DMSO Deintercalation in Kaolinite–DMSO Intercalate: Influence of Solution Polarity on Removal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results
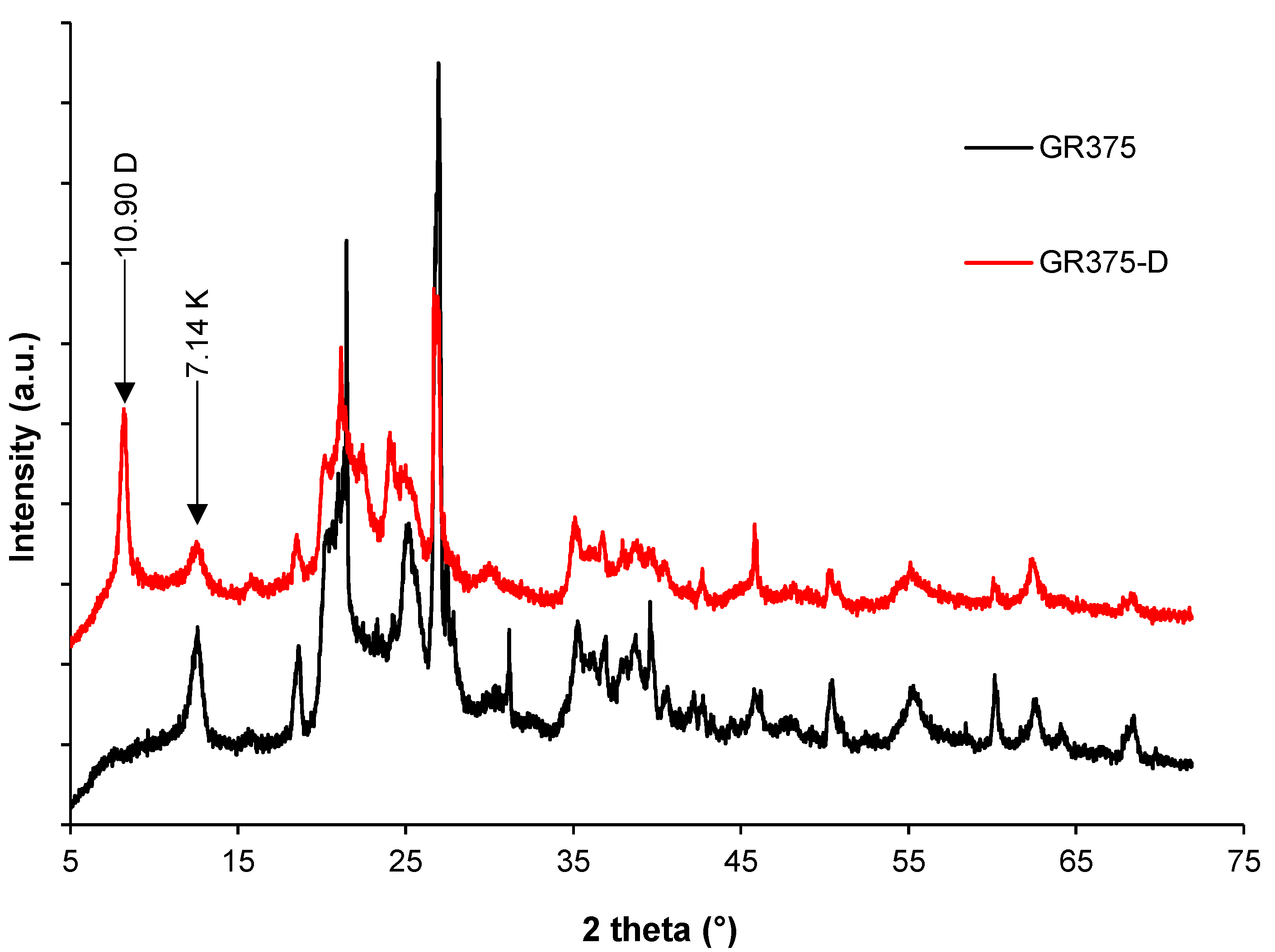
3.1. DMSO Intercalation: Structural Evolution by XRD, FTIR, and DSC
3.2. DMSO Deintercalation and Structural Evolution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Sadasivuni, K.K.; Saha, P.; Adhikari, J.; Deshmukh, K.; Ahmed, M.B.; Cabubuhan, J.-J. Recent advances in mechanical properties of biopolymer composites: A review. Polym. Compos. 2020, 4, 32–59. [Google Scholar] [CrossRef]
- Luo, J.-J.; Daniel, I.M. Characterization and modeling of mechanical behavior of polymer/clay nanocomposites. Compos. Sci. Technol. 2003, 63, 1607–1616. [Google Scholar] [CrossRef]
- Mbey, J.A.; Hoppe, S.; Thomas, F. Cassava starch-kaolinite composite films. Thermal and mechanical properties related to filler-matrix interactions. Polym. Compos. 2015, 36, 184–191. [Google Scholar] [CrossRef]
- De Carvalho, A.J.F.; Curvelo, A.A.S.; Agnelli, J.A.M. A first insight on composites of thermoplastic starch and kaolin. Carbohydr. Polym. 2001, 45, 189–194. [Google Scholar] [CrossRef]
- Wilhelm, H.M.; Sierakowski, M.R.; Souza, G.P.; Wypych, F. The influence of layered compounds on the properties of starch/layered compounds composites. Polym. Inter. 2003, 52, 1035–1044. [Google Scholar] [CrossRef]
- Chen, B.; Evans, J.R.G. Thermoplastic starch–clay nanocomposites and their characteristics. Carbohydr. Polym. 2005, 61, 455–463. [Google Scholar] [CrossRef]
- Dintcheva, N.T.; Al-Malaika, S. Photo-stabilization of biopolymers-based nanocomposites with UV-modified layered silicates. Polym. Degrad. Stab. 2020, 179, 109252. [Google Scholar] [CrossRef]
- Murray, H.H. Kaolin minerals: Their genesis and occurrences. In Hydrous Phyllosilicates; Bailey, S.W., Ed.; Mineralogical Society of America: Madison, WI, USA, 1988; Volume 19, pp. 67–89. [Google Scholar]
- Mbey, J.A.; Hoppe, S.; Thomas, F. Cassava starch-kaolinite composite film. Effect of clay content and clay modification on film properties. Carbohydr. Polym. 2012, 88, 213–222. [Google Scholar] [CrossRef]
- Giese, R.F. Kaolin Minerals: Structures and Stabilities. In Hydrous Phyllosilicates; Bailey, S.W., Ed.; Mineralogical Society of America: Chantilly, VA, USA, 1988; pp. 29–66. [Google Scholar]
- Cabedo, L.; Giménez, E.; Lagaron, J.M.; Gavara, R.; Saura, J.J. Development of EVOH kaolinite nanocomposites. Polymers 2004, 45, 5233–5238. [Google Scholar] [CrossRef]
- Mbey, J.A.; Thomas, F.; Ngally Sabouang, C.J.; Liboum Njopwouo, D. An insight on the weakening of the interlayer bonds in a Cameroonian kaolinite through DMSO intercalation. Appl. Clay Sci. 2013, 83–84, 327–335. [Google Scholar] [CrossRef]
