Abstract
Background/Objectives: Leishmania spp. are protozoan parasites transmitted by female sandflies (Phlebotomus or Lutzomyia). Clinical manifestations depend on species and host immunity. While cutaneous and visceral forms prevail, mucocutaneous involvement—particularly isolated nasal septum leishmaniasis—is rare and frequently misdiagnosed as an inflammatory, infectious, or neoplastic condition. Risk factors associated with mucocutaneous leishmaniasis include systemic or local immunodeficiency, prior renal transplantation, treatment with chronic inhaled steroids, residence in endemic areas or travel to such regions, and previous Leishmania infections. Immunosuppressed patients are at higher risk for atypical presentations and delayed diagnosis, which can result in extensive tissue destruction. Early clinical suspicion, histopathological confirmation, and prompt therapy are essential to prevent permanent mucosal damage. Therefore, a multidisciplinary approach is needed for adequate evaluation and effective treatment. Methods: A 67-year-old man with rheumatoid arthritis on methotrexate reported a two-year history of right-sided nasal obstruction and ulceration that failed to respond to antibiotics. He did not present systemic symptoms. Results: Facial CT revealed a septal deviation; the patient underwent septoplasty, and biopsy confirmed Leishmania amastigotes. Serology (rK39 immunochromatographic test) was positive. He was treated with liposomal amphotericin B at 4 mg/kg/day for five days, followed by miltefosine at 100 mg/day orally for 14 days. At an eight-week follow-up, the nasal mucosa was fully healed, obstruction was resolved, and there was no evidence of recurrence. Conclusions: Although nasal septum leishmaniasis is uncommon, it should be considered in the differential diagnosis of chronic nasal lesions, especially in immunocompromised patients or those from endemic regions. Definitive diagnosis requires biopsy with histological or molecular confirmation. Combined liposomal amphotericin B and miltefosine therapy yields high cure rates and prevents mucosal destruction. Early recognition is critical to avoid diagnostic delays and long-term sequelae.
1. Introduction and Clinical Significance
Leishmania spp. is a protozoan parasite primarily transmitted through the bite of female sandflies belonging to the genera Phlebotomus or Lutzomyia. This parasite can cause various clinical conditions, which differ considerably based on the specific Leishmania species involved, the inoculation site, and the host’s immune state. Clinical manifestations of leishmaniasis can affect the skin, mucocutaneous areas, or involve visceral organs. Each manifestation presents distinct clinical and pathological characteristics.
In the field of otolaryngology, mucocutaneous leishmaniasis (MCL) frequently involves the nasal mucosa, a site notably susceptible to chronic inflammatory processes and tissue destruction. Several documented cases in the literature highlight endonasal involvement as a significant clinical manifestation of MCL [1,2,3,4,5]. It typically presents ulceration, nasal obstruction, epistaxis, or even septal perforation, leading to considerable diagnostic challenges. Histopathological evaluation of nasal lesions often reveals chronic inflammatory infiltrates with or without detectable intracellular amastigotes, necessitating additional diagnostic modalities such as polymerase chain reaction (PCR) for confirmation.
Multiple risk factors predispose individuals to mucocutaneous leishmaniasis, including systemic or localized immunodeficiency, prior organ transplantation (notably renal), chronic inhaled steroid therapy, prolonged residence or recent travel to endemic regions, and previous infection with Leishmania species. Immunocompromised patients, particularly those receiving immunosuppressive therapies, exhibit an increased susceptibility to the disease as well as severe symptoms.
The present case highlights the clinical complexity inherent in diagnosing and managing chronic nasal obstruction and ulceration in immunocompromised patients. This complexity necessitates a multidisciplinary approach involving otolaryngologists, infectious disease specialists, pathologists, and other healthcare professionals to ensure accurate diagnosis, adequate therapeutic intervention, and comprehensive follow-up. Early recognition and treatment of MCL are essential to prevent significant morbidity and potential disfiguring complications, particularly in patients with an extensive latency period between initial infection and mucosal manifestation.
