Thermoplastic Composites: Modelling Melting, Decomposition and Combustion of Matrix Polymers
Abstract
:1. Introduction
2. Theoretical Model
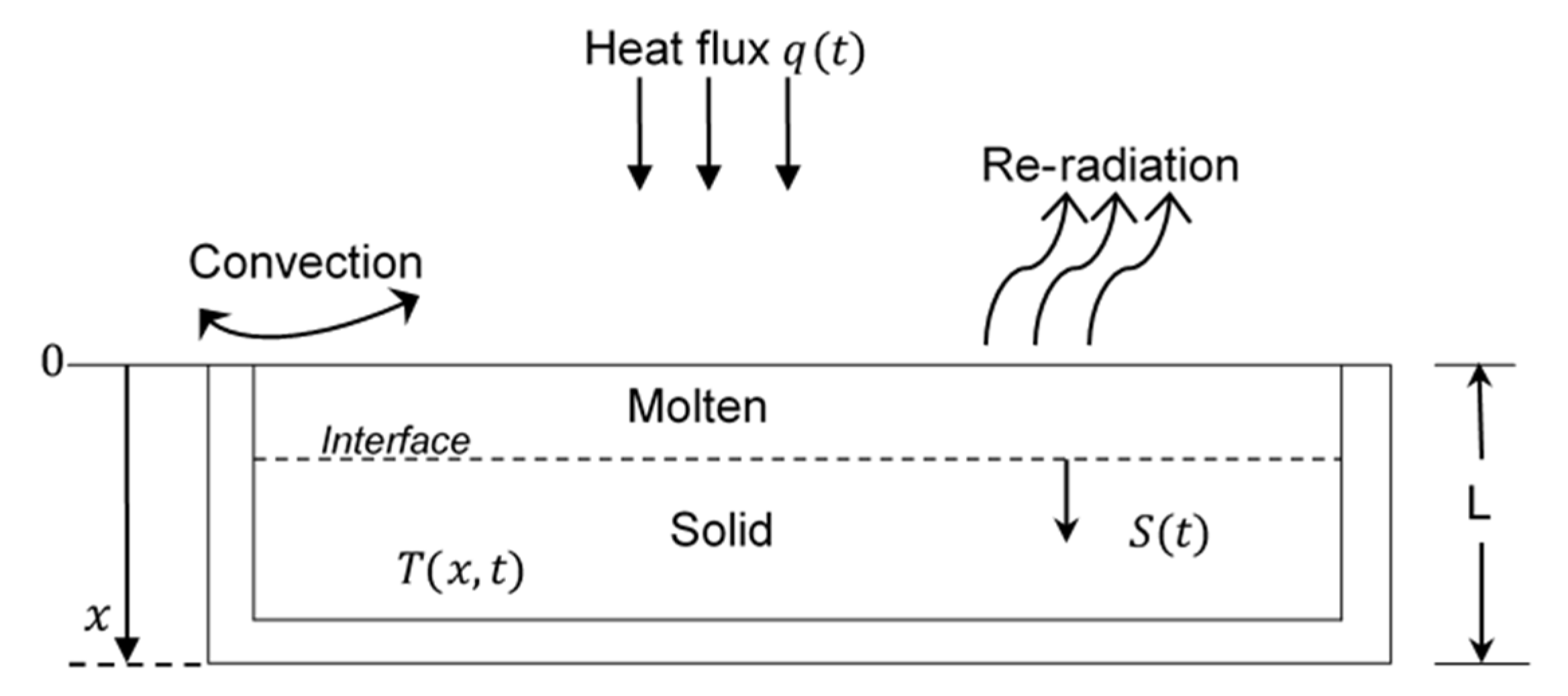
2.1. Heat Transfer Model with Moving Boundary Condition
2.1.1. Theoretical Approach
2.1.2. Model Description
2.2. Model Incorporating Ignition Behaviour
2.2.1. Theory
2.2.2. Numerical Method to Predict Temperature Behaviour
3. Materials and Methods
3.1. Materials and Sample Preparation
- Polypropylene (PP), Moplen HP516R, LyondellBasell, Warrington, UK;
- Polyamide 6 (PA6), Technyl C 301 Natural, Rhodia, Saint Frons, France;
- Polyethylene terephtalate (PET, polyester) received from Fibre Extrusion Technology, Leeds, UK.