- Olejnik, V.S.; Posner, A.M.; Quirk, J.P. The intercalation of polar organic compound into kaolinite. Clay Miner. 1970, 8, 421–434. [Google Scholar] [CrossRef]
- Tunney, J.J.; Detellier, C. Interlamellar covalent grafting of organic units on kaolinite. Chem. Mater. 1993, 5, 141–148. [Google Scholar] [CrossRef]
- Costanzo, P.M.; Giese, R.E. Ordered halloysite: Dimethylsulfoxide intercalate. Clays Clay. Miner. 1986, 34, 105–107. [Google Scholar] [CrossRef]
- Frost, R.L.; Kristóf, J.; Paroz, G.N.; Kloprogge, J.T. Intercalation of kaolinite with acetamide. Phys. Chem. Miner. 1999, 26, 257–263. [Google Scholar] [CrossRef]
- Frost, R.L.; Makó, E.; Kristóf, J.; Horváth, E.; Cseh, T. The effect of mechanochemical activation upon the intercalation of a high-defect kaolinite with formamide. J. Colloid Interface Sci. 2003, 265, 386–395. [Google Scholar] [CrossRef]
- Yanfeng, L.; Sun, D.; Pan, X.; Zhang, B. Kaolinite intercalation precursors. Clays Clay Miner. 2009, 57, 779–786. [Google Scholar]
- Zhang, S.; Liu, Q.; Yang, Y.; Wang, D.; He, J.; Sun, L. Preparation, morphology, and structure of kaolinites with various aspect ratios. Appl. Clay Sci. 2017, 147, 117–122. [Google Scholar] [CrossRef]
- Makó, E.; Kovács, A.; Kristóf, T. Influencing parameters of direct homogenization intercalation of kaolinite with urea, dimethyl sulfoxide, formamide, and N-methylformamide. Appl. Clay Sci. 2019, 182, 105287. [Google Scholar] [CrossRef]
- Makó, E.; Kristóf, J.; Horváth, E.; Vágvölgyi, V. Mechanochemical intercalation of low reactivity kaolinite. Appl. Clay Sci. 2013, 83–84, 24–31. [Google Scholar] [CrossRef]
- Mbey, J.A.; Siewe, J.M.; Ngally Sabouang, C.J.; Razafitianamaharavo, A.; Kong, S.; Thomas, F. DMSO Intercalation in Selected Kaolinites: Influence of the Crystallinity. ChemEngineering 2020, 4, 66. [Google Scholar] [CrossRef]
- Kaewtatip, K.; Tanrattanakul, V. Structure and properties of pregelatinized cassava starch/kaolin composites. Mater. Des. 2012, 37, 423–428. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Q.; Zhang, S.; Zhang, Y.; Zhang, Y.; Liang, P. Characterization of kaolinite/styrene butadiene rubber composite: Mechanical properties and thermal stability. Appl. Clay Sci. 2016, 124–125, 167–174. [Google Scholar] [CrossRef]
- Mbey, J.A.; Thomas, F.; Hoppe, S. Kaolinite dispersion in cassava starch based composite films. A photonic microscopy and x-ray tomography study. J. Polym. Eng. 2018, 38, 641–647. [Google Scholar] [CrossRef]
- Ndjigui, P.-D.; Onana, V.L.; Sababa, E.; Bayiga, E.C. Mineralogy and geochemistry of the Lokoundje alluvial clays from the Kribi deposits, Cameroonian Atlantic coast: Implications for their origin and depositional environment. J. Afr. Earth Sci. 2018, 143, 102–117. [Google Scholar] [CrossRef]
