Nonisothermal Crystallization Kinetics of Polylactic Acid under the Influence of Polyolefin Elastomers
Abstract
1. Introduction
2. Theoretical Background
3. Experimental
3.1. Materials
3.2. Sample Preparation
3.3. Characterization
4. Results and Discussions
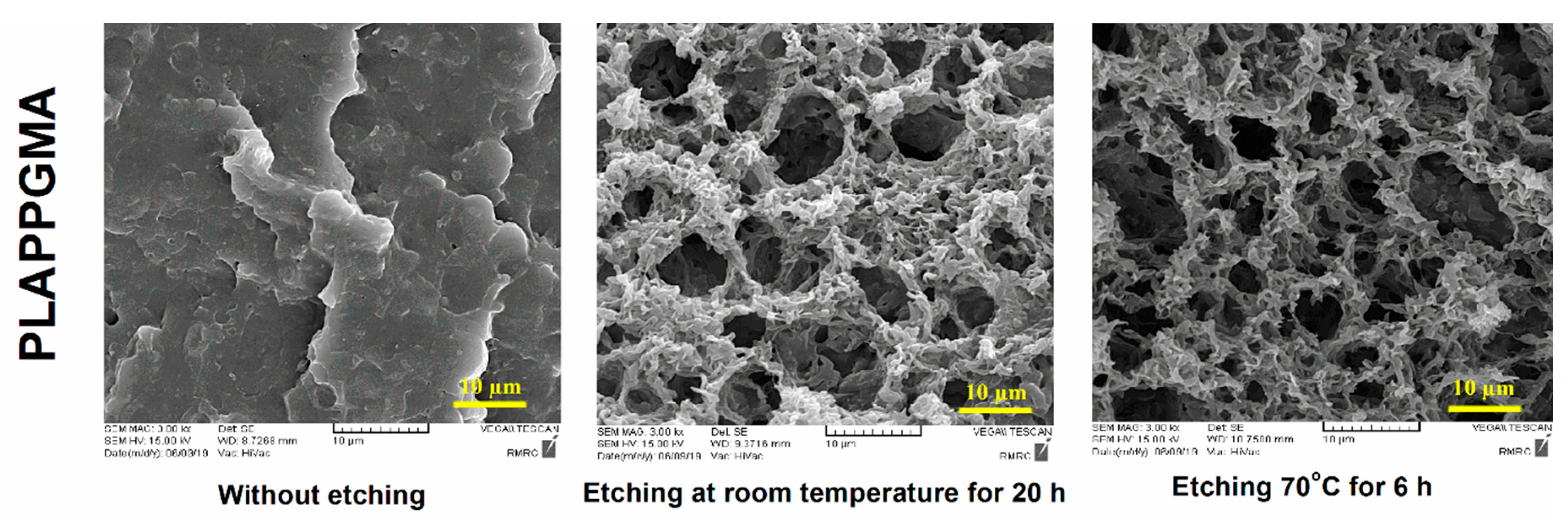
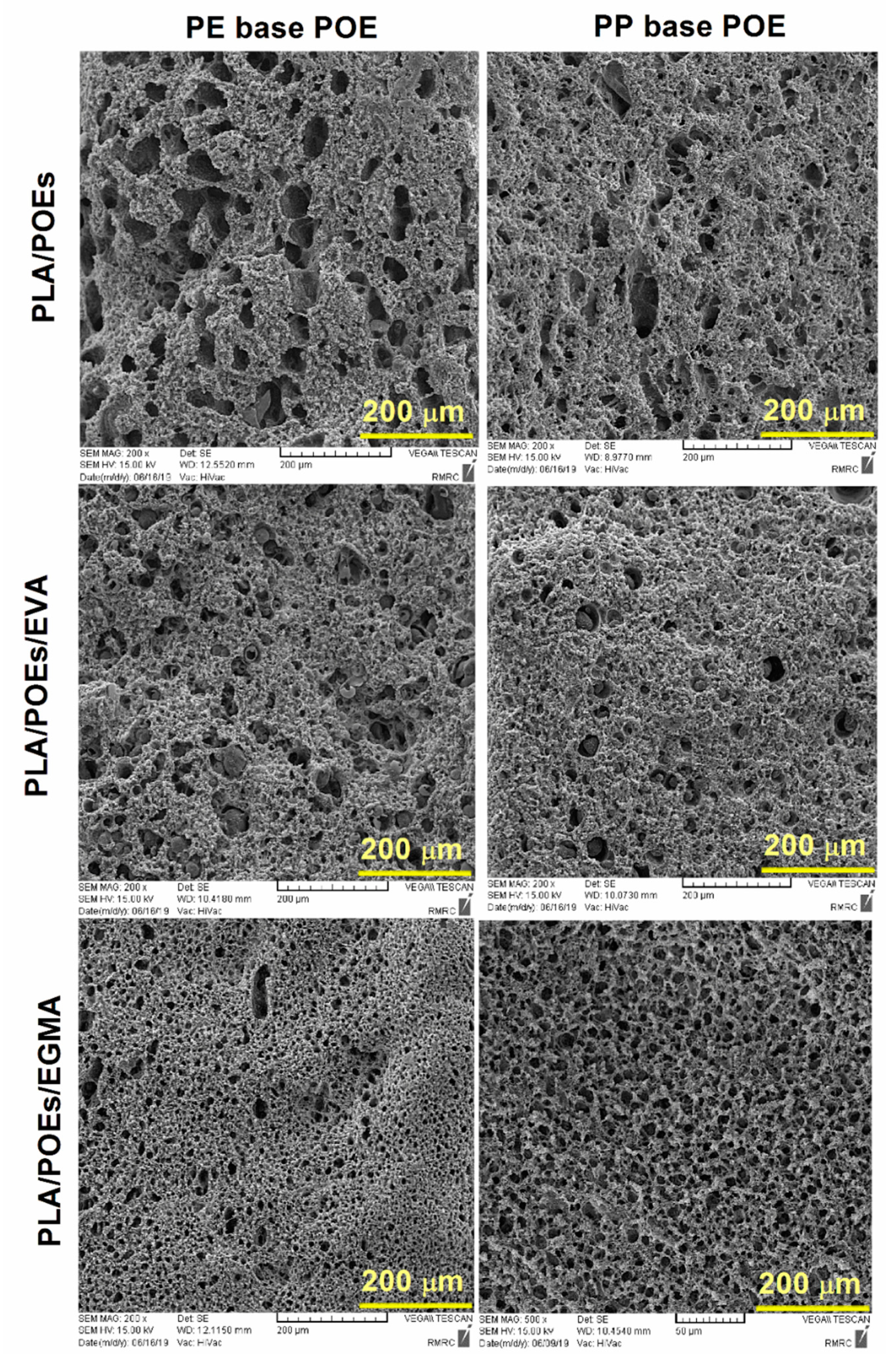
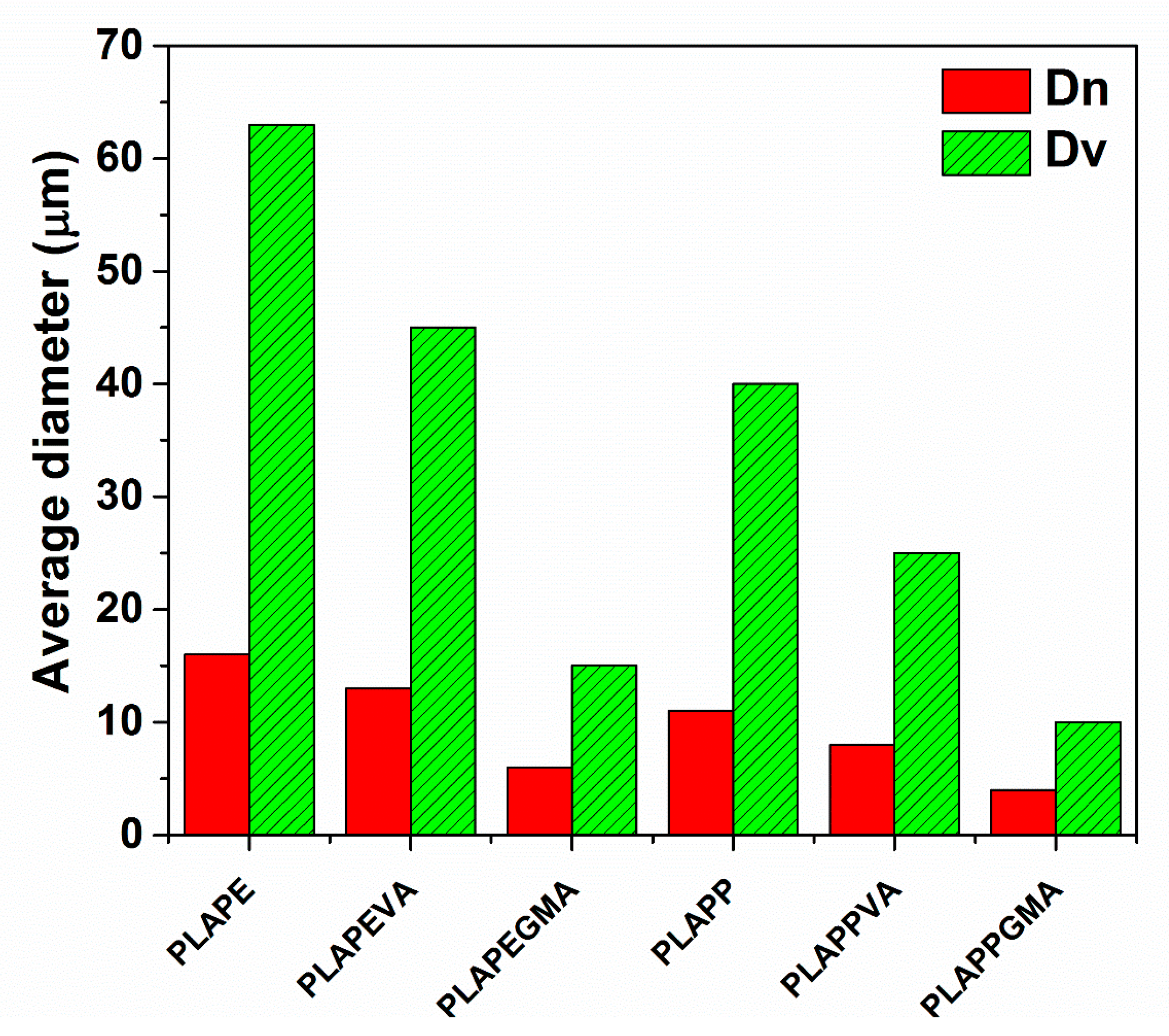
4.1. Morphological Observations
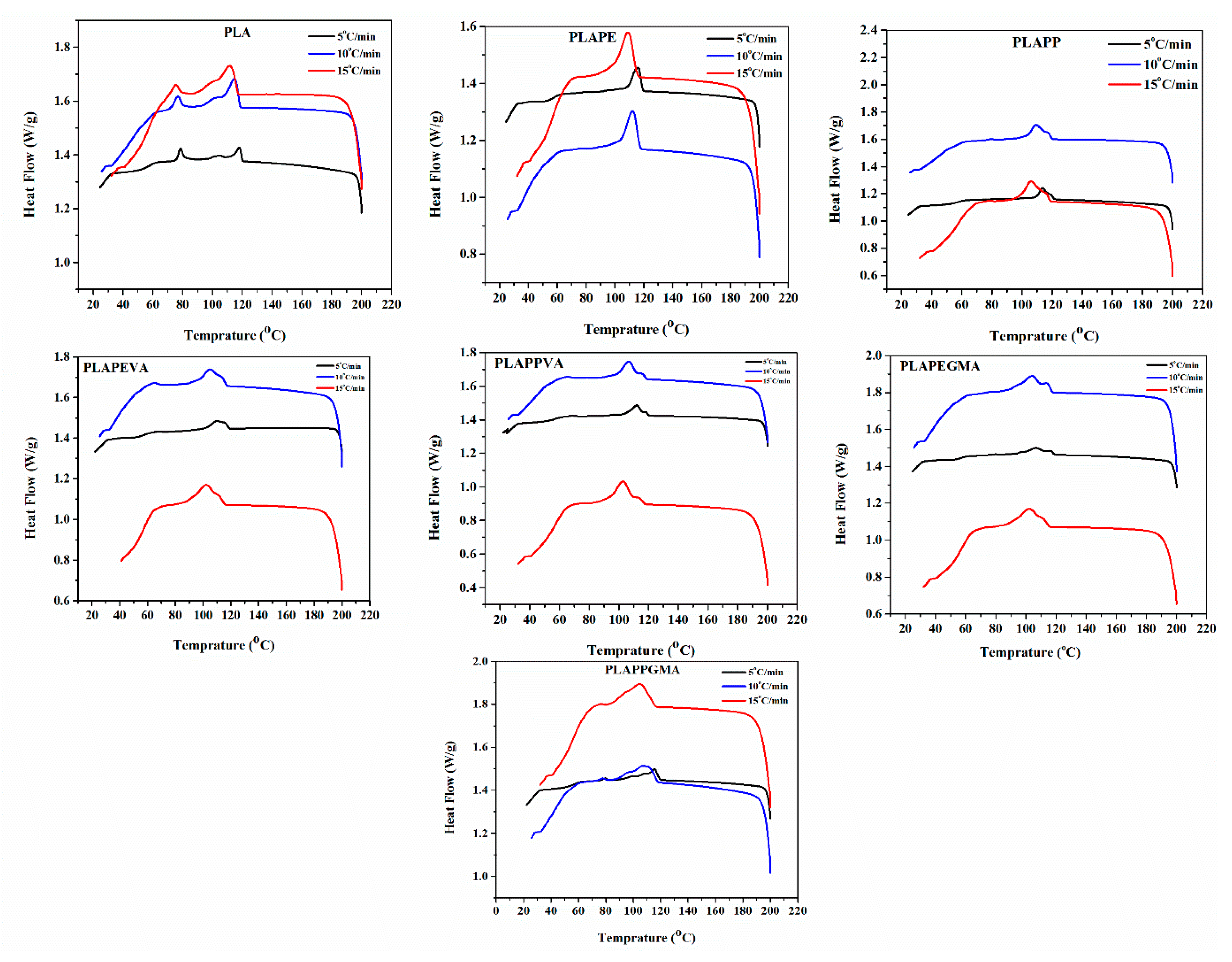
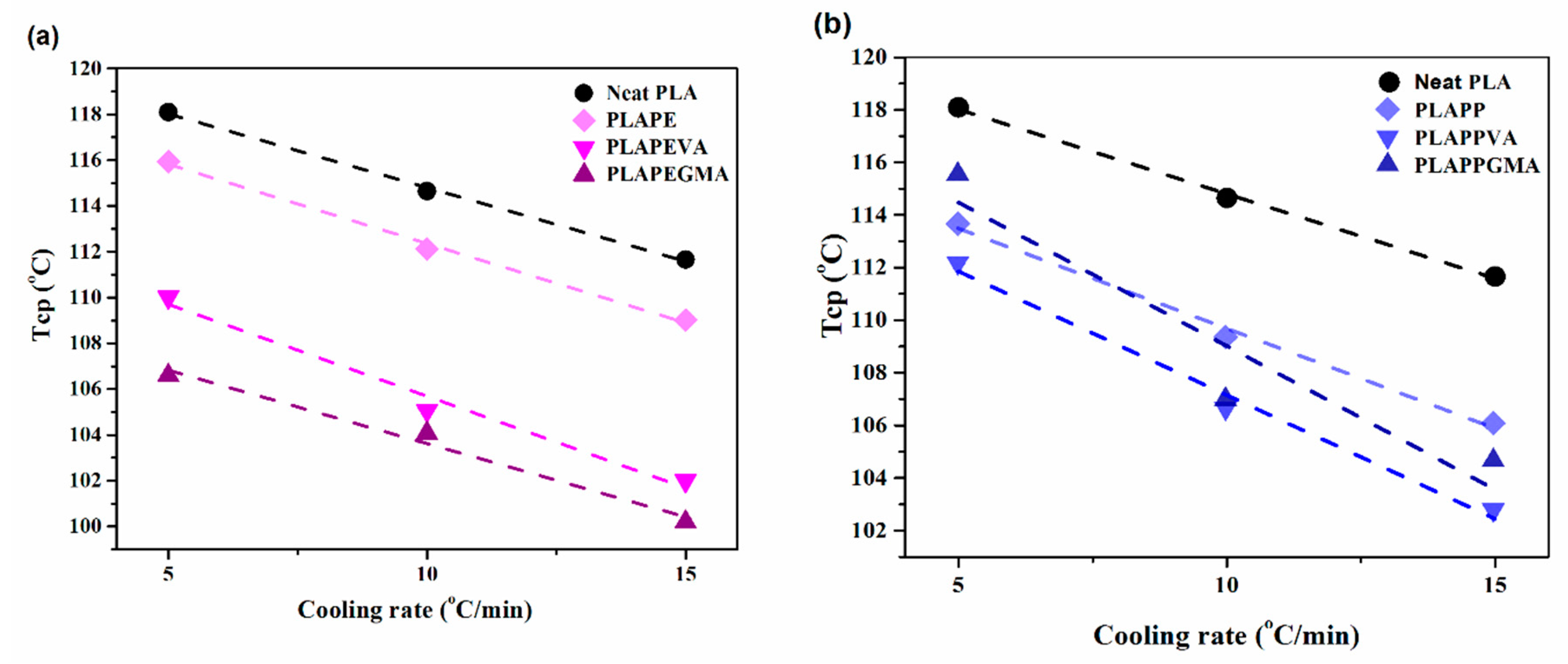
4.2. Nonisothermal Crystallizations
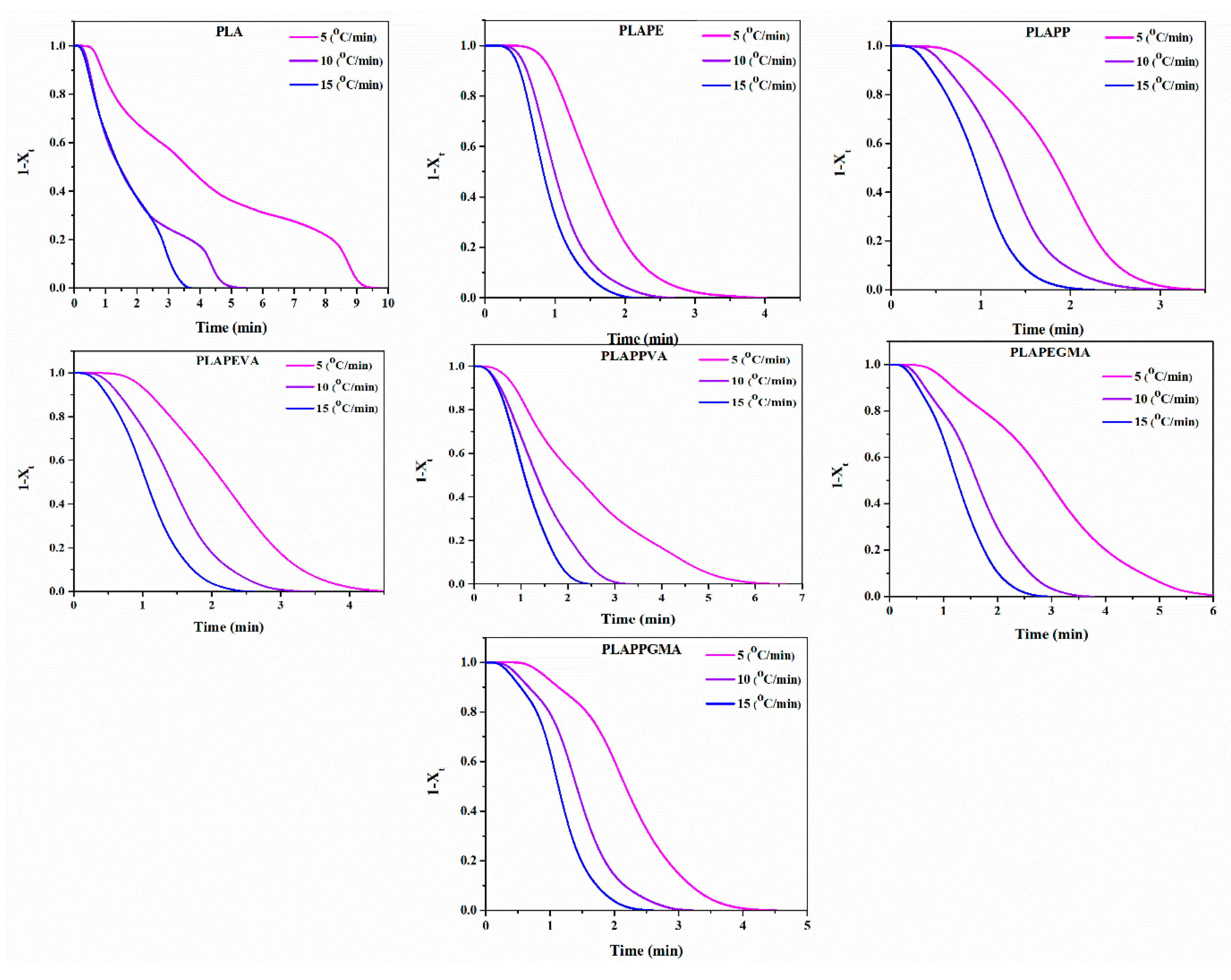
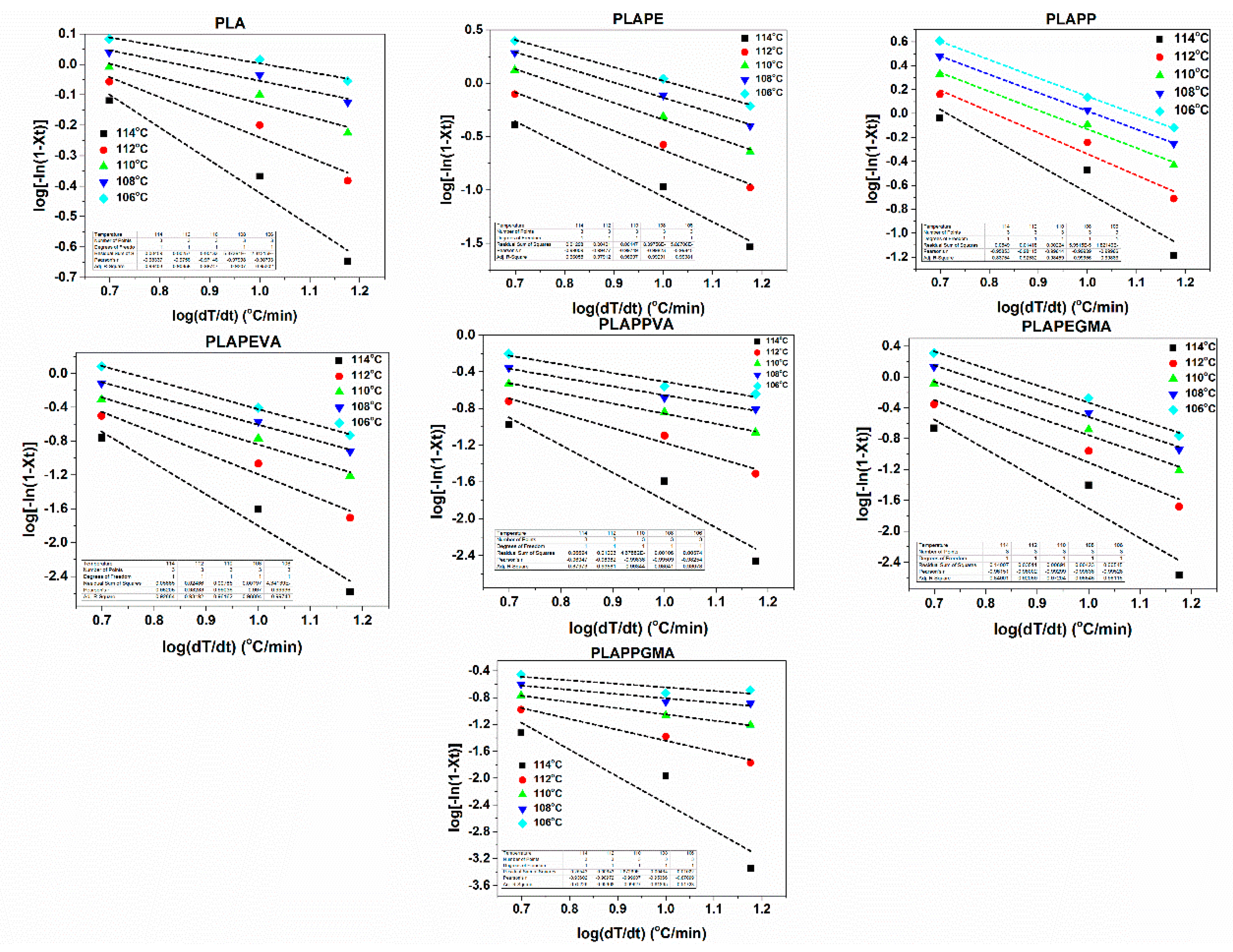
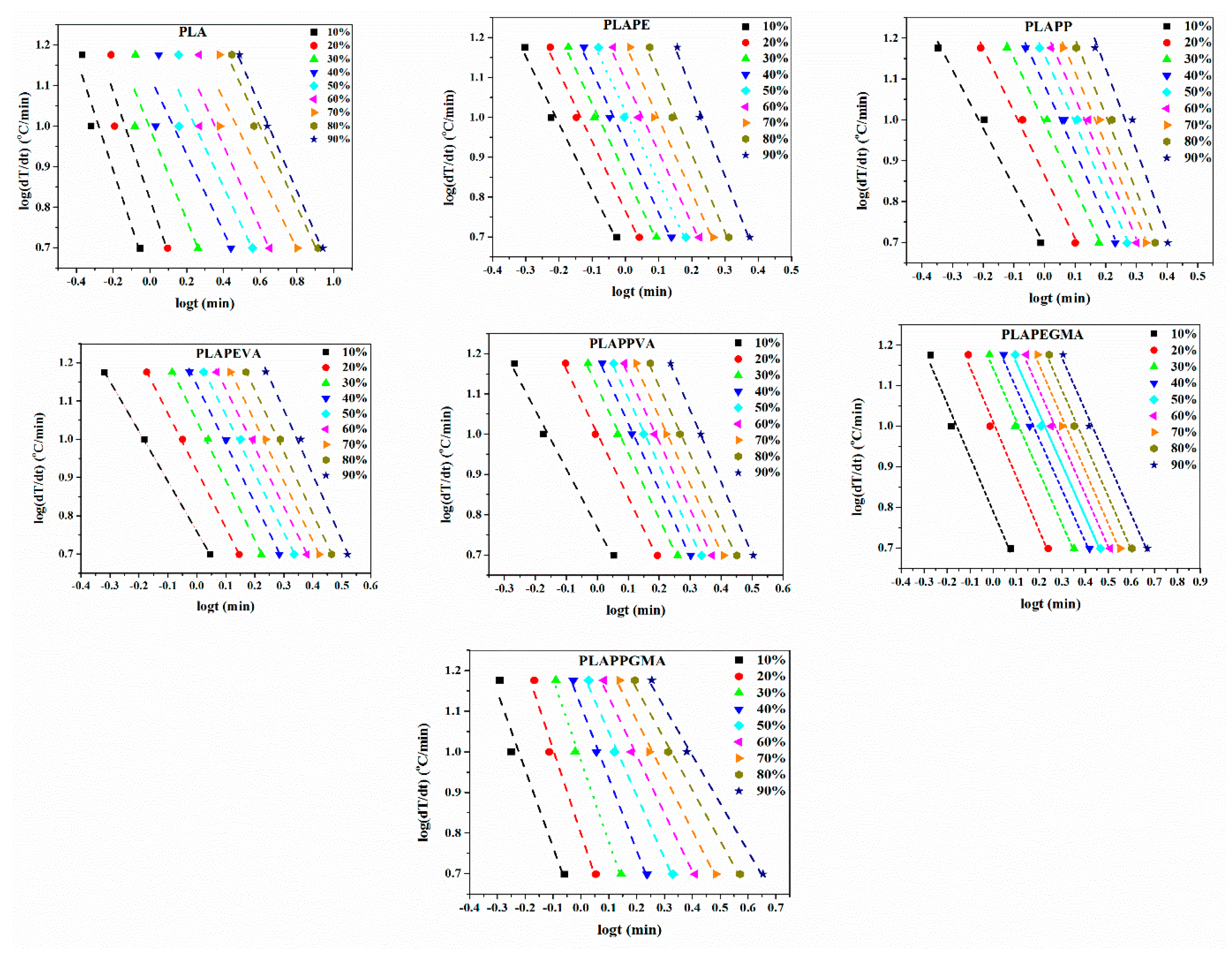
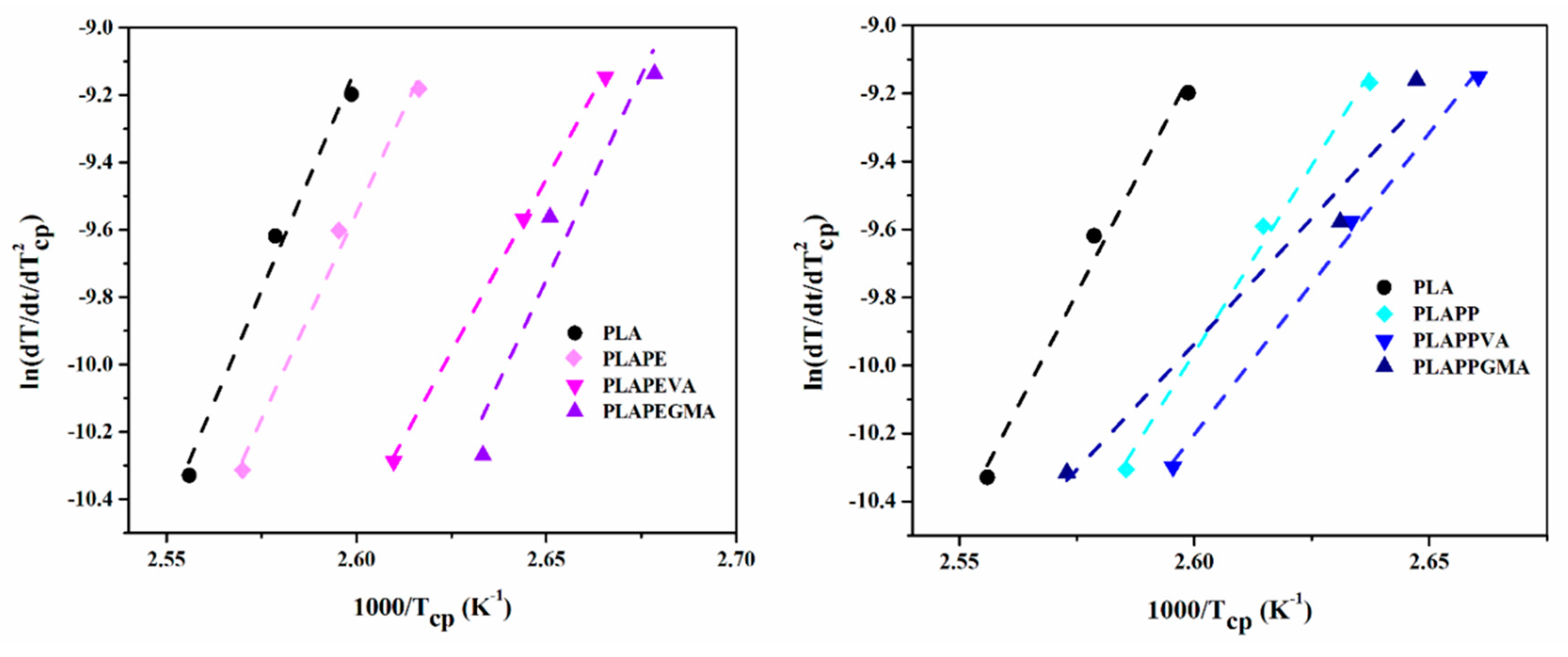
4.3. Crystallization Kinetics
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, S.-L.; Wu, Z.; Yang, W.; Yang, M.-B. Thermal and mechanical properties of chemical crosslinked polylactide (PLA). Polym. Test. 2008, 27, 957–963. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [PubMed]
- Myers, M.; Felty, E. Structural characterizations of vitreous inorganic polymers by thermal studies. Mater. Res. Bull. 1967, 2, 535–546. [Google Scholar] [CrossRef]
- Perego, G.; Cella, G.D.; Bastioli, C. Effect of molecular weight and crystallinity on poly (lactic acid) mechanical properties. J. Appl. Polym. Sci. 1996, 59, 37–43. [Google Scholar] [CrossRef]
- Kong, Y.; Hay, J. The measurement of the crystallinity of polymers by DSC. Polymer 2002, 43, 3873–3878. [Google Scholar] [CrossRef]
- Lu, X.; Hay, J. Isothermal crystallization kinetics and melting behaviour of poly(ethylene terephthalate). Polymer 2001, 42, 9423–9431. [Google Scholar] [CrossRef]
- Ozawa, T. Kinetics of non-isothermal crystallization. Polymer 1971, 12, 150–158. [Google Scholar] [CrossRef]
