Health and Safety Concerns Related to CNT and Graphene Products, and Related Composites
Abstract
:1. Introduction
2. State of the Art Review
2.1. Materials
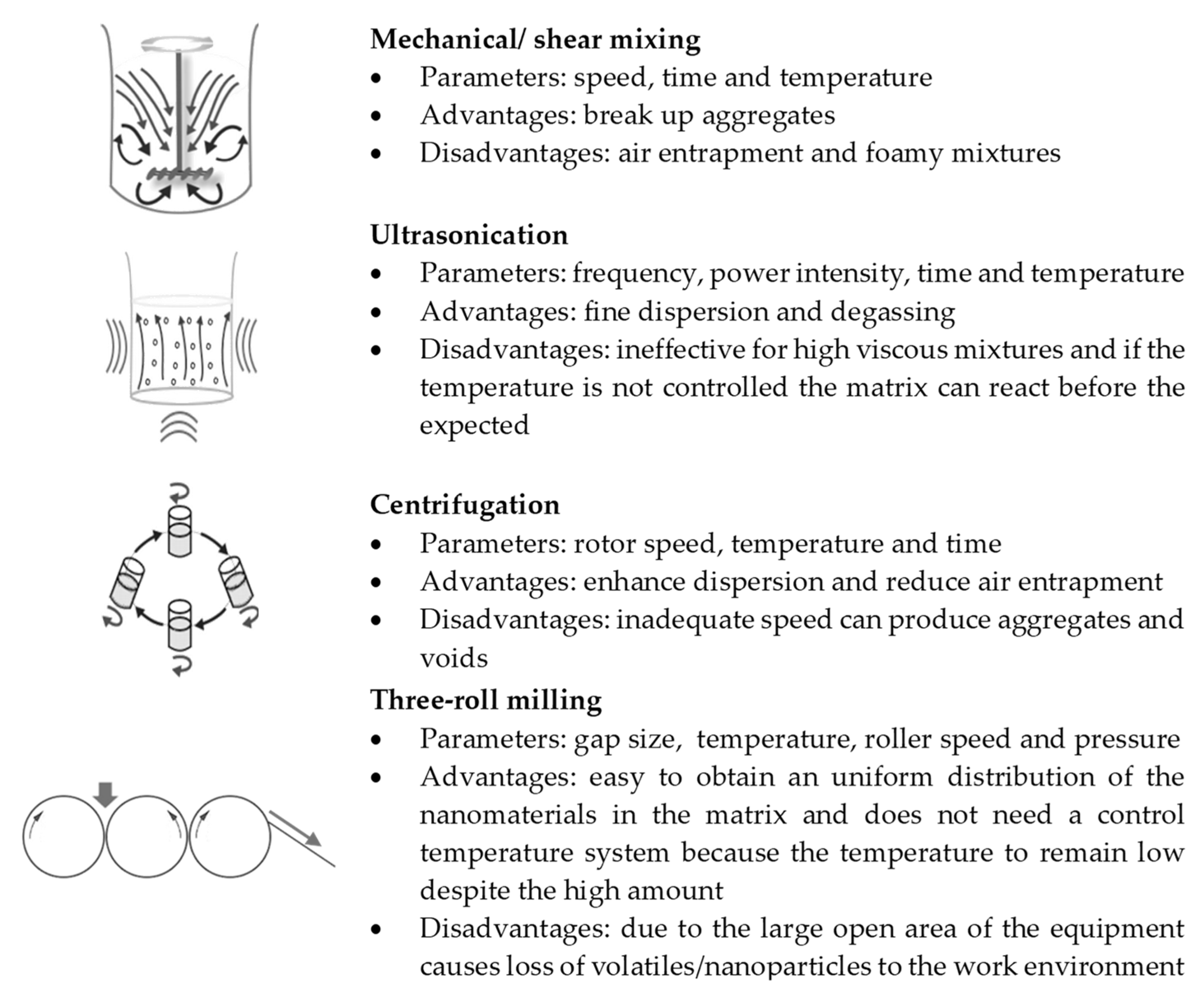
2.2. Processes
2.3. Products
2.4. Health
- Systemic—affecting semi permeable barriers (blood-brain and blood-spinal cord) of the central nervous system.
- Olfactory neurodegenerative diseases—due to the direct brain delivery of particles.
- Trigeminal—the route uses the trigeminal nerve as an important way to transport from nose to brain, i.e., intracellular or axonal transport.
2.5. Safety
- How to overcome limitations of current technologies and techniques?
- What are the most important physical and chemical properties with respect to biological behavior of ENM, in short, medium and long time?
- What should be the degree of accuracy and how to measure accurately?
- What are the ignition and explosive properties, and their potential risks?
2.6. Risk Management
2.6.1. Risk Assessment Methods
2.6.2. Research
2.6.3. Industry
2.6.4. Application
3. Discussion
- Employees,
- Identification and compliance with regulations,
- Risk and vulnerability analysis,
- Emergency response plans, related drills and training,
- Personnel training, refreshments, role compliance monitoring,
- Maintenance, inspection and monitoring of equipment and instrumentation,
- Fire and explosion protection,
- Personal protection equipment,
- Investigation of incidents and near misses,
- Hazardous material transportation,
- Alarms and control equipment,
- Passive barriers,
- Construction of code-certified buildings,
- Costs related to raw materials,
- Costs of equipment,
- Insurance cost,
- Security cost,
- Decommissioning costs,
- Software related costs.
