Effect of Hydrogels Containing Nanosilica on the Properties of Cement Pastes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Hydrogels
2.1.2. Cement Pastes
2.2. Experimental Methods
2.2.1. Absorption Test
2.2.2. Compressive Strength Test
2.2.3. Electrical Resistivity Test (EIS)
2.2.4. Autogenous Shrinkage Test
2.2.5. TGA
2.2.6. Interaction between Hydrogels and Cement Paste Matrix
2.2.7. Pozzolanic Potential of the Hydrogels
3. Results and Discussion
3.1. Absorption
3.2. Compressive Strength
3.3. Electrical Resistivity
3.4. Autogenous Shrinkage
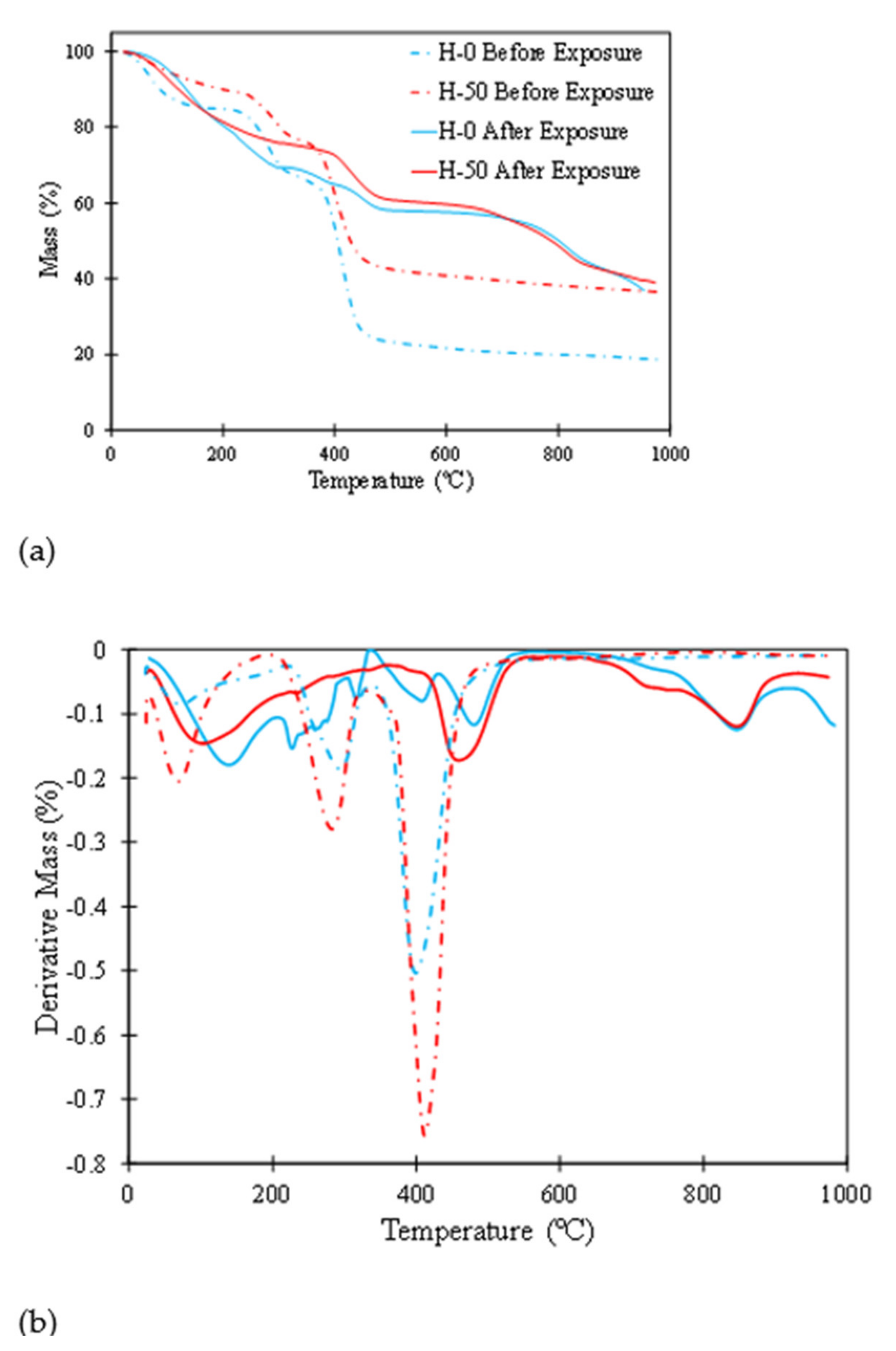
3.5. TGA
3.6. Interaction between Hydrogel Rod and Cement Paste
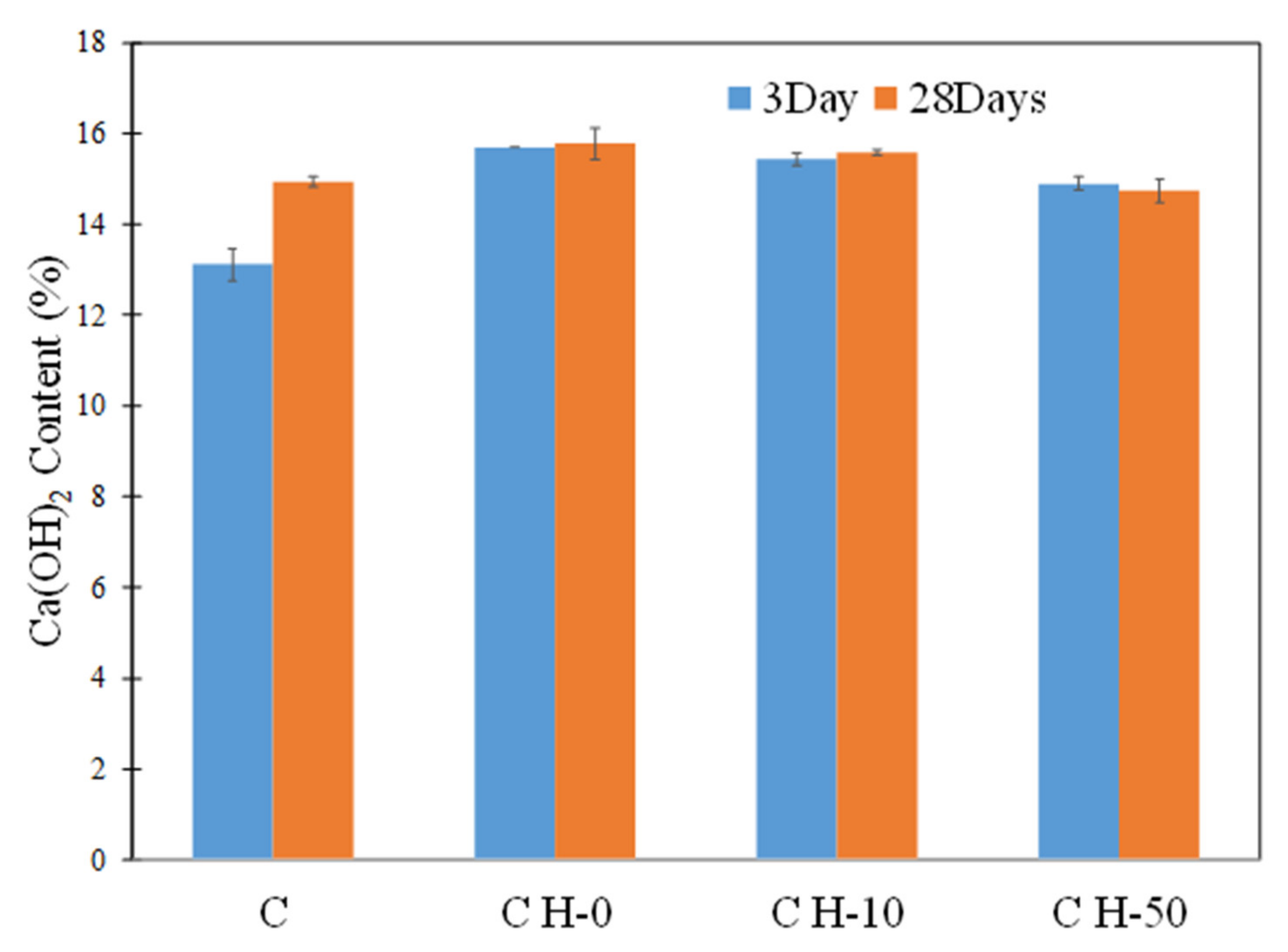
3.7. Pozzolanic Potential of Hydrogels
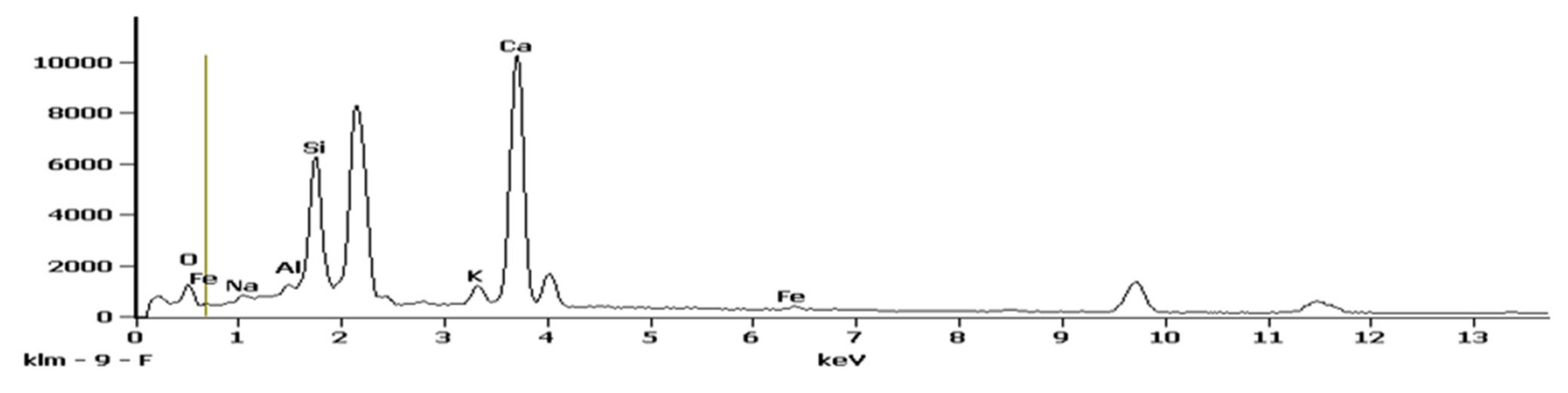
3.7.1. FTIR
3.7.2. TGA
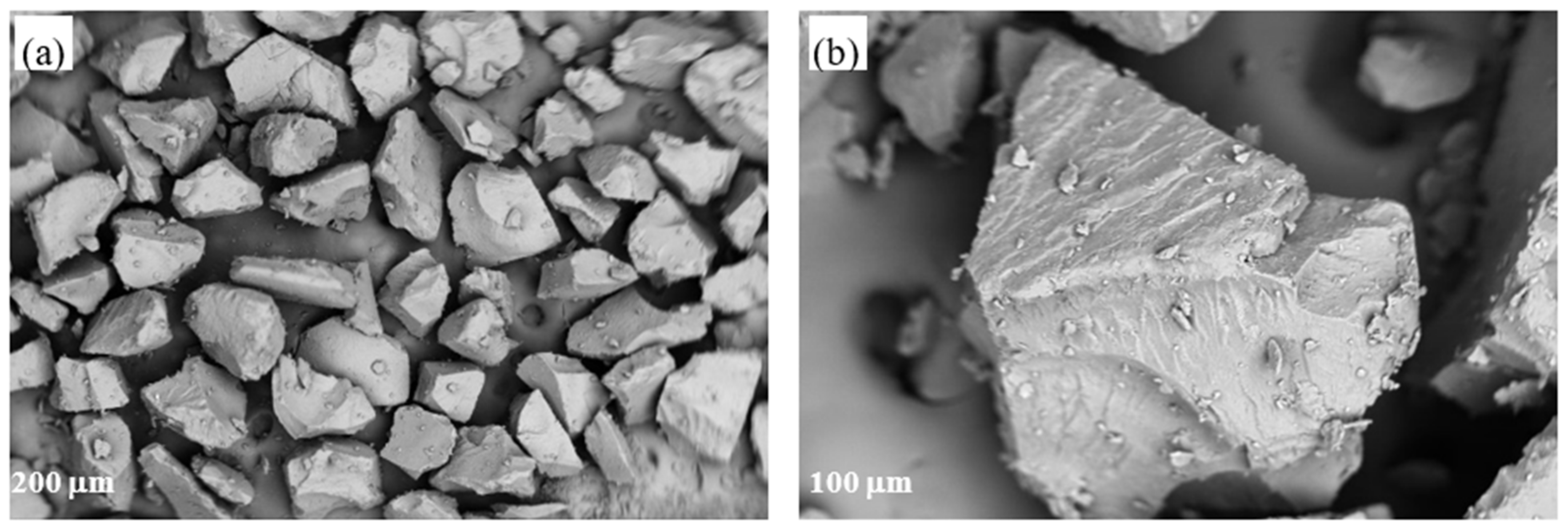
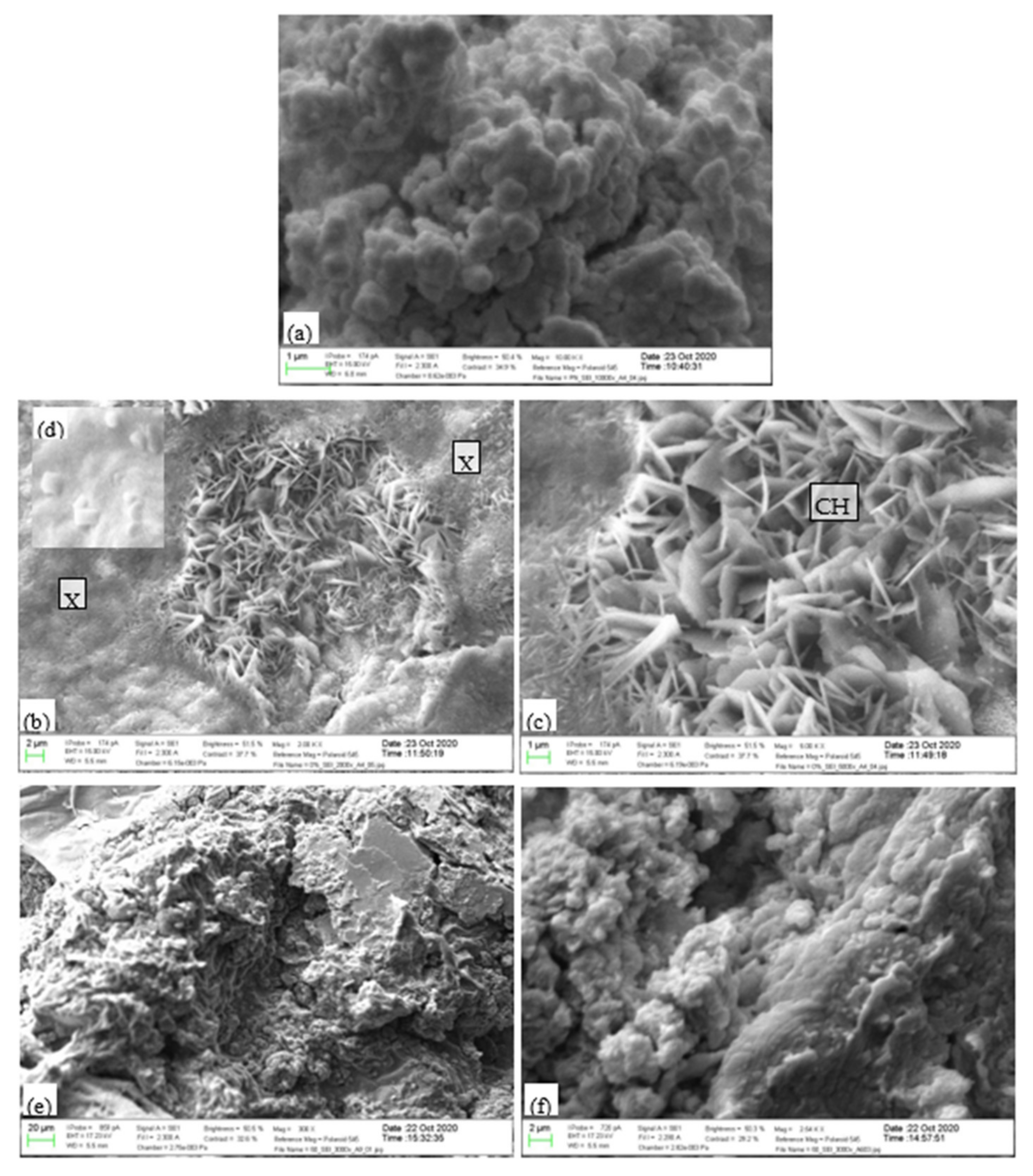
3.7.3. SEM Examination
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Weber, S.; Reinhardt, H.W. A New Generation of High Performance Concrete: Concrete with Autogenous Curing. Adv. Cem. Based Mater. 1997, 6, 59–68. [Google Scholar] [CrossRef]
- Walraven, J. High Performance Concrete: A Material with a Large Potential. J. Adv. Concr. Technol. 2009, 7, 145–156. [Google Scholar] [CrossRef]
- Lura, P.; Jensen, O.M.; van Breugel, K. Autogenous shrinkage in high-performance cement paste: An evaluation of basic mechanisms. Cem. Concr. Res. 2003, 33, 223–232. [Google Scholar] [CrossRef]
- Jensen, O.M.; Hansen, P.F. Water-entrained cement-based materials I. Principles and theoretical background. Cem. Concr. Res. 2001, 31, 647–654. [Google Scholar] [CrossRef]
- Wang, K.; Jansen, D.C.; Shah, S.P.; Karr, A.F. Permeability study of cracked concrete. Cem. Concr. Res. 1997, 27, 381–393. [Google Scholar] [CrossRef]
- Jensen, O.M.; Lura, P. Techniques and materials for internal water curing of concrete. Mater. Struct. Constr. 2006, 39, 817–825. [Google Scholar] [CrossRef]
- Beushausen, H.; Gillmer, M.; Alexander, M. The influence of superabsorbent polymers on strength and durability properties of blended cement mortars. Cem. Concr. Compos. 2014, 52, 73–80. [Google Scholar] [CrossRef]