- Chapleau, N.; Huneault, M.A.; Li, H. Biaxial Orientation of Polylactide/Thermoplastic Starch Blends. Int. Polym. Process. 2007, 22, 402–409. [Google Scholar] [CrossRef]
- Lee, R.E.; Guo, Y.; Tamber, H.; Planeta, M.; Leung, S.N.S. Thermoforming of polylactic acid foam sheets: Crystallization behaviors and thermal stability. Ind. Eng. Chem. Res. 2016, 55, 560–567. [Google Scholar] [CrossRef]
- Serra, T.; Planell, J.A.; Navarro, M. High-resolution PLA-based composite scaffolds via 3-D printing technology. Acta Biomater. 2013, 9, 5521–5530. [Google Scholar] [CrossRef]
- Harris, A.M.; Lee, E.C. Improving mechanical performance of injection molded PLA by controlling crystallinity. J. Appl. Polym. Sci. 2007, 107, 2246–2255. [Google Scholar] [CrossRef]
- Saeidlou, S.; Huneault, M.A.; Li, H.; Park, C.B. Poly(lactic acid) crystallization. Prog. Polym. Sci. 2012, 37, 1657–1677. [Google Scholar] [CrossRef]
- Kulinski, B.; Piorkowska, E. Crystallization, structure and properties of plasticized poly(l-lactide). Polymer 2005, 46, 10290–10300. [Google Scholar] [CrossRef]
- Yokohara, T.; Yamaguchi, M. Structure and properties for biomass-based polyester blends of PLA and PBS. Eur. Polym. J. 2008, 44, 677–685. [Google Scholar] [CrossRef]
- Pantani, R.; De Santis, F.; Sorrentino, A.; De Maio, F.; Titomanlio, G. Crystallization kinetics of virgin and processed poly (lactic acid). Polym. Degrad. Stab. 2010, 95, 1148–1159. [Google Scholar] [CrossRef]
- Jun, C.L. Reactive Blending of Biodegradable Polymers: PLA and Starch. J. Polym. Environ. 2000, 8, 33–37. [Google Scholar] [CrossRef]
- Liu, H.; Song, W.; Chen, F.; Guo, L.; Zhang, J. Interaction of microstructure and interfacial adhesion on impact performance of polylactide (PLA) ternary blends. Macromolecules 2011, 44, 1513–1522. [Google Scholar] [CrossRef]