2. Case Presentation
We present the case of a 67-year-old male patient, resident in Madrid, Spain, who presented with a two-year history of persistent nasal crusting, progressive obstruction, and a chronic ulcer located in the right nasal cavity. During this prolonged period, he had been treated empirically with various antibiotics without significant clinical improvement. He denied fever, chills, rigors, weight loss, night sweats, skin lesions, arthralgias, or rashes. His past medical history was relevant for rheumatoid arthritis, diagnosed in the early 2000s and considered clinically inactive, managed with long-term low-dose methotrexate and folic acid supplementation. The patient also had a history of hypertension, dyslipidemia, benign prostatic hyperplasia, mitral valve prolapses with mild regurgitation, and a cardiac ablation for Wolff–Parkinson–White syndrome. He was a light smoker (1–2 cigarettes per day), retired, and previously employed as a glass worker.
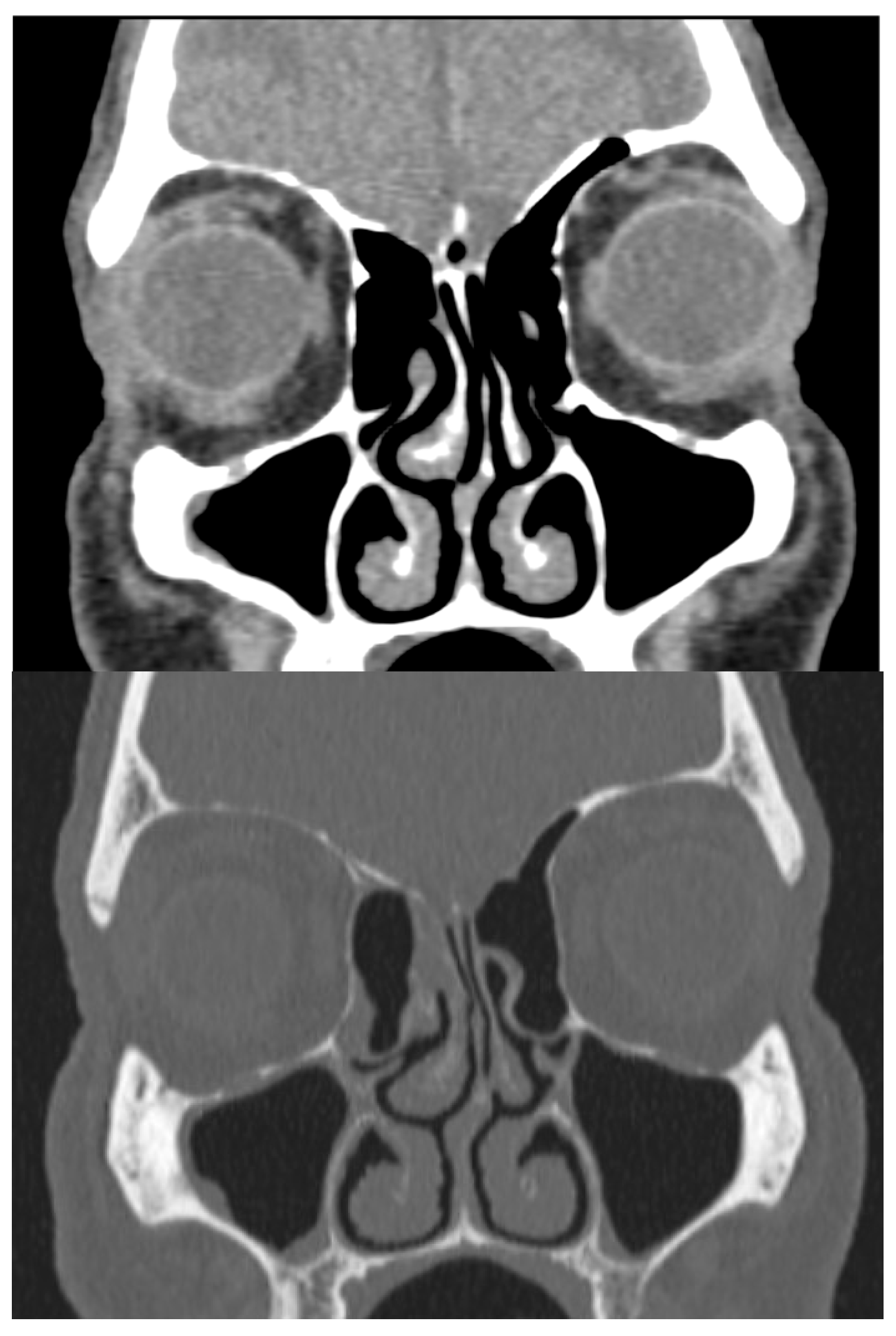
Physical examination revealed a large mass occupying the entire lumen of the right nasal cavity. There was no initial evidence of septal perforation. A computed tomography (CT) scan of the paranasal sinuses showed left septal deviation but failed to explain the origin of the mucosal mass (Figure 1). Due to persistent symptoms and obstructive findings, a septoplasty was performed.