4. Proposals
4.1. Technical
4.1.1. Laboratory
- Ventilation:
- ○
- With renewal without recycling 5–10×/h,
- ○
- With at least a sealed F7/H14 filter (EN 779) for exiting air, since these filters have 80–90% average efficiency for 0.4 μm particles,
- ○
- Low pressure room,
- ○
- Capture of the contaminate air at the source.
- A seamless and impermeable resin floor with coving between the floor and wall will minimize the risk of accumulation in cracks and gaps of nanomaterials, by making at the same time easier to clean and easily remove contaminants from the area.
- Manipulation under fume hood (compulsory).
- SAS entrance and exit (simple).
- Safety shower.
4.1.2. Industry
- Cooperation with academia and/or larger companies to have access to a high level of controls.
- Consider the acquisition of used laboratory equipment, but bear in mind to ensure they operate under the correct specifications prior to use.
- Work with local economic and development groups to promote the importance of engineering controls as enabler tools for nanotechnology commercialization for SMEs and promoting the benefits of protecting workers, environment and community at large.
- Include discussion of EHS – Environmental Health and Safety rules in the business plan and budget for high-level of controls possible needed.
4.2. Personal Protection Equipment
4.2.1. Laboratory
- Masks with P3 filters are advised for respiratory protection.
- To protect the eyes/face, use a face shield or lab goggles.
- Protection of the body parts with overalls with hood (Tyvek® style), since nonwoven clothing is more efficient against penetration of NP than woven cotton.
- The use of two or more pairs of disposable gloves (nitrile, vinyl, latex, Neoprene®) should be adopted and frequently replaced, especially for exposure to nanomaterials in liquid phase.
4.2.2. Industry
- A powered air respirator, because ensures better comfort for longer work periods (it is recommended this system if the work lasts over 2 h). However, for shorter periods of exposure a P3 filtering mask can be used.
- Similar to the laboratories, it should be used two or more pairs of disposable gloves (nitrile, vinyl, latex, Neoprene®), but they should be replaced with higher frequency.
4.3. Training
4.3.1. Laboratory
4.3.2. Industry
- Hazards are specific to MNMs and different from the bulk material.
- Hazard classes are assigned to MNMs.
- Routes of exposure are important which workplace exposures have been measured and which tasks put workers most at risk.
- How proposed OELs can be interpreted.
- When and how control banding, specific controls and PPE for MNMs can be used.
4.4. Maintenance
4.4.1. Laboratory
- Perform the separation of the laboratory clothes from the outside ones (compulsory).
- Use safety containers for transportation of nanomaterials.
- Store in specific areas and separate cabins the nanomaterials. These cabinets must carry an appropriate danger label.
- The workers that handle the nanomaterials in the laboratory should be the ones that cleans the laboratory.
- The cleaning process should be wet to avoid the dispersion to the working atmosphere of the nanoparticles.
- The residues should be treated as chemical/hazardous waste and deposited in double bagging with a label that indicates the presence of nanomaterials in those wastes and include available information characterizing known and suspected properties.
- In the case that nanoparticles are accidentally split, the workplace must be cleaned immediately with a wet damp towel. Under no circumstances may residual materials be blown off the surface, particularly in the case of nanomaterials.
- Use the same protective equipment’s during the cleaning and maintenance activities.
- Laboratory responsible must supervise the cleaning and maintenance activities.
4.4.2. Industry
4.5. Application of Safety Guidelines
4.5.1. Laboratory
- Restricted access to the room (magnetic card access control system)
- Evidence about exposed people + board to record presence
- Medical surveillance to all persons that works with nanomaterials (once a year).
4.5.2. Industry
4.6. Product Life Cycle
5. Conclusions
Funding
Conflicts of Interest
References
- Sousa, S.P.B.; Santos, R.M.; Rocha, N. Smart carbon fibre reinforced polymer (CFRP) composites with carbon allotropes: A brief reflection on potential safety and health issues. In Proceedings of the International Symposium on Occupational Safety and Hygiene SHO 2019, Guimarães, Portugal, 15–16 April 2019. [Google Scholar]