- Schröfl, C.; Mechtcherine, V.; Gorges, M. Relation between the molecular structure and the efficiency of superabsorbent polymers (SAP) as concrete admixture to mitigate autogenous shrinkage. Cem. Concr. Res. 2012, 42, 865–873. [Google Scholar] [CrossRef]
- Snoeck, D.; Schaubroeck, D.; Dubruel, P.; De Belie, N. Effect of high amounts of superabsorbent polymers and additional water on the workability, microstructure and strength of mortars with a water-to-cement ratio of 0.50. Constr. Build. Mater. 2014, 72, 148–157. [Google Scholar] [CrossRef]
- Mignon, A.; Snoeck, D.; Dubruel, P.; Van Vlierberghe, S.; De Belie, N. Crack mitigation in concrete: Superabsorbent polymers as key to success? Materials 2017, 10, 237. [Google Scholar] [CrossRef]
- Shen, D.; Jiang, J.; Zhang, M.; Yao, P.; Jiang, G. Tensile creep and cracking potential of high performance concrete internally cured with super absorbent polymers at early age. Constr. Build. Mater. 2018, 165, 451–461. [Google Scholar] [CrossRef]
- Liu, J.; Shi, C.; Ma, X.; Khayat, K.H.; Zhang, J.; Wang, D. An overview on the effect of internal curing on shrinkage of high performance cement-based materials. Constr. Build. Mater. 2017, 146, 702–712. [Google Scholar] [CrossRef]
- Reinhardt, H.; Assmann, A.; Mönnig, S. Superabsorbent polymers (SAPs)—An admixture to increase the durability of concrete. In International Conference on Microstructure Related Durability of Cementitious Composites; Sun, W., van Breugel, K., Miao, C., Ye, G., Eds.; RILEM Publications: Paris, France, 2008; pp. 313–322. ISBN 978-2-35158-084-4. [Google Scholar]
- Snoeck, D.; Steuperaert, S.; Van Tittelboom, K.; Dubruel, P.; De Belie, N. Visualization of water penetration in cementitious materials with superabsorbent polymers by means of neutron radiography. Cem. Concr. Res. 2012, 42, 1113–1121. [Google Scholar] [CrossRef]
- Mignon, A.; Graulus, G.J.; Snoeck, D.; Martins, J.; De Belie, N.; Dubruel, P.; Van Vlierberghe, S. pH-sensitive superabsorbent polymers: A potential candidate material for self-healing concrete. J. Mater. Sci. 2015, 50, 970–979. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Fakoorpoor, S.M.; Hosseini, P.; Khaloo, A. Interactions between superabsorbent polymers and cement-based composites incorporating colloidal silica nanoparticles. Cem. Concr. Compos. 2013, 37, 196–204. [Google Scholar] [CrossRef]