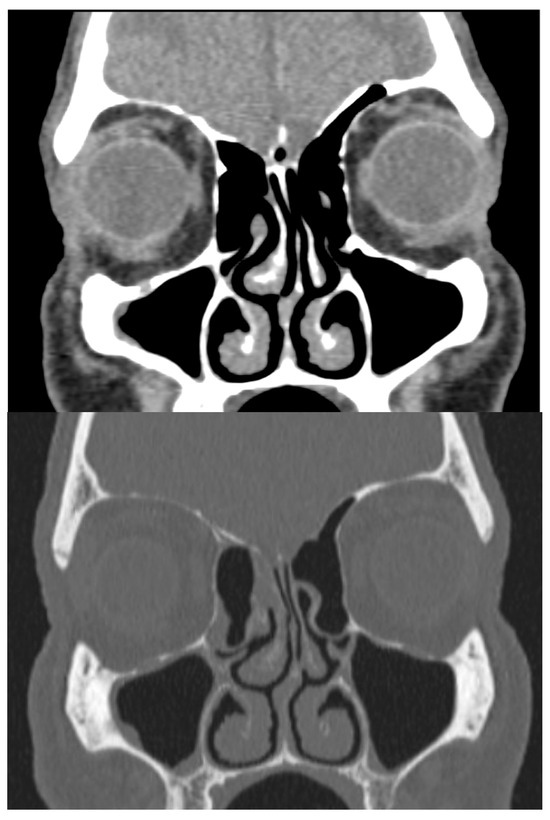
Figure 1.
CT scan in bone and soft-tissue windows showing septal deviation. Anterior curving is shown towards the left nasal fossa with a right inferior spur in contact with the ipsilateral middle turbinate and hypertrophy of the inferior turbinates.
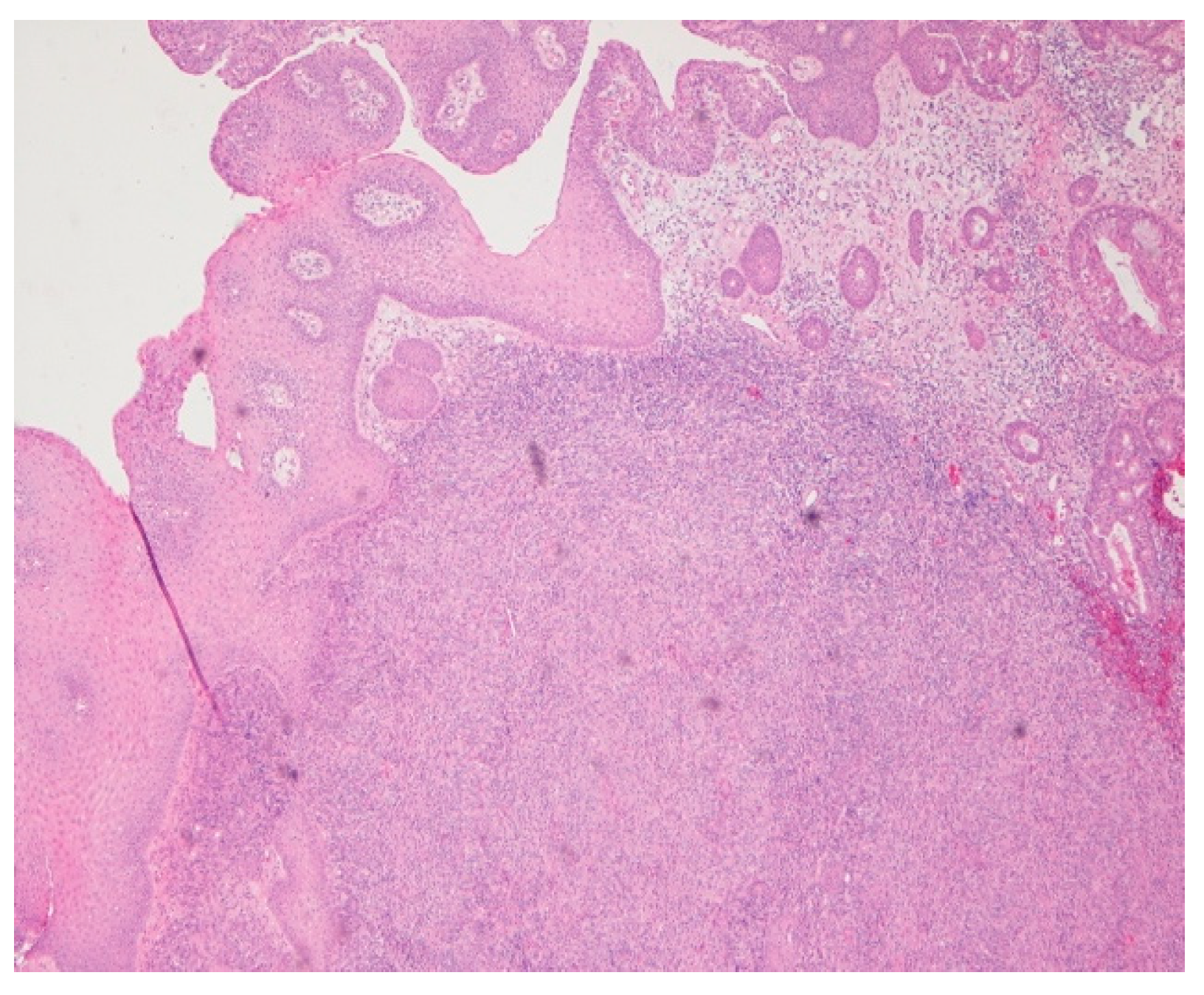
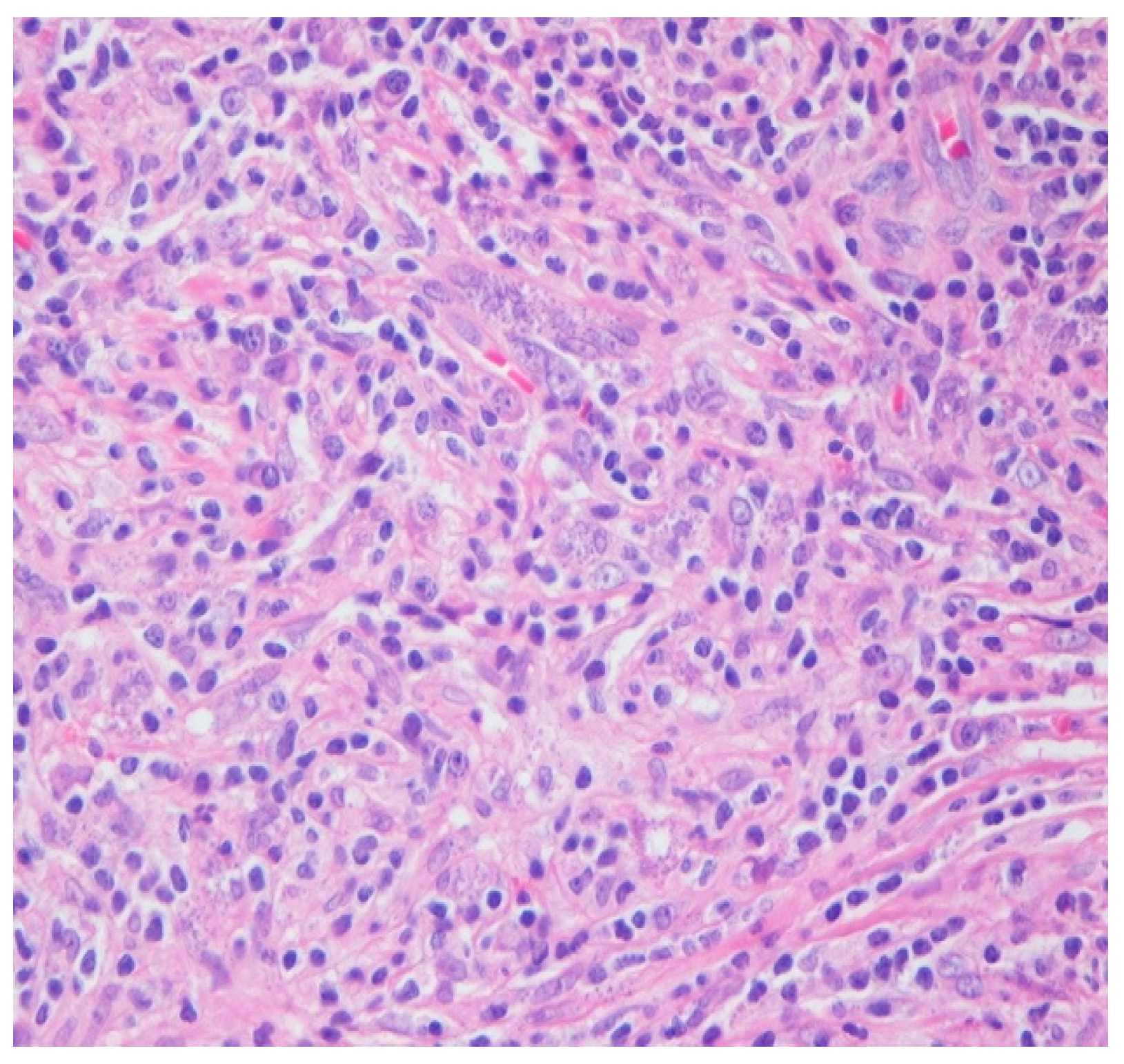
Intraoperatively, the nasal mucosa appeared friable, and the cartilaginous septum was fragmented and necrotic. Tissue samples were collected for histological