.Y.; Duan, L.; Wang, D.P.; et al. Adaptable Hydrogels Mediate Cofactor-Assisted Activation of Biomarker-Responsive Drug Delivery via Positive Feedback for Enhanced Tissue Regeneration. Adv. Sci. 2018, 5, 1800875. [Google Scholar] [CrossRef]
- Huang, S.S.; Liu, H.L.; Liao, K.D.; Hu, Q.Q.; Guo, R.; Deng, K.X. Functionalized GO Nanovehicles with Nitric Oxide Release and Photothermal Activity-Based Hydrogels for Bacteria-Infected Wound Healing. ACS Appl. Mater. Inter. 2020, 12, 28952–28964. [Google Scholar] [CrossRef]
- Huang, C.T.; Shrestha, L.K.; Ariga, K.; Hsu, S.H. A graphene-polyurethane composite hydrogel as a potential bioink for 3D bioprinting and differentiation of neural stem cells. J. Mater. Chem. B 2017, 5, 8854–8864. [Google Scholar] [CrossRef] [PubMed]
- Asadi, N.; Alizadeh, E.; Salehi, R.; Khalandi, B.; Davaran, S.; Akbarzadeh, A. Nanocomposite hydrogels for cartilage tissue engineering: A review. Artif. Cells Nanomed. Biotechnol. 2018, 46, 465–471. [Google Scholar] [CrossRef]
- Naranda, J.; Bracic, M.; Vogrin, M.; Maver, U. Recent Advancements in 3D Printing of Polysaccharide Hydrogels in Cartilage Tissue Engineering. Materials 2021, 14, 3977. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Shi, D.; Dong, L.; Zhang, Z.; Li, X.; Chen, M. Fabrication of polydopamine nanoparticles knotted alginate scaffolds and their properties. J. Biomed. Mater. Res. A 2018, 106, 3255–3266. [Google Scholar] [CrossRef] [PubMed]
- Piluso, S.; Labet, M.; Zhou, C.; Seo, J.W.; Thielemans, W.; Patterson, J. Engineered Three-Dimensional Microenvironments with Starch Nanocrystals as Cell-Instructive Materials. Biomacromolecules 2019, 20, 3819–3830. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xiong, J.; Wang, D.; Zhang, J.; Yang, L.; Sun, S.; Liang, Y. 3D Bioprinting of Hydrogels for Cartilage Tissue Engineering. Gels 2021, 7, 144. [Google Scholar] [CrossRef]
- Sultan, S.; Mathew, A.P. 3D Printed Porous Cellulose Nanocomposite Hydrogel Scaffolds. J. Vis. Exp. 2019, 146, e59401. [Google Scholar] [CrossRef]
- Tang, G.; Liu, Z.; Liu, Y.; Yu, J.; Wang, X.; Tan, Z.; Ye, X. Recent Trends in the Development of Bone Regenerative Biomaterials. Front. Cell Dev. Biol. 2021, 9, 665813. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, M.; He, J. A review of biomimetic scaffolds for bone regeneration: Toward a cell-free strategy. Bioeng. Transl. Med. 2021, 6, e10206. [Google Scholar] [CrossRef]
- Azami, M.; Moosavifar, M.J.; Baheiraei, N.; Moztarzadeh, F.; Ai, J. Preparation of a biomimetic nanocomposite scaffold for bone tissue engineering via mineralization of gelatin hydrogel and study of mineral transformation in simulated body fluid. J. Biomed. Mater. Res.-Part A 2012, 100, 1347–1355. [Google Scholar] [CrossRef]
- Yang, X.; Akhtar, S.; Rubino, S.; Leifer, K.; Hilborn, J.; Ossipov, D. Direct “Click” Synthesis of Hybrid Bisphosphonate–Hyaluronic Acid Hydrogel in Aqueous Solution for Biomineralization. Chem. Mater. 2012, 24, 1690–1697. [Google Scholar] [CrossRef]
- Barros, J.; Ferraz, M.P.; Azeredo, J.; Fernandes, M.H.; Gomes, P.S.; Monteiro, F.J. Alginate-nanohydroxyapatite hydrogel system: Optimizing the formulation for enhanced bone regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 105, 109985. [Google Scholar] [CrossRef] [PubMed]
- Diba, M.; Wang, H.; Kodger, T.E.; Parsa, S.; Leeuwenburgh, S.C.G. Highly Elastic and Self-Healing Composite Colloidal Gels. Adv. Mater. 2017, 29, 1604672. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.K.; Kim, S.; Baljon, J.J.; Wu, B.M.; Aghaloo, T.; Lee, M. Microporous methacrylated glycol chitosan-montmorillonite nanocomposite hydrogel for bone tissue engineering. Nat. Commun. 2019, 10, 3523. [Google Scholar] [CrossRef] [PubMed]