- Farzanian, K.; Pimenta Teixeira, K.; Perdiago Rocha, I.; De Sa Carneiro, L.; Ghahremaninezhad, A. The mechanical strength, degree of hydration, and electrical resistivity of cement pastes modified with superabsorbent polymers. Constr. Build. Mater. 2016, 109, 156–165. [Google Scholar] [CrossRef]
- Mechtcherine, V.; Gorges, M.; Schroefl, C.; Assmann, A. Effect of internal curing by using superabsorbent polymers ( SAP ) on autogenous shrinkage and other properties of a high-performance fine-grained concrete: Results of a RILEM round-robin test. Mater. Struct. 2014, 47, 541–562. [Google Scholar] [CrossRef]
- Mechtcherine, V.; Secrieru, E.; Schröfl, C. Effect of superabsorbent polymers (SAPs) on rheological properties of fresh cement-based mortars—Development of yield stress and plastic viscosity over time. Cem. Concr. Res. 2015, 67, 52–65. [Google Scholar] [CrossRef]
- Jensen, O.M.; Hansen, P.F. Water-entrained cement-based materials II. Experimental observations. Cem. Concr. Res. 2002, 32, 973–978. [Google Scholar] [CrossRef]
- Mechtcherine, V.; Reinhardt, H.-W. Application of Super Absorbent Polymers (SAP) in Concrete Construction: State-of-the-Art Report Prepared by Technical Committee 225-SAP; Springer Science & Business Media: Berlin, Germany, 2012; Volume 2, ISBN 9400727321. [Google Scholar]
- Esteves, L.P. Superabsorbent polymers: On their interaction with water and pore fluid. Cem. Concr. Compos. 2011, 33, 717–724. [Google Scholar] [CrossRef]
- Zhu, Q.; Barney, C.W.; Erk, K.A. Effect of ionic crosslinking on the swelling and mechanical response of model superabsorbent polymer hydrogels for internally cured concrete. Mater. Struct. 2015, 48, 2261–2276. [Google Scholar] [CrossRef]
- Farzanian, K.; Ghahremaninezhad, A. On the effect of chemical composition on the desorption of superabsorbent hydrogels in contact with a porous cementitious material. Gels 2018, 4, 70. [Google Scholar] [CrossRef]