- Brasinika, D.; Kyriakidou, K.; Sousa, S.P.B.; Rocha, N.; Koumoulos, E.P.; Charitidis, C.A. Health and Safety Issues in the Development of (nano) carbon-based materials and composites: The case of novel multifunctional carbon fibre reinforced polymer composites. In Proceedings of the International Symposium on Occupational Safety and Hygiene SHO 2019, Guimarães, Portugal, 15–16 March 2018. [Google Scholar]
- Wan, B.; Hou, J.; Guo, L.-H. Safety of Carbon Nanotubes. In Industrial Applications of Carbon Nanotubes; Elsevier: Amsterdam, The Netherlands, 2017; pp. 405–431. [Google Scholar]
- Narei, H.; Ghasempour, R.; Akhavan, O. Toxicity and Safety Issues of Carbon Nanotubes. In Carbon Nanotube-Reinforced Polymers; Rafiee, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 145–171. [Google Scholar] [CrossRef]
- Guseva Canu, I.; Batsungnoen, K.; Maynard, A.; Hopf, N.B. State of knowledge on the occupational exposure to carbon nanotubes. Int. J. Hyg. Environ. Health 2020, 225, 113472. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-T.; Park, D.H.; Baac, H.W.; Han, S. Graphene-and carbon-nanotube-based transparent electrodes for semitransparent solar cells. Materials 2018, 11, 1503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Searl, A.; Crawford, J. Review of Health Risks for Workers in the Waste and Recycling Industry; Institute of Occupational Medicine: London, UK, 2012. [Google Scholar]
- Mohan, V.B. Handling and Risk Mitigation of Nanoscale Graphene and Related Materials: Some Considerations and Recommendations. C—J. Carbon Res. 2019, 5, 36. [Google Scholar] [CrossRef] [Green Version]
- Ye, R.; Tour, J.M. Graphene at Fifteen. ACS Nano 2019, 13, 10872–10878. [Google Scholar] [CrossRef]
- Novello, A.M.; Buitrago, E.; Groso, A.; Meyer, T. Efficient management of nanomaterial hazards in a large number of research laboratories in an academic environment. Saf. Sci. 2020, 121, 158–164. [Google Scholar] [CrossRef]
- Freeland, J.; Hulme, J.; Kinnison, D.; Mitchell, A.; Veitch, P.; Aitken, R.; Hankin, S.; Poland, C.; Bard, D.; Gibson, R.; et al. Working Safely with Nanomaterials in Research & Development; The UK NanoSafety Partnership Group: Edinburgh, UK, 2012. [Google Scholar]
- Savolainen, K. Nanosafety in Europe 2015–2025: Towards Safe and Sustainable Nanomaterials and Nanotechnology Innovations; Finnish Institute of Occupational Health: Helsinki, Finland, 2013. [Google Scholar]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Pervin, F.; Jeelani, S.; Mallick, P.K. Improvement in mechanical properties of carbon fabric–epoxy composite using carbon nanofibers. J. Mater. Process. Technol. 2008, 198, 445–453. [Google Scholar] [CrossRef]
- Chen, Q.; Wu, W.; Zhao, Y.; Xi, M.; Xu, T.; Fong, H. Nano-epoxy resins containing electrospun carbon nanofibers and the resulting hybrid multi-scale composites. Compos. Part B Eng. 2014, 58, 43–53. [Google Scholar] [CrossRef]
- Barua, B.; Saha, M.C. Ultrasound assisted hybrid carbon epoxy composites containing carbon nanotubes. J. Eng. Mater. Technol. 2013, 135. [Google Scholar] [CrossRef]
- Godara, A.; Mezzo, L.; Luizi, F.; Warrier, A.; Lomov, S.V.; van Vuure, A.W.; Gorbatikh, L.; Moldenaers, P.; Verpoest, I. Influence of carbon nanotube reinforcement on the processing and the mechanical behaviour of carbon fiber/epoxy composites. Carbon 2009, 47, 2914–2923. [Google Scholar] [CrossRef]
- Üstün, T.; Ulus, H.; Karabulut, S.E.; Eskizeybek, V.; Şahin, Ö.S.; Avcı, A.; Demir, O. Evaluating the effectiveness of nanofillers in filament wound carbon/epoxy multiscale composite pipes. Compos. Part B Eng. 2016, 96, 1–6. [Google Scholar] [CrossRef]
- Hawkins, D.A., Jr.; Haque, A. Fracture toughness of carbon-graphene/epoxy hybrid nanocomposites. Procedia Eng. 2014, 90, 176–181. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.K.; Chowdhury, M.M.R.; Bolden, N.W. Optimized Mechanical Performance of Carbon Fiber-Epoxy Composite Using Amino-Functionalized Graphene Nanoplatelets. In Proceedings of the ASME International Mechanical Engineering Congress and Exposition, Houston, TX, USA, 13–19 November 2015; p. V009T012A057. [Google Scholar]
- Qin, W.; Vautard, F.; Drzal, L.T.; Yu, J. Mechanical and electrical properties of carbon fiber composites with incorporation of graphene nanoplatelets at the fiber–matrix interphase. Compos. Part B Eng. 2015, 69, 335–341. [Google Scholar] [CrossRef]
- Dahm, M.M.; Schubauer-Berigan, M.K.; Evans, D.E.; Birch, M.E.; Bertke, S.; Beard, J.D.; Erdely, A.; Fernback, J.E.; Mercer, R.R.; Grinshpun, S.A. Exposure assessments for a cross-sectional epidemiologic study of US carbon nanotube and nanofiber workers. Int. J. Hyg. Environ. Health 2018, 221, 429–440. [Google Scholar] [CrossRef]
- Gurkan, I.; Cebeci, H. An approach to identify complex CNT reinforcement effect on the interlaminar shear strength of prepreg composites by Taguchi method. Compos. Struct. 2016, 141, 172–178. [Google Scholar] [CrossRef]