- Esmizadeh, E.; Sadeghi, T.; Vahidifar, A.; Naderi, G.; Ghoreishy, M.H.R.; Paran, S.M.R. Nano Graphene-Reinforced Bio-nanocomposites Based on NR/PLA: The Morphological, Thermal and Rheological Perspective. J. Polym. Environ. 2019, 27, 1529–1541. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, W.; Huang, Y.; Pan, Y.; Jiang, L.; Dan, Y.; Luo, Y.; Peng, Z. Thermal, mechanical and rheological properties of polylactide toughened by expoxidized natural rubber. Mater. Des. 2013, 45, 198–205. [Google Scholar] [CrossRef]
- Balakrishnan, H.; Hassan, A.; Wahit, M.U. Mechanical, thermal, and morphological properties of polylactic acid/linear low density polyethylene blends. J. Elastomers Plast. 2010, 42, 223–239. [Google Scholar] [CrossRef]
- Xu, Y.; Delgado, P.; Todd, A.D.; Loi, J.; Saba, S.A.; McEneany, R.J.; Tower, T.; Topolkaraev, V.; Macosko, C.W.; Hillmyer, M. Lightweight micro-cellular plastics from polylactide/polyolefin hybrids. Polymer 2016, 102, 73–83. [Google Scholar] [CrossRef]
- Heshmati, V.; Zolali, A.M.; Favis, B.D. Morphology development in poly (lactic acid)/polyamide11 biobased blends: Chain mobility and interfacial interactions. Polymer 2017, 120, 197–208. [Google Scholar] [CrossRef]
- Imre, B.; Bedő, D.; Domján, A.; Schön, P.; Vancso, G.J.; Pukánszky, B. Structure, properties and interfacial interactions in poly (lactic acid)/polyurethane blends prepared by reactive processing. Eur. Polym. J. 2013, 49, 3104–3113. [Google Scholar] [CrossRef][Green Version]
- Wen, S.; Yan, L.; Gong, C.; Zheng, G.; Zhou, Y. Preparation and performance of the polylactic acid/polyolefin-elastomer/thermoplastic starch composite materials. Chin. J. Colloid Polym. 2011, 29, 81–83. [Google Scholar]