- Vafaei, B.; Farzanian, K.; Ghahremaninezhad, A. The influence of superabsorbent polymer on the properties of alkali-activated slag pastes. Constr. Build. Mater. 2020, 236, 117525. [Google Scholar] [CrossRef]
- Farzanian, K.; Ghahremaninezhad, A. Desorption of superabsorbent hydrogels with varied chemical compositions in cementitious materials. Mater. Struct. 2018, 51, 3. [Google Scholar] [CrossRef]
- Snoeck, D.; Jensen, O.M.; De Belie, N. The influence of superabsorbent polymers on the autogenous shrinkage properties of cement pastes with supplementary cementitious materials. Cem. Concr. Res. 2015, 74, 59–67. [Google Scholar] [CrossRef]
- Hou, P.K.; Kawashima, S.; Wang, K.J.; Corr, D.J.; Qian, J.S.; Shah, S.P. Effects of colloidal nanosilica on rheological and mechanical properties of fly ash-cement mortar. Cem. Concr. Compos. 2013, 35, 12–22. [Google Scholar] [CrossRef]
- Mazloom, M.; Ramezanianpour, A.A.; Brooks, J.J. Effect of silica fume on mechanical properties of high-strength concrete. Cem. Concr. Compos. 2004, 26, 347–357. [Google Scholar] [CrossRef]
- Baloch, H.; Usman, M.; Rizwan, S.A.; Hanif, A. Properties enhancement of super absorbent polymer (SAP) incorporated self-compacting cement pastes modified by nano silica (NS) addition. Constr. Build. Mater. 2019, 203, 18–26. [Google Scholar] [CrossRef]
- Sikora, K.S.; Klemm, A.J. Effect of Superabsorbent Polymers on Workability and Hydration Process in Fly Ash Cementitious Composites. J. Mater. Civ. Eng. 2015, 27, 04014170. [Google Scholar] [CrossRef]
- Almeida, F.C.R.; Klemm, A.J. Efficiency of internal curing by superabsorbent polymers (SAP) in PC-GGBS mortars. Cem. Concr. Compos. 2018, 88, 41–51. [Google Scholar] [CrossRef]
- Farzanian, K.; Vafaei, B.; Ghahremaninezhad, A. The behavior of superabsorbent polymers (SAPs) in cement mixtures with glass powders as supplementary cementitious materials. Materials 2019, 12, 3597. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Spiesz, P.; Brouwers, H.J.H. Effect of nano-silica on the hydration and microstructure development of Ultra-High Performance Concrete (UHPC) with a low binder amount. Constr. Build. Mater. 2014, 65, 140–150. [Google Scholar] [CrossRef]