- Dehghan, M.; Al-Mahaidi, R.; Sbarski, I. Investigation of CNT modification of epoxy resin in CFRP strengthening systems. Polym. Compos. 2016, 37, 1021–1033. [Google Scholar] [CrossRef]
- Santos, R.M.; Vale, D.; Rocha, J.; Martins, C.; Mould, S.T.; Rocha, N. Multiscale carbon fibre-reinforced polymer (CFRP) composites containing carbon nanotubes with tailored interfaces. Fatigue Fract. Eng. Mater. Struct. 2019, 42, 1521–1533. [Google Scholar] [CrossRef]
- Thostenson, E.T.; Li, W.Z.; Wang, D.Z.; Ren, Z.F.; Chou, T.W. Carbon nanotube/carbon fiber hybrid multiscale composites. J. Appl. Phys. 2002, 91, 6034–6037. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Mittal, V. Hybrid Nanomaterials: Advances in Energy, Environment, and Polymer Nanocomposites; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Thostenson, E.T.; Chou, T.-W. Processing-structure-multi-functional property relationship in carbon nanotube/epoxy composites. Carbon 2006, 44, 3022–3029. [Google Scholar] [CrossRef]
- Salam, H.; Dong, Y.; Davies, I.J.; Pramanik, A. The effects of material formulation and manufacturing process on mechanical and thermal properties of epoxy/clay nanocomposites. Int. J. Adv. Manuf. Technol. 2016, 87, 1999–2012. [Google Scholar] [CrossRef] [Green Version]
- Anand, A.; Joshi, M. Structural Composites Hybridized with Nanofillers: An Overview. J. Indian Inst. Sci. 2015, 95, 233–247. [Google Scholar]
- Qian, H.; Greenhalgh, E.S.; Shaffer, M.S.P.; Bismarck, A. Carbon nanotube-based hierarchical composites: A review. J. Mater. Chem. 2010, 20, 4751–4762. [Google Scholar] [CrossRef]
- Fleury, D.; Bomfim, J.A.S.; Vignes, A.; Girard, C.; Metz, S.; Muñoz, F.; R’Mili, B.; Ustache, A.; Guiot, A.; Bouillard, J.X. Identification of the main exposure scenarios in the production of CNT-polymer nanocomposites by melt-moulding process. J. Clean. Prod. 2013, 53, 22–36. [Google Scholar] [CrossRef]
- Kausar, A.; Rafique, I.; Muhammad, B. Electromagnetic Interference Shielding of Polymer/Nanodiamond, Polymer/Carbon Nanotube, and Polymer/Nanodiamond–Carbon Nanotube Nanobifiller Composite: A Review. Polym.-Plast. Technol. Eng. 2017, 56, 347–363. [Google Scholar] [CrossRef]
- Osada, R.; Suzuki, K. Development of a Flexible Tactile Sensor Using Area-Arrayed Bundle Structures of Multi-Walled Carbon Nanotubes. In Proceedings of the ASME 2018 International Mechanical Engineering Congress and Exposition, Pittsburgh, PA, USA, 9–15 November 2018. [Google Scholar]
- Beaumont, P.W.; Soutis, C.; Hodzic, A. The Structural Integrity of Carbon Fiber Composites; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Khan, S.U.; Kim, J.-K. Impact and delamination failure of multiscale carbon nanotube-fiber reinforced polymer composites: A review. Int. J. Aeronaut. Space Sci. 2011, 12, 115–133. [Google Scholar] [CrossRef] [Green Version]
- Lubineau, G.; Rahaman, A. A review of strategies for improving the degradation properties of laminated continuous-fiber/epoxy composites with carbon-based nanoreinforcements. Carbon 2012, 50, 2377–2395. [Google Scholar] [CrossRef]
- Tang, Y.H.; Ye, L.; Zhang, Z.; Friedrich, K. Interlaminar fracture toughness and CAI strength of fibre-reinforced composites with nanoparticles—A review. Compos. Sci. Technol. 2013, 86, 26–37. [Google Scholar] [CrossRef]
- StatNano. Nanomaterials and Morphologies. Available online: https://statnano.com/nanomaterials (accessed on 20 July 2020).
- Comfort, K.K. The rise of nanotoxicology: A successful collaboration between engineering and biology. AIMS Bioeng. 2016, 3, 230. [Google Scholar] [CrossRef]
- Emerce, E.; Ghosh, M.; Öner, D.; Duca, R.-C.; Vanoirbeek, J.; Bekaert, B.; Hoet, P.H.; Godderis, L. Carbon Nanotube-and Asbestos-Induced DNA and RNA Methylation Changes in Bronchial Epithelial Cells. Chem. Res. Toxicol. 2019, 32, 850–860. [Google Scholar] [CrossRef]
- Gangoli, V.S.; Raja, P.M.V.; Esquenazi, G.L.; Barron, A.R. The safe handling of bulk low-density nanomaterials. SN Appl. Sci. 2019, 1, 644. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, G.; Valapa, R.B.; Pugazhenthi, G.; Katiyar, V. Investigating the properties of poly (lactic acid)/exfoliated graphene based nanocomposites fabricated by versatile coating approach. Int. J. Biol. Macromol. 2018, 113, 1080–1091. [Google Scholar] [CrossRef] [PubMed]
- Pelin, M.; Sosa, S.; Prato, M.; Tubaro, A. Occupational exposure to graphene based nanomaterials: Risk assessment. Nanoscale 2018, 10, 15894–15903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Facciolà, A.; Visalli, G.; La Maestra, S.; Ceccarelli, M.; D’Aleo, F.; Nunnari, G.; Pellicanò, G.F.; Di Pietro, A. Carbon nanotubes and central nervous system: Environmental risks, toxicological aspects and future perspectives. Environ. Toxicol. Pharmacol. 2019, 65, 23–30. [Google Scholar] [CrossRef]