- Barbu, A.; Neamtu, B.; Zahan, M.; Iancu, G.M.; Bacila, C.; Miresan, V. Current Trends in Advanced Alginate-Based Wound Dressings for Chronic Wounds. J. Pers. Med. 2021, 11, 890. [Google Scholar] [CrossRef]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef]
- Shi, L.; Zhao, Y.; Xie, Q.; Fan, C.; Hilborn, J.; Dai, J.; Ossipov, D.A. Moldable Hyaluronan Hydrogel Enabled by Dynamic Metal-Bisphosphonate Coordination Chemistry for Wound Healing. Adv. Healthc. Mater. 2018, 7, 1–9. [Google Scholar] [CrossRef]
- Rakhshaei, R.; Namazi, H.; Hamishehkar, H.; Kafil, H.S.; Salehi, R. In situ synthesized chitosan-gelatin/ZnO nanocomposite scaffold with drug delivery properties: Higher antibacterial and lower cytotoxicity effects. J. Appl. Polym. Sci. 2019, 136, 47590. [Google Scholar] [CrossRef]
- Boni, R.; Ali, A.; Shavandi, A.; Clarkson, A.N. Current and novel polymeric biomaterials for neural tissue engineering. J. Biomed. Sci. 2018, 25, 90. [Google Scholar] [CrossRef]
- Yao, Z.; Yan, L.W.; Wang, T.; Qiu, S.; Lin, T.; He, F.L.; Yuan, R.H.; Liu, X.L.; Qi, J.; Zhu, Q.T. A rapid micro-magnetic resonance imaging scanning for three-dimensional reconstruction of peripheral nerve fascicles. Neural Regen. Res. 2018, 13, 1953–1960. [Google Scholar]
- Dalamagkas, K.; Tsintou, M.; Seifalian, A. Advances in peripheral nervous system regenerative therapeutic strategies: A biomaterials approach. Mater. Sci. Eng. C 2016, 65, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.C.; Chen, Q.; Dai, Z.W.; Dai, Y.; Xia, F.; Zhang, X.J. Nanocomposite adhesive hydrogels: From design to application. J. Mater. Chem. B 2021, 9, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.P.; Zhao, X.; Hu, T.L.; Chen, B.J.; Yin, Z.H.; Ma, P.X.; Guo, B.L. Adhesive Hemostatic Conducting Injectable Composite Hydrogels with Sustained Drug Release and Photothermal Antibacterial Activity to Promote Full-Thickness Skin Regeneration During Wound Healing. Small 2019, 15, e1900046. [Google Scholar] [CrossRef]
- Sun, Z.; Chen, X.Y.; Ma, X.M.; Cui, X.X.; Yi, Z.; Li, X.D. Cellulose/keratin-catechin nanocomposite hydrogel for wound hemostasis. J. Mater. Chem. B 2018, 6, 6133–6141. [Google Scholar] [CrossRef] [PubMed]
- Li, F.B.; Zhang, G.Z.; Wang, Z.S.; Jiang, H.Y.; Yan, S.; Zhang, L.; Li, H.J. Strong Wet Adhesion of Tough Transparent Nanocomposite Hydrogels for Fast Tunable Focus Lenses. ACS Appl. Mater. Inter. 2019, 11, 15071–15078. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, Z.; Xu, J.; Shen, J.; Qin, L.; Yuan, W. Biomimetic Hierarchical Nanocomposite Hydrogels: From Design to Biomedical Applications. J. Compos. Sci. 2022, 6, 340. https://doi.org/10.3390/jcs6110340
Yao Z, Xu J, Shen J, Qin L, Yuan W. Biomimetic Hierarchical Nanocomposite Hydrogels: From Design to Biomedical Applications. Journal of Composites Science. 2022; 6(11):340. https://doi.org/10.3390/jcs6110340
Chicago/Turabian StyleYao, Zhi, Jiankun Xu, Jun Shen, Ling Qin, and Weihao Yuan. 2022. "Biomimetic Hierarchical Nanocomposite Hydrogels: From Design to Biomedical Applications" Journal of Composites Science 6, no. 11: 340. https://doi.org/10.3390/jcs6110340
APA StyleYao, Z., Xu, J., Shen, J., Qin, L., & Yuan, W. (2022). Biomimetic Hierarchical Nanocomposite Hydrogels: From Design to Biomedical Applications. Journal of Composites Science, 6(11), 340. https://doi.org/10.3390/jcs6110340