- Björnström, J.; Martinelli, A.; Matic, A.; Börjesson, L.; Panas, I. Accelerating effects of colloidal nano-silica for beneficial calcium-silicate-hydrate formation in cement. Chem. Phys. Lett. 2004, 392, 242–248. [Google Scholar] [CrossRef]
- Van, V.T.A.; Rößler, C.; Bui, D.D.; Ludwig, H.M. Rice husk ash as both pozzolanic admixture and internal curing agent in ultra-high performance concrete. Cem. Concr. Compos. 2014, 53, 270–278. [Google Scholar] [CrossRef]
- Nie, S.; Hu, S.; Wang, F.; Hu, C.; Li, X.; Zhu, Y. Pozzolanic reaction of lightweight fine aggregate and its influence on the hydration of cement. Constr. Build. Mater. 2017, 153, 165–173. [Google Scholar] [CrossRef]
- Cândido, J.D.S.; Pereira, A.G.B.; Fajardo, A.R.; Ricardo, N.M.P.S.; Feitosa, J.P.A.; Muniz, E.C.; Rodrigues, F.H.A. Poly(acrylamide-co-acrylate)/rice husk ash hydrogel composites. II. Temperature effect on rice husk ash obtention. Compos. Part B Eng. 2013, 51, 246–253. [Google Scholar] [CrossRef]
- Adewunmi, A.A.; Ismail, S.; Owolabi, T.O.; Sultan, A.S.; Olatunji, S.O.; Ahmad, Z. Modeling the thermal behavior of coal fly ash based polymer gel system for water reduction in oil and gas wells. J. Pet. Sci. Eng. 2017, 157, 430–440. [Google Scholar] [CrossRef]
- Cheraghian, G.; Wu, Q.; Mosto, M.; Li, M.; Afrand, M.; Sangwai, J.S. Effect of a novel clay / silica nanocomposite on water-based drilling fl uids: Improvements in rheological and fi ltration properties. Colloids Surf. A J. 2018, 555, 339–350. [Google Scholar] [CrossRef]
- Cheraghian, G. Application of nano-fumed silica in heavy oil recovery. Pet. Sci. Technol. 2016, 34, 12–18. [Google Scholar] [CrossRef]
- Cheraghian, G. Evaluation of Clay and Fumed Silica Nanoparticles on Adsorption of Surfactant Polymer during Enhanced Oil Recovery. J. Japan Pet. Inst. 2017, 60, 85–94. [Google Scholar] [CrossRef]
- Krafcik, M.J.; Bose, B.; Erk, K.A. Synthesis and Characterization of Polymer-Silica Composite Hydrogel Particles and Influence of Hydrogel Composition on Cement Paste Microstructure. Adv. Civ. Eng. Mater. 2018, 7, 590–613. [Google Scholar] [CrossRef]
- Farzanian, K.; Ghahremaninezhad, A. On the effect of the capillary forces on the desorption of hydrogels in contact with a porous cementitious material. Mater. Struct. Constr. 2017, 50, 216. [Google Scholar] [CrossRef]