- Koppens, F.; Coleman, J.; Morandi, V.; Tredicucci, A. Science and technology roadmap for graphene, related two-dimensional crystals, and hybrid systems. Nanoscale 2015, 7, 4598–4810. [Google Scholar]
- Bianco, A.; Prato, M. Safety concerns on graphene and 2D materials: A Flagship perspective. 2d Mater. 2015, 2, 30201. [Google Scholar] [CrossRef] [Green Version]
- Sardoiwala, M.N.; Kaundal, B.; Choudhury, S.R. Toxic impact of nanomaterials on microbes, plants and animals. Environ. Chem. Lett. 2018, 16, 147–160. [Google Scholar] [CrossRef]
- Selvaraj, K.; Gowthamarajan, K.; Karri, V.V.S.R. Nose to brain transport pathways an overview: Potential of nanostructured lipid carriers in nose to brain targeting. Artif. CellsNanomed. Biotechnol. 2018, 46, 2088–2095. [Google Scholar] [CrossRef]
- Giusti, A.; Atluri, R.; Tsekovska, R.; Gajewicz, A.; Apostolova, M.D.; Battistelli, C.L.; Bleeker, E.A.J.; Bossa, C.; Bouillard, J.; Dusinska, M.; et al. Nanomaterial grouping: Existing approaches and future recommendations. NanoImpact 2019, 16, 100182. [Google Scholar] [CrossRef]
- Cao, Y.; Luo, Y. Pharmacological and toxicological aspects of carbon nanotubes (CNTs) to vascular system: A review. Toxicol. Appl. Pharmacol. 2019, 385, 114801. [Google Scholar] [CrossRef]
- Bonner, J.C. Nanoparticles as a potential cause of pleural and interstitial lung disease. Proc. Am. Thorac. Soc. 2010, 7, 138–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryman-Rasmussen, J.P.; Tewksbury, E.W.; Moss, O.R.; Cesta, M.F.; Wong, B.A.; Bonner, J.C. Inhaled multiwalled carbon nanotubes potentiate airway fibrosis in murine allergic asthma. Am. J. Respir. Cell Mol. Biol. 2009, 40, 349–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loos, M. Carbon Nanotube Reinforced Composites: CNT Polymer Science and Technology; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- NIOSH. Current Intelligence Bulletin 65: Occupational Exposure to Carbon Nanotubes and Nanofibers; Department of Health and Human Services, Public Health Service, Center for Disease Control and Prevention, National Institute for Occupational Safety and Health DHHS (NIOSH): Cincinnati, OH, USA, 2013. [Google Scholar]
- Gaffet, E. NanoMaterials & Responsible Development How to conciliate Research, Innovation and Safety. In Proceedings of the Nanotechitaly 2011, Venice, Italy, 28–30 November 2011. [Google Scholar]
- Aitken, R.; Hankin, S.; Ross, B.; Tran, C.; Stone, V.; Fernandes, T.; Donaldson, K.; Duffin, R.; Chaudhry, Q.; Wilkins, T. EMERGNANO: A Review of Completed and Near Completed Environment, Health and Safety Research on Nanomaterials and Nanotechnology Defra Project CB0409; Report TM/09/01; Institute of Occupational Medicine: Edinburgh, UK, 2009. [Google Scholar]
- Dazon, C.; Witschger, O.; Bau, S.; Fierro, V.; Llewellyn, P.L. Toward an operational methodology to identify industrial-scaled nanomaterial powders with the volume specific surface area criterion. Nanoscale Adv. 2019, 1, 3232–3242. [Google Scholar] [CrossRef] [Green Version]
- Sousa, S.P.; Baptista, J.S.; Ribeiro, M. Polymer nano and submicro composites risk assessment. Int. J. Work. Cond. 2014, 7, 103–119. [Google Scholar]
- World Health Organization. WHO Guidelines on Protecting Workers from Potential Risks of Manufactured Nanomaterials; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Silva, F.; Sousa, S.; Arezes, P.; Swuste, P.; Ribeiro, M.; Baptista, J.S. Qualitative risk assessment during polymer mortar test specimens preparation-methods comparison. J. Phys. Conf. Ser. 2015, 617, 12037. [Google Scholar] [CrossRef] [Green Version]
- Nanotechnologies–Part, B. 2: Guide to Safe Handling and Disposal of Manufactured Nanomaterials; PD 6699-2-2007; British Standards Institution: London, UK, 2007.
- CDC. NIOSH: Nanotechnology—Field Studies Effort. Available online: https://www.cdc.gov/niosh/topics/nanotech/field.html (accessed on 17 June 2020).
- Wohlleben, W.; Hellack, B.; Nickel, C.; Herrchen, M.; Hund-Rinke, K.; Kettler, K.; Riebeling, C.; Haase, A.; Funk, B.; Kühnel, D. The nanoGRAVUR framework to group (nano) materials for their occupational, consumer, environmental risks based on a harmonized set of material properties, applied to 34 case studies. Nanoscale 2019, 11, 17637–17654. [Google Scholar] [CrossRef]
- European Commission. Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), establishing a European Chemicals Agency, amending Directive 1999/45/EC and repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC. Off. J. Eur. Union 2006, 396, 1–849.