3.2. Thermal Analysis
3.3. Measurement of Temperatures of Horizontally Oriented Samples Exposed to Radiant Heat in a Cone Calorimeter
4. Results and Discussion
4.1. Thermal Analytical Results
4.2. Input Parameter and Sensitivity Analysis
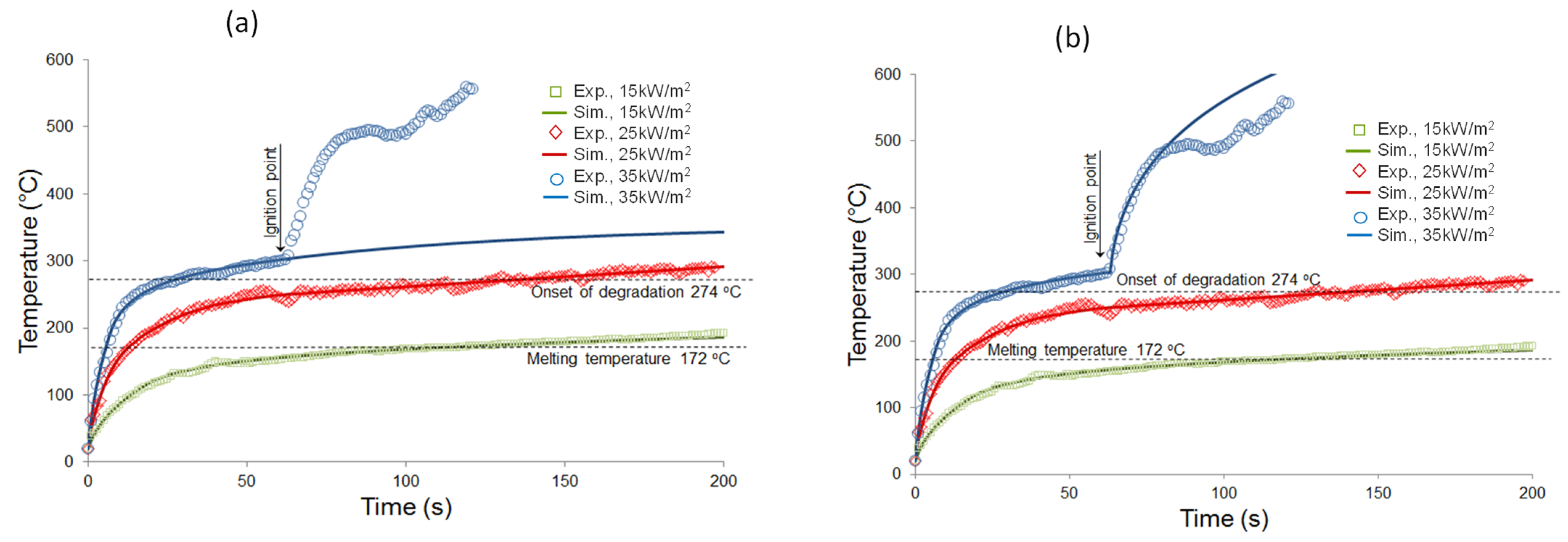
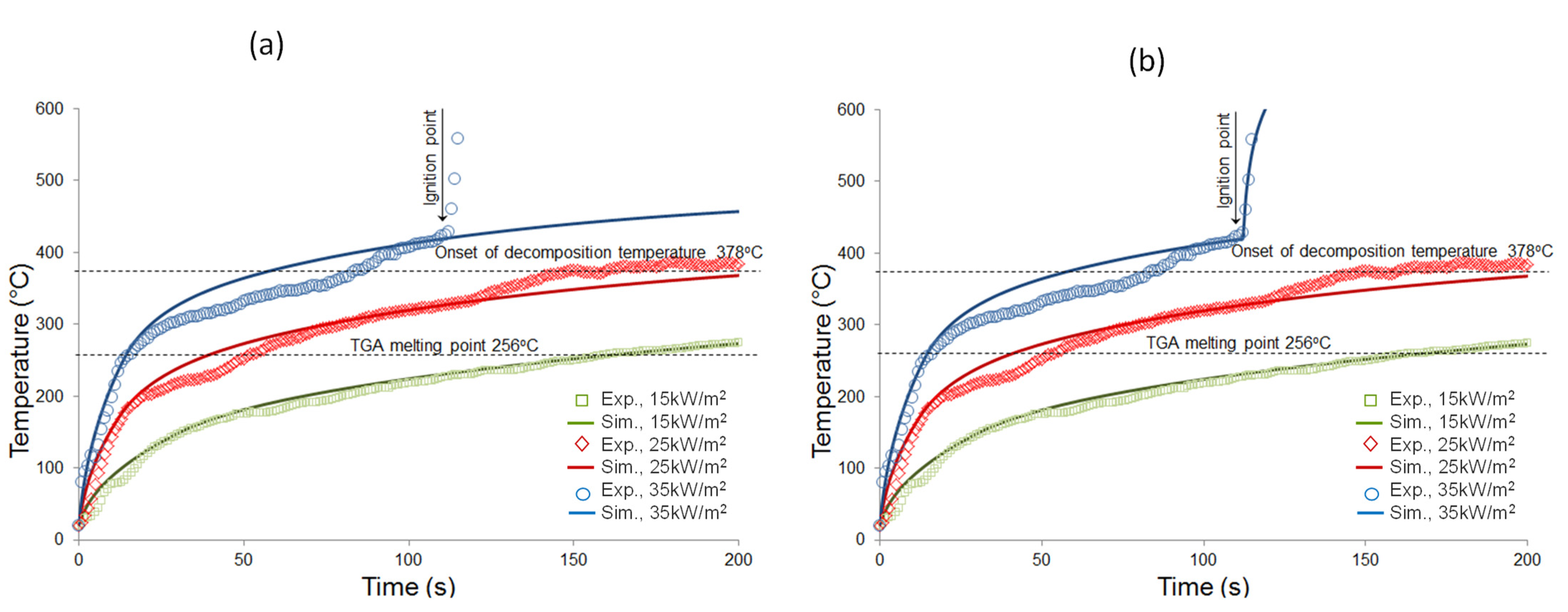
4.3. Simulation Results
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.; Shields, T.J.; Silcock, G.W.H. Effect of melting behaviour on upward flame spread of thermoplastics. Fire Mater. 1997, 21, 1–6. [Google Scholar] [CrossRef]
- Ohlemiller, T.J.; Butler, K. Influence of Polymer Melt Behaviour on Flammability 2000. Available online: https://nvlpubs.nist.gov/nistpubs/Legacy/IR/nistir6588v1.pdf (accessed on 18 December 2020).