- Tunstall, L.E.; Scherer, G.W.; Prud’homme, R.K. Studying AEA interaction in cement systems using tensiometry. Cem. Concr. Res. 2017, 92, 29–36. [Google Scholar] [CrossRef]
- Cabinets, M.; Rooms, M.; Statements, B.; Mass, D. Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (Using 2-in or [50 mm] Cube Specimens); ASTM International: West Conshohocken, PA, USA, 2020; Volume 4, pp. 1–12. [Google Scholar]
- Neithalath, N.; Weiss, J.; Olek, J. Predicting the Permeability of Pervious Concrete (Enhanced Porosity Concrete) from Non-Destructive Electrical Measurements; United States Purdue University: West Lafayette, IN, USA, 2006. [Google Scholar]
- Snyder, K.A.; Ferraris, C.; Martys, N.S.; Garboczi, E.J. Using impedance spectroscopy to assess the viability of the rapid chloride test for determining concrete conductivity. J. Res. Natl. Inst. Stand. Technol. 2000, 105, 497. [Google Scholar] [CrossRef]
- Mass, D.; Cements, W.; Ag, C.; Mortar, H.; Test, C.C. Standard Test Method for Autogenous Strain of Cement Paste and Mortar; ASTM International: West Conshohocken, PA, USA, 2020; pp. 1–8. [Google Scholar]
- Wehbe, Y.; Ghahremaninezhad, A. Combined effect of shrinkage reducing admixtures (SRA) and superabsorbent polymers (SAP) on the autogenous shrinkage and properties of cementitious materials. Constr. Build. Mater. 2017, 138, 151–162. [Google Scholar] [CrossRef]
- Xia, M.; Yao, Z.; Ge, L.; Chen, T.; Li, H. A potential bio-filler: The substitution effect of furfural modified clam shell for carbonate calcium in polypropylene. J. Compos. Mater. 2015, 49, 807–816. [Google Scholar] [CrossRef]
- Fernández-Carrasco, L.; Torrens-Martín, D.; Morales, L.M.; Martínez-Ramírez, S. Infrared spectroscopy in the analysis of building and construction materials. In Infrared Spectroscopy—Materials Science, Engineering and Technology; InTech: Rijeka, Croatia, 2012; pp. 369–382. [Google Scholar]
- Chu, D.H.; Vinoba, M.; Bhagiyalakshmi, M.; Baek, I.H.; Nam, S.C.; Yoon, Y.; Kim, S.H.; Jeong, S.K. CO2 mineralization into different polymorphs of CaCO3 using an aqueous-CO2 system. RSC Adv. 2013, 3, 21722–21729. [Google Scholar] [CrossRef]