- ECHA. Read-Across Assessment Framework (RAAF); European Chemicals Agency: Helsinki, Finland, 2017. [Google Scholar]
- Schimpel, C.; Resch, S.; Flament, G.; Carlander, D.; Vaquero, C.; Bustero, I.; Falk, A. A methodology on how to create a real-life relevant risk profile for a given nanomaterial. J. Chem. Health Saf. 2018, 25, 12–23. [Google Scholar] [CrossRef] [Green Version]
- Sweet, L.; Strohm, B. Nanotechnology—Life-cycle risk management. Hum. Ecol. Risk Assess. 2006, 12, 528–551. [Google Scholar] [CrossRef]
- Lövestam, G.; Rauscher, H.; Roebben, G.; Klüttgen, B.S.; Gibson, N.; Putaud, J.-P.; Stamm, H. Considerations on a definition of nanomaterial for regulatory purposes. Jt. Res. Cent. (JRC) Ref. Rep. 2010, 80, 1–41. [Google Scholar]
- Roes, L.; Patel, M.K.; Worrell, E.; Ludwig, C. Preliminary evaluation of risks related to waste incineration of polymer nanocomposites. Sci. Total Environ. 2012, 417, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Wardak, A.; Gorman, M.E.; Swami, N.; Deshpande, S. Identification of risks in the life cycle of nanotechnology-based products. J. Ind. Ecol. 2008, 12, 435–448. [Google Scholar] [CrossRef]
- Gupta, J.; Hendershot, D.; Mannan, M. The real cost of process safety—A clear case for inherent safety. Process Saf. Environ. Prot. 2003, 81, 406–413. [Google Scholar] [CrossRef]
- Hodson, L.L.; Geraci, C.L. Chapter 11—Managing nanotechnology risks in small business—A National Institute for Occupational Safety and Health Perspectiv. In Nanotechnology Environmental Health and Safety; Elsevier: Amsterdam, The Netherlands, 2018; pp. 251–280. [Google Scholar] [CrossRef]
- Jaurand, M.-C.F.; Renier, A.; Daubriac, J. Mesothelioma: Do asbestos and carbon nanotubes pose the same health risk? Part. Fibre Toxicol. 2009, 6, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laux, P.; Tentschert, J.; Riebeling, C.; Braeuning, A.; Creutzenberg, O.; Epp, A.; Fessard, V.; Haas, K.-H.; Haase, A.; Hund-Rinke, K. Nanomaterials: Certain aspects of application, risk assessment and risk communication. Arch. Toxicol. 2018, 92, 121–141. [Google Scholar] [CrossRef] [Green Version]
- Donaldson, K.; Poland, C.; Bonner, J.; Duffin, R. The Toxicology of Carbon Nanotubes; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- Bussy, C.; Ali-Boucetta, H.; Kostarelos, K. Safety considerations for graphene: Lessons learnt from carbon nanotubes. Acc. Chem. Res. 2013, 46, 692–701. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Sung, J.H.; Song, K.S.; Kim, J.K.; Choi, B.S.; Yu, I.J.; Park, J.D. Derivation of occupational exposure limits for multi-walled carbon nanotubes and graphene using subchronic inhalation toxicity data and a multi-path particle dosimetry model. Toxicol. Res. 2019, 8, 580–586. [Google Scholar] [CrossRef]
- OECD. OECD Countries Address the Safety of Manufactured Nanomaterials. Available online: https://www.oecd.org/chemicalsafety/oecd-countries-address-the-safety-of-manufactured-nanomaterials.htm (accessed on 17 June 2020).
- Iyiegbuniwe, E.A.; Nwosu, U.U.; Kodali, S. A Review of Occupational Health Implications of Exposure and Risk Management of Carbon Nanotubes and Carbon Nanofibers. Int. J. Environ. Sci. Dev. 2016, 7, 849. [Google Scholar] [CrossRef]
- Schulte, P.; Kuempel, E.; Drew, N. Characterizing risk assessments for the development of occupational exposure limits for engineered nanomaterials. Regul. Toxicol. Pharmacol. 2018, 95, 207–219. [Google Scholar] [CrossRef]
- Firme, C.P., III; Bandaru, P.R. Toxicity issues in the application of carbon nanotubes to biological systems. Nanomed: Nanotechnol. Biol. Med. 2010, 6, 245–256. [Google Scholar] [CrossRef]
- Dahm, M.M.; Evans, D.E.; Schubauer-Berigan, M.K.; Birch, M.E.; Fernback, J.E. Occupational exposure assessment in carbon nanotube and nanofiber primary and secondary manufacturers. Ann. Occup. Hyg. 2012, 56, 542–556. [Google Scholar] [PubMed] [Green Version]
- Chen, M.; Zhou, S.; Zhu, Y.; Sun, Y.; Zeng, G.; Yang, C.; Xu, P.; Yan, M.; Liu, Z.; Zhang, W. Toxicity of carbon nanomaterials to plants, animals and microbes: Recent progress from 2015-present. Chemosphere 2018, 206, 255–264. [Google Scholar] [CrossRef] [PubMed]
- European Comission. Employment, Social Affairs & Inclusion, Guidance on the Protection of the Health and Safety of Workers from the Potential Risks Related to Nanomaterials at Work; European Comission: Brussels, Belgium, 2013. [Google Scholar]
- NANO. Resources for Nanotechnology Laboratory Safety. Available online: https://www.nano.gov/LabSafety (accessed on 20 May 2020).