- Kandola, B.K.; Price, D.; Milnes, G.J.; Da Silva, A. Development of a novel experimental technique for quantitative study of melt dripping of thermoplastic polymers. Polym. Degrad. Stab. 2013, 98, 52–63. [Google Scholar] [CrossRef]
- Kandola, B.K.; Ndiaye, M.; Price, D. Quantification of polymer degradation during melt dripping of thermoplastic polymers. Polym. Degrad. Stab. 2014, 106, 16–25. [Google Scholar] [CrossRef]
- Wisniak, J. Josef Stefan: Radiation, conductivity, diffusion, and other phenomena. Rev. CENIC Cienc. Químicas 2006, 37, 188–195. [Google Scholar]
- Di Blasi, C.; Crescitelli, S.; Russo, G.; Cinque, G. Numerical model of ignition processes of polymeric materials including gas-phase absorption of radiation. Combust. Flame 1991, 83, 333–344. [Google Scholar] [CrossRef]
- Di Blasi, C. Modeling of solid- and gas-phase processes during thermal degradation of composite materials. Polym. Degrad. Stab. 1996, 54, 241–248. [Google Scholar] [CrossRef]
- Di Blasi, C. Numerical simulation of cellulose pyrolysis. Biomass Bioenergy 1994, 7, 87–98. [Google Scholar] [CrossRef]
- Criado, J.M.; Pérez-Maqueda, L.A. Sample controlled thermal analysis and kinetics. J. Therm. Anal. Calorim. 2005, 80, 27–33. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Wight, C.A. Isothermal and nonisothermal reaction kinetics in solids: In search of ways toward consensus. J. Phys. Chem. A 1979, 101, 8279–8284. [Google Scholar] [CrossRef]
- Henderson, J.B.; Wiebelt, J.A.; Tant, M.R. A model for the thermal response of polymer composite materials with experimental verification. J. Comp. Mat. 1985, 19, 579–595. [Google Scholar] [CrossRef]
- Staggs, J.E.J. Ignition of char-forming polymers at a critical heat flux. Polym. Degrad. Stab. 2001, 74, 433–439. [Google Scholar] [CrossRef]
- Di Blasi, C. Transition between regimes in the degradation of thermoplastic polymers. Polym. Degrad. Stab. 1999, 64, 359–367. [Google Scholar] [CrossRef]
- Chartoff, R.C. Thermoplastic polymers. In Thermal Characterization of Polymeric Materials, 2nd ed.; Turi, E.A., Ed.; Academic Press: New York, NY, USA, 1997; pp. 621–625. [Google Scholar]
- Stefan, J. Ueber die theorie der eisbildung, insbesondere uber die eisbildung im polaemeere. Ann. Der Phys. Chem. 1891, 42, 269–286. [Google Scholar] [CrossRef] [Green Version]
- Nyazika, T.; Jimenez, M.; Samyn, F.; Bourbigot, S. Pyrolysis modelling, sensitivity analysis, and optimization techniques for combustible materials: A review. J. Fire Sci. 2019, 37, 377–433. [Google Scholar] [CrossRef]
- Salva, N.N.; Tarzia, D.A. A sensitivity analysis for the determination of unknown thermal coefficients through a phase-change process with temperature-dependent thermal conductivity. Int. Commun. Heat Mass Transf. 2011, 38, 418–424. [Google Scholar] [CrossRef]
- Kurschner, P.; Maki-Marttunen, T.; Vestergaard, S.; Wandl, S. Modelling and simulation of ice/snow melting. In Proceedings of the 22nd ECMI Modelling Week, Eindhoven, The Netherlands, 31 October 2008. [Google Scholar]
- Lyon, R.E.; Quintiere, J.G. Criteria for piloted ignition of combustible solids. Combust. Flame 2007, 151, 551–559. [Google Scholar] [CrossRef]
- Thompson, E.V. Thermal properties. In Encyclopedia of Polymer Science and Engineering; Mark, H.F., Ed.; John Wiley & Sons: New York, NY, USA, 1989; Volume 16, pp. 711–747. [Google Scholar]
- Crank, J. Two method for the numerical solution of moving-boundary problems in diffusion and heat flow. Quart. J. Mech. Appl. Math. 1957, 10, 220–231. [Google Scholar] [CrossRef]