- Yu, P.; Kirkpatrick, R.J.; Poe, B.; McMillan, P.F.; Cong, X. Structure of calcium silicate hydrate (C-S-H): Near-, Mid-, and Far-infrared spectroscopy. J. Am. Ceram. Soc. 1999, 82, 742–748. [Google Scholar] [CrossRef]
- Huang, H.; Ye, G.; Damidot, D. Characterization and quantification of self-healing behaviors of microcracks due to further hydration in cement paste. Cem. Concr. Res. 2013, 52, 71–81. [Google Scholar] [CrossRef]
- Delgado, A.H.; Paroli, R.M.; Beaudoin, J.J. Comparison of IR techniques for the characterization of construction cement minerals and hydrated products. Appl. Spectrosc. 1996, 50, 970–976. [Google Scholar] [CrossRef]
- Chavda, H.V.; Patel, R.D.; Modhia, I.P.; Patel, C.N. Preparation and characterization of superporous hydrogel based on different polymers. Int. J. Pharm. Investig. 2012, 2, 134. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, A.S.G.; Neto, M.P.A. Application of Ftir in the Determination of Acrylate Content in Poly(Sodium Acrylate-Co-Acrylamide) Superabsorbent Hydrogels. Quim. Nov. 2012, 35, 1464–1467. [Google Scholar] [CrossRef]
- Mignon, A.; Snoeck, D.; Schaubroeck, D.; Luickx, N.; Dubruel, P.; Van Vlierberghe, S.; De Belie, N. pH-responsive superabsorbent polymers: A pathway to self-healing of mortar. React. Funct. Polym. 2015, 93, 68–76. [Google Scholar] [CrossRef]
- Mahdavinia, G.R.; Pourjavadi, A.; Hosseinzadeh, H.; Zohuriaan, M.J. Modified chitosan 4. Superabsorbent hydrogels from poly (acrylic acid-co-acrylamide) grafted chitosan with salt-and pH-responsiveness properties. Eur. Polym. J. 2004, 40, 1399–1407. [Google Scholar] [CrossRef]
- Ghosh, S.N.; Handoo, S.K. Infrared and Raman spectral studies in cement and concrete. Cem. Concr. Res. 1980, 10, 771–782. [Google Scholar] [CrossRef]
- Leung, W.M.; Axelson, D.E.; Van Dyke, J.D. Thermal degradation of polyacrylamide and poly(acrylamide-co-acrylate). J. Polym. Sci. Part A Polym. Chem. 1987, 25, 1825–1846. [Google Scholar] [CrossRef]
- Vance, K.; Falzone, G.; Pignatelli, I.; Bauchy, M.; Balonis, M.; Sant, G. Direct Carbonation of Ca(OH)2 Using Liquid and Supercritical CO2: Implications for Carbon-Neutral Cementation. Ind. Eng. Chem. Res. 2015, 54, 8908–8918. [Google Scholar] [CrossRef]