examination. Histopathological evaluation of the biopsied cartilage and mucosa revealed squamous epithelial hyperplasia and surface erosion with associated crusting. The lamina propria was markedly expanded and infiltrated by a dense, diffuse inflammatory infiltrate consisting of lymphocytes, histiocytes, plasma cells, and occasional multinucleated giant cells. Notably, numerous intracellular amastigotes, consistent with Leishmania spp., were identified within histiocytes (Figure 2 and Figure 3). These findings were highly suggestive of mucosal leishmaniasis in the context of chronic nasal inflammation. Laboratory tests revealed normal blood counts and renal function, with minimal inflammatory marker elevation. Serologic testing was positive for rK39, supporting a diagnosis of active Leishmania infection, although PCR testing was negative, and CPA was indeterminate.
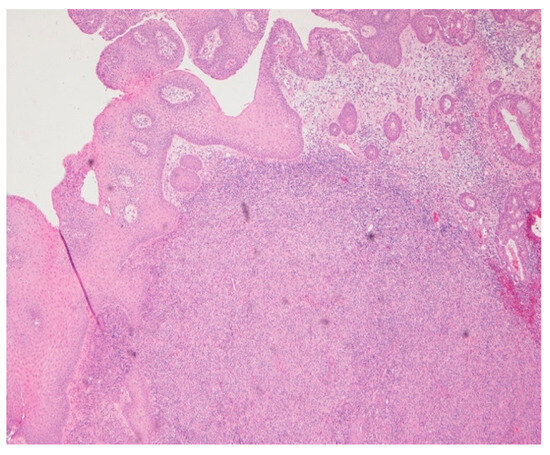
Figure 2.
Hematoxylin and eosin (HE) 4×. Chronic inflammatory infiltration, with dense diffuse lymphohistioplasmocytic infiltrates.