- Reichardt, C.; Welten, T. Solvents and Solvent Effects in Organic Chemistry, 4th ed.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011; 718p, ISBN 978-3-527-32473-6. [Google Scholar]
- Fang, Q.; Huang, S.; Wang, W. Intercalation of dimethylsulfoxide in kaolinite: Molecular dynamics simulation study. Chem. Physic. Lett. 2005, 411, 233–237. [Google Scholar] [CrossRef]
- Wang, B.X.; Zhao, X.P. The influence of intercalation rate and degree of substitution on the electrorheological activity of a novel ternary intercalated nanocomposite. J. Solid State Chem. 2006, 179, 949–954. [Google Scholar] [CrossRef]
- Ledoux, R.L.; White, J.L. Infrared studies of hydrogen bonding interaction between kaolinite surfaces and intercalated potassium acetate, hydrazine, formamide, and urea. J. Colloid Interface Sci. 1966, 21, 127–152. [Google Scholar] [CrossRef]
- Cases, J.M.; Lietard, O.; Yvon, J.; Delon, J.F. Etude des propriétés cristallochimiques, morphologiques, superficielles de kaolinites désordonnées. Bull. Minéral. 1982, 105, 439–455. [Google Scholar] [CrossRef]





| Solvent | Polarity | Chemical Grade (Purity) |
|---|---|---|
| Distilled water | 1 | |
| Acetone | 0.355 | Analytical (98%) |
| Ethyl acetate | 0.228 | Analytical (>98%) |
| Sample | Crystallite Size L (Å) | Number of Layer/Sheet |
|---|---|---|
| GR375 | 80 | 11 |
| GR375 D | 130 | 12 |
| GR375 AC | 50 | 07 |
| GR375 AE | 48 | 07 |
| GR375 E | 45 | 06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
ZOGO MFEGUE, B.; MBEY, J.A.; COULIBALY, S.L.; ONANA, V.L.; NDJIGUI, P.-D. DMSO Deintercalation in Kaolinite–DMSO Intercalate: Influence of Solution Polarity on Removal. J. Compos. Sci. 2021, 5, 97. https://doi.org/10.3390/jcs5040097
ZOGO MFEGUE B, MBEY JA, COULIBALY SL, ONANA VL, NDJIGUI P-D. DMSO Deintercalation in Kaolinite–DMSO Intercalate: Influence of Solution Polarity on Removal. Journal of Composites Science. 2021; 5(4):97. https://doi.org/10.3390/jcs5040097
Chicago/Turabian StyleZOGO MFEGUE, Berenger, Jean Aimé MBEY, Sandotin Lassina COULIBALY, Vincent Laurent ONANA, and Paul-Désiré NDJIGUI. 2021. "DMSO Deintercalation in Kaolinite–DMSO Intercalate: Influence of Solution Polarity on Removal" Journal of Composites Science 5, no. 4: 97. https://doi.org/10.3390/jcs5040097
APA StyleZOGO MFEGUE, B., MBEY, J. A., COULIBALY, S. L., ONANA, V. L., & NDJIGUI, P.-D. (2021). DMSO Deintercalation in Kaolinite–DMSO Intercalate: Influence of Solution Polarity on Removal. Journal of Composites Science, 5(4), 97. https://doi.org/10.3390/jcs5040097