- Wu, M.; Wu, Z.; Wang, K.; Zhang, Q.; Fu, Q. Simultaneous the thermodynamics favorable compatibility and morphology to achieve excellent comprehensive mechanics in PLA/OBC blend. Polymer 2014, 55, 6409–6417. [Google Scholar] [CrossRef]
- Jiang, J.; Su, L.; Zhang, K.; Wu, G. Rubber-toughened PLA blends with low thermal expansion. J. Appl. Polym. Sci. 2012, 128, 3993–4000. [Google Scholar] [CrossRef]
- Wu, M.; Wang, K.; Zhang, Q.; Fu, Q. Manipulation of multiphase morphology in the reactive blending system OBC/PLA/EGMA. RSC Adv. 2015, 5, 96353–96359. [Google Scholar] [CrossRef]
- Paran, S.M.R.; Vahabi, H.; Ducos, F.; Formela, K.; Zarrintaj, P.; Laachachi, A.; Saeb, M.R. Crystallization kinetics study of dynamically vulcanized PA6/NBR/HNTs nanocomposites by nonisothermal differential scanning calorimetry. J. Appl. Polym. Sci. 2018, 135, 46488. [Google Scholar] [CrossRef]
- Balazs, A. Crystallization Kinetics of Commercial PLA Filament. Commun.-Sci. Lett. Univ. Zilina 2017, 19, 15–19. [Google Scholar]
- Bianchi, O.; Oliveira, R.; Fiorio, R.; Martins, J.D.N.; Zattera, A.; Canto, L. Assessment of Avrami, Ozawa and Avrami–Ozawa equations for determination of EVA crosslinking kinetics from DSC measurements. Polym. Test. 2008, 27, 722–729. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, J.; Liu, H.; Xiao, C. Applicability of Kissinger model in nonisothermal crystallization assessed using a computer simulation method. J. Therm. Anal. Calorim. 2014, 117, 783–787. [Google Scholar] [CrossRef]
- Rastin, H.; Jafari, S.H.; Saeb, M.R.; Khonakdar, H.A.; Wagenknecht, U.; Heinrich, G. Mechanicalrheological, and thermal behavior assessments in HDPE/PA-6/EVOH ternary blends with variable morphology. J. Polym. Res. 2014, 21, 352. [Google Scholar] [CrossRef]