- Crank, J. Fire and Moving Boundary Problem; Clarendon Press: Oxford, UK, 1984. [Google Scholar]
- Poirier, D.; Salcudean, M. On numerical methods used in mathematical modelling of phase change in liquid metal. J. Heat Transf. 1988, 110, 562–569. [Google Scholar] [CrossRef]
- Bradean, R.; Ingham, D.B.; Heggs, P.J. Stefan problem in blow moulding operations. In Mathematics of Heat Transfer; Tupholme, G.E., Wood, A.S., Eds.; Oxford Clarendon Press: Oxford, UK, 1998; pp. 89–96. [Google Scholar]
- Ohlemiller, T.; Shields, J.; Butler, K.; Collins, B.; Seck, M. Exploring the role of polymer melt viscosity in melt flow and flammability behavior. In Proceedings of the Fall Conference of the Fire Retardant Chemicals Association, Ponte Vehdra, FL, USA, 15–18 October 2000. [Google Scholar]
- Šarler, B. Stefan’s work on solid-liquid phase change. Eng. Anal. Bound. Elem. 1995, 16, 83–92. [Google Scholar] [CrossRef]
- ISO 5660-1:2015. Reaction-To-Fire Tests—Heat Release, Smoke Production and Mass Loss Rate—Part 1: Heat Release Rate (Cone Calorimeter Method) and Smoke Production Rate (Dynamic Measurement); International Organization for Standardization: Geneva, Switzerland, 2015. [Google Scholar]
- Zhang, S.; Horrocks, A.R. A Review of fire retardant polypropylene Fibres. Prog. Polym. Sci. 2003, 28, 1517–1538. [Google Scholar] [CrossRef]
- Mark, J.E. (Ed.) Physical Properties of Polymers Handbook, 2nd ed.; Springer Science: New York, NY, USA, 2007; ISBN 978-0-387-69002-5. [Google Scholar]
- Vyazovkin, S. Modification of the integral isoconversional method to account for variation in the activation energy. J. Compt. Chem. 2001, 22, 178–183. [Google Scholar] [CrossRef]
- Onate, E.; Marti, J.; Ryzhakov, P.; Rossi, R.; Idelsohn, S.R. Analysis of the melting, burning and flame spread of polymers with the particle finite element method. Comput. Assist. Methods Eng. Sci. 2013, 20, 165–184. [Google Scholar]




| Polymer | TGA Analysis | Glass Transition Temp. b (°C) | Melting Temp. b (°C) | |
|---|---|---|---|---|
| TOnset a | DTG Maxima (°C) | |||
| PP | 274 (415) | 367 (459) | −26 | 172 |
| PA6 | 372 (375) | 434, 458 (456) | 54 | 225 |
| PET | 378 (397) | 429, 446, 538 (439) | 68 | 256 |
| Polymer | Thermal Conductivity, k (W/m·k) | Specific Heat Capacity, cp (J/kg·k) | Density, ρ (kg/m3) |
|---|---|---|---|
| PP | 0.12 | 1622 | 910 |
| PA6 | 0.15 | 1172 | 1350 |
| PET | 0.24 | 1502 | 1177 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ndiaye, M.; Myler, P.; Kandola, B.K. Thermoplastic Composites: Modelling Melting, Decomposition and Combustion of Matrix Polymers. J. Compos. Sci. 2022, 6, 27. https://doi.org/10.3390/jcs6010027
Ndiaye M, Myler P, Kandola BK. Thermoplastic Composites: Modelling Melting, Decomposition and Combustion of Matrix Polymers. Journal of Composites Science. 2022; 6(1):27. https://doi.org/10.3390/jcs6010027
Chicago/Turabian StyleNdiaye, Mamadou, Peter Myler, and Baljinder K. Kandola. 2022. "Thermoplastic Composites: Modelling Melting, Decomposition and Combustion of Matrix Polymers" Journal of Composites Science 6, no. 1: 27. https://doi.org/10.3390/jcs6010027
APA StyleNdiaye, M., Myler, P., & Kandola, B. K. (2022). Thermoplastic Composites: Modelling Melting, Decomposition and Combustion of Matrix Polymers. Journal of Composites Science, 6(1), 27. https://doi.org/10.3390/jcs6010027