- Kumar, N.; Kumbhat, S. Chapter 11—Toxicity and Environmental Issues. In Essentials in Nanoscience and Nanotechnology; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 447–448. [Google Scholar]
- Hallock, M.F.; Greenley, P.; DiBerardinis, L.; Kallin, D. Potential risks of nanomaterials and how to safely handle materials of uncertain toxicity. J. Chem. Health Saf. 2009, 16, 16–23. [Google Scholar] [CrossRef]
- Groso, A.; Petri-Fink, A.; Rothen-Rutishauser, B.; Hofmann, H.; Meyer, T. Engineered nanomaterials: Toward effective safety management in research laboratories. J. Nanobiotechnology 2016, 14, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golanski, L.; Guiot, A.; Rouillon, F.; Pocachard, J.; Tardif, F. Experimental evaluation of personal protection devices against graphite nanoaerosols: Fibrous filter media, masks, protective clothing, and gloves. Hum. Exp. Toxicol. 2009, 28, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Dhasmana, A.; Firdaus, S.; Singh, K.P.; Raza, S.; Jamal, Q.M.S.; Kesari, K.K.; Rahman, Q.; Lohani, M. Nanoparticles: Applications, Toxicology and Safety Aspects. In Perspectives in Environmental Toxicology; Springer: Berlin/Heidelberg, Germany, 2017; pp. 47–70. [Google Scholar]
- Park, M.V.; Bleeker, E.A.; Brand, W.; Cassee, F.R.; van Elk, M.; Gosens, I.; de Jong, W.H.; Meesters, J.A.; Peijnenburg, W.J.; Quik, J.T. Considerations for safe innovation: The case of Graphene. ACS Nano 2017, 11, 9574–9593. [Google Scholar] [CrossRef]
- Bałazy, A.; Toivola, M.; Reponen, T.; Podgórski, A.; Zimmer, A.; Grinshpun, S.A. Manikin-based performance evaluation of N95 filtering-facepiece respirators challenged with nanoparticles. Ann. Occup. Hyg. 2006, 50, 259–269. [Google Scholar]
- Dolez, P.; Vinches, L.; Wilkinson, K.; Plamondon, P.; Vu-Khanh, T. Development of a test method for protective gloves against nanoparticles in conditions simulating occupational use. J. Phys. Conf. Ser. 2011, 304, 12066. [Google Scholar] [CrossRef]
- Schug, T.T.; Johnson, A.F.; Balshaw, D.M.; Garantziotis, S.; Walker, N.J.; Weis, C.; Nadadur, S.S.; Birnbaum, L.S. ONE Nano: NIEHS’s strategic initiative on the health and safety effects of engineered nanomaterials. Environ. Health Perspect. 2013, 121, 410–414. [Google Scholar] [CrossRef] [Green Version]
- Dhawan, A.; Shanker, R.; Das, M.; Gupta, K.C. Guidance for safe handling of nanomaterials. J. Biomed. Nanotechnol. 2011, 7, 218–224. [Google Scholar] [CrossRef]
- Ellenbecker, M.; Tsai, S.; Jacobs, M.; Riediker, M.; Peters, T.; Liou, S.; Avila, A.; FossHansen, S. The difficulties in establishing an occupational exposure limit for carbon nanotubes. J. Nanoparticle Res. 2018, 20, 131. [Google Scholar] [CrossRef]
- Boyes, W.K.; Thornton, B.L.M.; Al-Abed, S.R.; Andersen, C.P.; Bouchard, D.C.; Burgess, R.M.; Hubal, E.A.C.; Ho, K.T.; Hughes, M.F.; Kitchin, K. A comprehensive framework for evaluating the environmental health and safety implications of engineered nanomaterials. Crit. Rev. Toxicol. 2017, 47, 771–814. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-G.; Yeo, M.-K. Nanomaterial regulatory policy for human health and environment. Mol. Cell. Toxicol. 2016, 12, 223–236. [Google Scholar] [CrossRef]






| Type | ϕ a (nm) | Content (wt. %) | Relevant Property Improvements |
|---|---|---|---|
| Carbon Nanofibres | 60 to 200 | 2.0 | +22% Flexural strength +11% Tensile strength +4% Glass transition temperature [15] |
| ~200 | 0.3 | +79% Impact absorption energy +42% Inter-laminar shear strength +14% Flexural strength [16] | |
| Multiwalled carbon nanotubes | 10 to 20 | 0.3 | +25 % Flexural strength [17] |
| ~10 | 0.5 | +3% Tensile strength [18] | |
| 30 to 50 | 0.3 | +53% Flexural modulus [19] | |
| Graphene | 8 | 0.1 | +11% Fracture toughness [20] |
| 5 | 0.4 | +19% Tensile modulus +18% Tensile strength +19% Flexural modulus +15% Flexural strength [21] | |
| 100 | 4 | +19% Interlaminar Shear Strength +26% Flexural modulus (90°) +82% Flexural strength (90°) +4% Flexural modulus (0°) +7% Flexural strength (0°) +167% Electrical conductivity [22] |
| Milestone | Topic | 2025 |
|---|---|---|
| Risk assessment (RA) | Proactive risk management | With the use of risk banding tools and effective control measures development practice in 201, high throughput screening approaches will be validated by 2020 |
| Tools | RA-enabled LCA/Integration into decision tools | |
| Epidemiology and health surveillance | Health effect | Implementation of the markers |
| Register | Implementation of results for regulations | |
| Study design | Longitudinal studies started | |
| Databases | Infrastructure | IT procedures for automatic uploading |
| Ontologies | Automatization of ontologies | |
| Risk management | Risk perception and guidance | Guidance on risk evaluation |