- Battegazzore, D. Crystallization kinetics of poly(lactic acid)-talc composites. Express Polym. Lett. 2011, 5, 849–858. [Google Scholar] [CrossRef]
- Garlotta, D. A Literature Review of Poly(Lactic Acid). J. Polym. Environ. 2001, 9, 63–84. [Google Scholar] [CrossRef]
- Rosli, N.A.; Ahmad, I.; Anuar, F.H.; Abdullah, I. Mechanical and thermal properties of natural rubber-modified poly(lactic acid) compatibilized with telechelic liquid natural rubber. Polym. Test. 2016, 54, 196–202. [Google Scholar] [CrossRef]
- Hay, J.N. Application of the modified avrami equations to polymer crystallisation kinetics. Br. Polym. J. 1971, 3, 74–82. [Google Scholar] [CrossRef]
- Cahn, J.W. Transformation kinetics during continuous cooling. Acta Met. 1956, 4, 572–575. [Google Scholar] [CrossRef]
- Sharples, A. Overall kinetics of crystallization. In Introduction to Polymer Crystallization; St. Martin’s Press: New York, NY, USA, 1966; pp. 44–59. [Google Scholar]

| Sample Code | PLA (phr) | POE (phr) | EVA (phr) | EGMA (phr) |
|---|---|---|---|---|
| PLA | 100 | - | - | - |
| PLAPE | 80 | 20 | - | - |
| PLAPEVA | 80 | 20 | 4 | - |
| PLAPEGMA | 80 | 20 | - | 4 |
| PLAPP | 80 | 20 | - | - |
| PLAPPVA | 80 | 20 | 4 | - |
| PLAPPGMA | 80 | 20 | - | 4 |
| Samples | Cooling Rate (°C/min) | Initial Crystallization Temperature, | Peak Temperature, | Crystallization Enthalpy, | Crystallization Temperature Range, | Crystallization Time, (s) | Degree of Crystallization, (%) |
|---|---|---|---|---|---|---|---|
| PLA | 5 | 122.29 | 118.10 | 9.50 | 48.41 | 9.68 | 10.22 |
| 10 | 120.39 | 114.65 | 10.64 | 54.65 | 5.46 | 11.44 | |
| 15 | 118.87 | 111.66 | 12.52 | 55.84 | 3.72 | 13.46 | |
| PLAPE | 5 | 122.19 | 115.94 | 6.62 | 20.08 | 4.02 | 8.89 |
| 10 | 120.71 | 112.12 | 7.14 | 26.99 | 2.70 | 9.60 | |