- Balcerowiak, W.; Hetper, J. Study of the Thermal Behaviours of some metal acrylates. J. Therm. Anal. 1977, 11, 101–107. [Google Scholar] [CrossRef]
- Mondal, P.; Shah, S.; Marks, L. Nanoscale Characterization of Cementitious Materials. ACI Mater. J. 2008, 105, 174–179. [Google Scholar]
- Shahsavari, R.; Hoon Hwang, S. Morphogenesis of Cement Hydrate: From Natural C-S-H to Synthetic C-S-H. In Materials; Rahman, R.O.A., Saleh, H.E.-D.M., Eds.; IntechOpen: Rijeka, Croatia, 2018; pp. 79–90. [Google Scholar]








| Hydrogel | Distilled Water (g) | AM (g) | MBA (g) | APS (g) | NSi (g) |
|---|---|---|---|---|---|
| H-0 | 100 | 20 | 0.05 | 0.64 | 0 |
| H-10 | 100 | 20 | 0.05 | 0.64 | 2 |
| H-20 | 100 | 20 | 0.05 | 0.64 | 4 |
| H-25 | 100 | 20 | 0.05 | 0.64 | 5 |
| H-50 | 100 | 20 | 0.05 | 0.64 | 10 |
| Composition | % |
|---|---|
| SiO2 | 20.6 |
| Al2O3 | 4.8 |
| Fe2O3 | 3.5 |
| CaO | 64 |
| MgO | 0.9 |
| Na2O | 0.1 |
| K2O | 0.3 |
| SO3 | 3.4 |
| Designation | Hydrogel | W/C | Superplasticizer (% per cement) | Hydrogel (% per cement) | Flow (cm) |
|---|---|---|---|---|---|
| C | - | 0.3 | 0.5 | - | 20 |
| C H-0 | H-0 | 0.35 | 0.5 | 0.3 | 20 |
| C H-10 | H-10 | 0.35 | 0.5 | 0.4 | 20 |
| C H-50 | H-50 | 0.35 | 0.5 | 0.7 | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vafaei, B.; Farzanian, K.; Ghahremaninezhad, A. Effect of Hydrogels Containing Nanosilica on the Properties of Cement Pastes. J. Compos. Sci. 2021, 5, 105. https://doi.org/10.3390/jcs5040105
Vafaei B, Farzanian K, Ghahremaninezhad A. Effect of Hydrogels Containing Nanosilica on the Properties of Cement Pastes. Journal of Composites Science. 2021; 5(4):105. https://doi.org/10.3390/jcs5040105
Chicago/Turabian StyleVafaei, Babak, Khashayar Farzanian, and Ali Ghahremaninezhad. 2021. "Effect of Hydrogels Containing Nanosilica on the Properties of Cement Pastes" Journal of Composites Science 5, no. 4: 105. https://doi.org/10.3390/jcs5040105
APA StyleVafaei, B., Farzanian, K., & Ghahremaninezhad, A. (2021). Effect of Hydrogels Containing Nanosilica on the Properties of Cement Pastes. Journal of Composites Science, 5(4), 105. https://doi.org/10.3390/jcs5040105