| Prevention through design approach | Integration of safe-by-design approaches into development stages of new NMs and their applications |
| Description | Benchmark Exposure Levels |
|---|---|
| Fibrous; a high aspect ratio insoluble NM | 0.01 fibres/ml |
| Any NM which is already classified in its molecular or in its larger particle form as carcinogenic, mutagenic, reproductive toxin or as sensitizing (CMRS) | 0.1 × OEL |
| Insoluble or poorly soluble NM not in the fibrous or CMRS category | 0.066 × OEL |
| Soluble NM not in the fibrous or CMRS category | 0.5 × OEL |
| Hazard Factor | ANSES | CB Nanotool | EPFL | GWSNN | ISPESL | PMSN | Stoffenmanager |
|---|---|---|---|---|---|---|---|
| Toxicity (nano and/or bulk material) | ● | ● | ● | ● | ● | ||
| Solubility | ● | ● | ● | ● | ● | ||
| Fibre form (particle shape) | ● | ● | ● | ● | |||
| Reactivity | ● | ● | ● | ||||
| Size | ● | ● | ● | ||||
| Fire and explosion | ● |
| Exposure Factor | ANSES | CB Nanotool | EPFL | GWSNN | ISPESL | PMSN | Stoffenmanager |
|---|---|---|---|---|---|---|---|
| Quantity | ● | ● | ● | ● | |||
| Duration/ frequency (time) factor | ● | ● | ● | ● | |||
| State of material (e.g., solid, liquid) | ● | ● | ● | ||||
| Release of nano-objects (e.g., dustiness) | ● | ● | ● | ● | ● | ||
| Aggregation/ agglomeration | ● | ● |
| Category | NM and Specifications | OEL Name | Mass Concentration (µg/m3) | Particle Concentration (particle/mL, fibres/cm3) | Surface Concentration (nm2/cm3) |
|---|---|---|---|---|---|
| Inhalation exposure: general MNM approach | |||||
| MNM | Fine particle matter ≤2500 nm | BOEL | 30 | ND | ND |
| MNM | Airborne particles from nanotechnology process | PCVs | ND | 3 times L3PC for more than 30 min | ND |
| Inhalation exposure: categorical approach | |||||
| Fibres | Non-entangled fibrous NM | Acceptance level (default), respirable fraction | ND | 0.01 | ND |
| Fibres | Fibrous NM | BEL | ND | 0.01 | ND |
| Fibres | Carbon nanofibers CNFs | OEL | ND | 0.01 | ND |
| Fibres | Carbon nanotubes CNTs, insoluble NM with high aspect ratio < 3:1 | NRV | ND | 0.01 | ND |
| Inhalation exposure: specific MNM approach | |||||
| Carbon | Multi-walled carbon nanotubes MWCNT 10nm | INEL | 1 | ND | ND |
| Carbon | MWCNT 140 nm | INEL | 2 | ND | ND |
| Carbon | Carbon nanotubes CNTs | No effect concentration in air | 2.5 | ND | ND |
| Carbon | Carbon nanotube group, SWCNT, DWCNT, MWCNT | OEL 15 years | 30 | ND | ND |
| Carbon | All CNTs and nanofibres | REL respirable elemental carbon | <1 | ND | ND |
| Carbon | MWCNT bay tubes | OEL Inhalable fraction | 50 | ND | ND |
| Carbon | MWCNT | DNEL chronic inhalation, systemic immune effect | 0.67 | ND | ND |
| Carbon | Fullerenes C60 | INEL | 7.4 | ND | ND |
| Carbon | Fullerenes C60 | OEL (PL) 15 years | 390 | ND | ND |
| Dermal exposure | |||||
| Carbon | MWCNT | DNEL dermal chronic exposure, assessment factor 3 | 5.9 µg/kg body weight | ND | ND |
| Carbon | MWCNT | DNEL dermal chronic exposure | 17.7 µg/kg body weight | ND | ND |
| Acute short-term exposure | |||||
| MNM | Airborne particles from nanotechnology processes | PCVs single short-term measurement | 5 times the local particle reference value | ND | ND |
| Carbon | MWCNT | DNEL acute inhalation, systemic immune effect | 4.02 | ND | ND |
| Carbon | Fullerenes C60 | INEL short-term, inhalable fraction | 44.4 | ND | ND |
| Carbon | MWCNT | DNEL acute inhalation, pulmonary effect | 201 | ND | ND |
| Carbon | MWCNT | DNEL dermal acute exposure | 106 µg/kg body weight | ND | ND |
| Carbon | MWCNT | DNEL dermal acute exposure, assessment factor 3 | 35.5 µg/kg body weight | ND | ND |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa, S.P.B.; Peixoto, T.; Santos, R.M.; Lopes, A.; Paiva, M.d.C.; Marques, A.T. Health and Safety Concerns Related to CNT and Graphene Products, and Related Composites. J. Compos. Sci. 2020, 4, 106. https://doi.org/10.3390/jcs4030106
Sousa SPB, Peixoto T, Santos RM, Lopes A, Paiva MdC, Marques AT. Health and Safety Concerns Related to CNT and Graphene Products, and Related Composites. Journal of Composites Science. 2020; 4(3):106. https://doi.org/10.3390/jcs4030106
Chicago/Turabian StyleSousa, Susana P.B., Tânia Peixoto, Raquel M. Santos, Ascensão Lopes, Maria da Conceição Paiva, and António T. Marques. 2020. "Health and Safety Concerns Related to CNT and Graphene Products, and Related Composites" Journal of Composites Science 4, no. 3: 106. https://doi.org/10.3390/jcs4030106
APA StyleSousa, S. P. B., Peixoto, T., Santos, R. M., Lopes, A., Paiva, M. d. C., & Marques, A. T. (2020). Health and Safety Concerns Related to CNT and Graphene Products, and Related Composites. Journal of Composites Science, 4(3), 106. https://doi.org/10.3390/jcs4030106