| 15 | 119.59 | 109.01 | 7.06 | 32.73 | 2.18 | 9.48 | |
| PLAPEVA | 5 | 121.62 | 110.02 | 5.37 | 23.83 | 4.77 | 7.22 |
| 10 | 119.55 | 105.06 | 6.92 | 34.83 | 3.48 | 9.30 | |
| 15 | 117.59 | 102.00 | 6.61 | 39.21 | 2.61 | 8.89 | |
| PLAPEGMA | 5 | 121.61 | 106.60 | 6.31 | 33.00 | 6.60 | 8.49 |
| 10 | 119.86 | 104.07 | 8.89 | 37.66 | 3.77 | 11.95 | |
| 15 | 117.80 | 100.19 | 8.94 | 44.43 | 2.96 | 12.02 | |
| PLAPP | 5 | 123.78 | 113.63 | 6.46 | 17.93 | 3.59 | 8.68 |
| 10 | 122.87 | 109.31 | 7.03 | 31.66 | 3.17 | 9.45 | |
| 15 | 121.32 | 106.02 | 7.86 | 33.98 | 2.27 | 10.56 | |
| PLAPPVA | 5 | 122.61 | 112.14 | 6.04 | 22.58 | 4.52 | 8.11 |
| 10 | 120.55 | 106.59 | 7.07 | 32.16 | 3.22 | 9.50 | |
| 15 | 119.33 | 102.72 | 7.53 | 38.97 | 2.60 | 10.12 | |
| PLAPPGMA | 5 | 121.02 | 115.52 | 7.60 | 33.25 | 6.65 | 10.21 |
| 10 | 119.03 | 106.93 | 7.92 | 33.49 | 3.35 | 10.65 | |
| 15 | 118.08 | 104.60 | 7.70 | 37.97 | 2.53 | 10.35 |
| Cooling Rate (°C/min) | Samples | n | (min) | |
|---|---|---|---|---|
| 5 | PLA | 1.20 | 0.68 | 3.61 |
| 10 | 1.27 | 0.91 | 2.04 | |
| 15 | 1.41 | 0.94 | 1.39 | |
| 5 | PLAPE | 3.28 | 0.69 | 1.52 |
| 10 | 2.98 | 0.95 | 1.02 | |
| 15 | 2.89 | 1.00 | 0.83 | |
| 5 | PLAPP | 3.22 | 0.64 | 1.86 |
| 10 | 2.84 | 0.90 | 1.64 | |
| 15 | 2.66 | 0.99 | 1.18 | |
| 5 | PLAPEVA | 2.77 | 0.61 | 2.17 |
| 10 | 2.52 | 0.88 | 1.58 | |
| 15 | 2.45 | 0.97 | 1.19 | |
| 5 | PLAPPVA | 3.09 | 0.58 | 2.17 |
| 10 | 2.77 | 0.88 | 1.55 | |
| 15 | 2.79 | 0.95 | 1.25 | |
| 5 | PLAPEGMA | 2.29 | 0.58 | 2.93 |
| 10 | 2.28 | 0.87 | 1.67 | |
| 15 | 2.36 | 0.94 | 1.31 | |
| 5 | PLAPPGMA | 1.77 | 0.70 | 2.14 |
| 10 | 2.08 | 0.91 | 1.08 | |
| 15 | 2.43 | 0.96 | 0.81 |
| Samples | T (°C) | m | Samples | T (°C) | m | ||
|---|---|---|---|---|---|---|---|
| PLA | 118 | 0.01 | 0.10 | PLAPEVA | 116 | 0.23 | 0.11 |
| 116 | 0.03 | 0.22 | 115 | 0.05 | 0.22 | ||
| 113 | 0.08 | 0.36 | 113 | 0.21 | 0.35 | ||
| 108 | 0.07 | 0.51 | 112 | 0.06 | 0.51 | ||
| 104 | 0.00 | 0.69 | 111 | 0.08 | 0.69 | ||
| 100 | 0.01 | 0.92 | 110 | 0.02 | 0.92 | ||
| 91 | 0.00 | 1.20 | 108 | 0.09 | 1.20 | ||
| 81 | 0.06 | 1.61 | 107 | 0.10 | 1.61 | ||
| 79 | 0.04 | 2.30 | 105 | 0.06 | 2.30 | ||
| PLAPE | 117 | 0.92 | 0.10 | PLAPPEVA | 117 | 0.06 | 0.11 |
| 117 | 0.09 | 0.22 | 115 | 0.04 | 0.22 | ||
| 116 | 0.18 | 0.36 | 114 | 0.02 | 0.36 | ||
| 115 | 0.05 | 0.51 | 113 | 0.29 | 0.51 | ||
| 115 | 0.01 | 0.69 | 112 | 0.07 | 0.69 | ||
| 114 | 0.13 | 0.91 | 111 | 0.20 | 0.92 | ||
| 113 | 0.19 | 1.20 | 110 | 0.17 | 1.21 | ||
| 112 | 0.06 | 1.60 | 108 | 0.08 | 1.61 | ||
| 110 | 0.00 | 2.30 | 107 | 0.01 | 2.30 | ||
| PLAPP | 119 | 0.26 | 0.11 | PLAPEGMA | 116 | 0.33 | 0.10 |
| 117 | 0.18 | 0.22 | 113 | 0.08 | 0.22 | ||
| 116 | 0.14 | 0.35 | 110 | 0.15 | 0.36 | ||
| 115 | 0.18 | 0.51 | 108 | 0.09 | 0.51 | ||
| 114 | 0.12 | 0.69 | 107 | 0.00 | 0.69 | ||
| 114 | 0.21 | 0.92 | 105 | 0.00 | 0.91 | ||
| 113 | 0.18 | 1.21 | 104 | 0.17 | 1.20 | ||
| 112 | 0.09 | 1.60 | 102 | 0.03 | 1.61 | ||
| 111 | 0.02 | 2.30 | 98 | 0.08 | 2.30 | ||
| PLAPPGMA | 117 | 0.17 | 0.11 | PLAPPGMA | 117 | 0.17 | 0.11 |
| 115 | 0.22 | 0.22 | 115 | 0.22 | 0.22 | ||
| 114 | 0.08 | 0.36 | 114 | 0.08 | 0.36 | ||
| 112 | 0.01 | 0.51 | 112 | 0.01 | 0.51 | ||
| 110 | 0.03 | 0.69 | 110 | 0.03 | 0.69 | ||
| 108 | 0.09 | 0.91 | 108 | 0.09 | 0.91 | ||
| 106 | 0.03 | 1.20 | 106 | 0.03 | 1.20 | ||
| 102 | 0.12 | 1.61 | 102 | 0.12 | 1.61 | ||
| 98 | 0.09 | 2.30 | 98 | 0.09 | 2.30 |
| Cooling Rate (°C/min) | Samples | Samples | Samples | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 5 | PLA | 2.19 | 0.69 | PLAPEVA | 1.43 | 0.69 | PLAPPGMA | 1.38 | 0.62 |
| 10 | 2.39 | 0.99 | 1.55 | 0.87 | 1.33 | 0.82 | |||
| 15 | 2.24 | 1.21 | 1.60 | 0.99 | 1.13 | 1.00 | |||
| 5 | PLAPE | 1.33 | 0.79 | PLAPPVA | 1.45 | 0.54 | |||
| 10 | 1.34 | 1.01 | 1.54 | 0.67 | |||||
| 15 | 1.28 | 1.14 | 1.57 | 0.79 | |||||
| 5 | PLAPP | 1.31 | 0.76 | PLAPEGMA | 1.69 | 0.65 | |||
| 10 | 1.50 | 0.92 | 1.74 | 0.77 | |||||
| 15 | 1.56 | 1.05 | 1.77 | 0.86 | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khosravi, A.; Fereidoon, A.; Khorasani, M.M.; Berthe, V.; Vahabi, H.; Paran, S.M.R.; Naderi, G.; Saeb, M.R. Nonisothermal Crystallization Kinetics of Polylactic Acid under the Influence of Polyolefin Elastomers. J. Compos. Sci. 2020, 4, 65. https://doi.org/10.3390/jcs4020065
Khosravi A, Fereidoon A, Khorasani MM, Berthe V, Vahabi H, Paran SMR, Naderi G, Saeb MR. Nonisothermal Crystallization Kinetics of Polylactic Acid under the Influence of Polyolefin Elastomers. Journal of Composites Science. 2020; 4(2):65. https://doi.org/10.3390/jcs4020065
Chicago/Turabian StyleKhosravi, Azadeh, Abdolhossein Fereidoon, Mohammad Mehdi Khorasani, Vincent Berthe, Henri Vahabi, Seyed Mohammad Reza Paran, Ghasem Naderi, and Mohammad Reza Saeb. 2020. "Nonisothermal Crystallization Kinetics of Polylactic Acid under the Influence of Polyolefin Elastomers" Journal of Composites Science 4, no. 2: 65. https://doi.org/10.3390/jcs4020065
APA StyleKhosravi, A., Fereidoon, A., Khorasani, M. M., Berthe, V., Vahabi, H., Paran, S. M. R., Naderi, G., & Saeb, M. R. (2020). Nonisothermal Crystallization Kinetics of Polylactic Acid under the Influence of Polyolefin Elastomers. Journal of Composites Science, 4(2), 65. https://doi.org/10.3390/jcs4020065