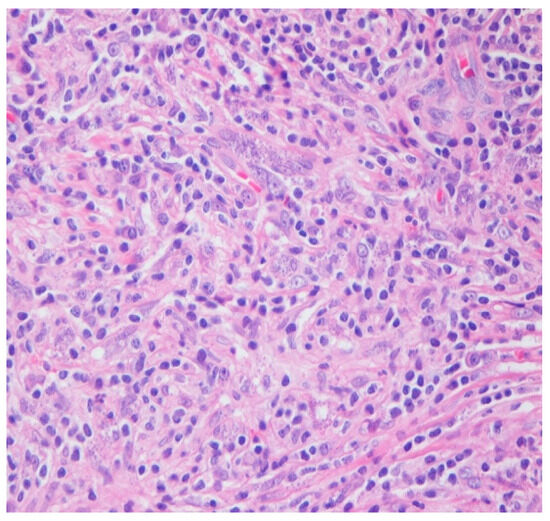
Figure 3.
HE 40×. Admixed histocytes, lymphocytes, and plasma cells, as well as intracellular organisms with round, basophilic structures and rod-like kinetoplasts scattered haphazardly and filling the histocytes (amastigotes).
Given the patient’s immunosuppressed status due to the use of methotrexate, a treatment plan consistent with regimens used in visceral leishmaniasis and HIV-associated disease was adopted.
Afterwards, the patient was treated with liposomal amphotericin B at a dose of 4 mg/kg/day for five consecutive days, reaching a cumulative dose of 20 mg/kg, followed by a 14-day course of miltefosine. This combination led to significant clinical improvement and complete resolution of the septal mucosal findings.
During treatment, the patient experienced transient side effects, including mild dizziness following amphotericin B infusions and gastrointestinal discomfort—mainly nausea and abdominal bloating—without vomiting or diarrhea. More notably, renal function deteriorated during the treatment with amphotericin B, with a temporary increase in serum creatinine and a corresponding decline in the estimated glomerular filtration rate. These changes were closely monitored, and despite the renal effects, the treatment was completed successfully. Renal function showed gradual improvement after therapy, supporting the safety and efficacy of this therapeutic approach under proper surveillance.
During the three-month and twelve-month follow-up, the nasal lesion was completely resolved with no recurrence observed on endoscopic examination, and the patient remained asymptomatic.
3. Discussion
Leishmaniasis is a vector-borne disease caused by protozoan parasites of the genus Leishmania, which are transmitted through the bite of infected female sandflies. It is endemic in over 80 countries, with high incidence across multiple continents, including Asia, South America, and parts of Africa and Europe. Countries notably affected include India, Iran, Afghanistan, Pakistan, Brazil, Bolivia, Peru, Colombia, Algeria, Tunisia, and Morocco. In Southern Europe, cases have been reported in France, Italy, Spain, Greece, and Portugal, typically linked to travel from endemic regions. Illustratively, one patient from the United Kingdom presented with nasal lesions after traveling to South America, reinforcing the clinical importance of detailed travel histories [1]. Another notable case was reported in Vienna, involving a patient who had traveled through South America and Spain [2].
The mucocutaneous form of leishmaniasis (MCL) represents one of the most severe clinical presentations of the disease. Clinical manifestations are largely dependent on the infecting Leishmania species, parasite virulence, and host immune response. Typically, two Leishmania species are responsible for MCL: Leishmania (Viannia) braziliensis and Leishmania (Viannia) panamensis. However, other species can occasionally cause mucosal involvement [6,7].
In Italy, a noteworthy case reported by Di Lella et al. (2006) involved an elderly man who had lived extensively in Latin America, specifically Brazil, and presented mucosal lesions several years after returning to Italy [7]. This patient exhibited significant involvement of the nasal, pharyngeal, laryngeal, and tracheal mucosa. Clinically, he experienced episodes of recurrent epistaxis, nasal congestion, progressive dysphagia, weight loss, and ulcerating lesions. A physical examination revealed extensive nasal septal perforation, destruction of the inferior nasal turbinates, and multiple granulomatous lesions throughout the upper aerodigestive tract. Diagnostic imaging with CT demonstrated substantial tissue damage, including subtotal loss of the cartilaginous septum. The lesions were histologically characterized by a chronic inflammatory infiltrate with histiocytes containing Leishman–Donovan bodies. Isoenzyme electrophoresis ultimately confirmed the causative agent as L. (Viannia) braziliensis. He was treated with liposomal amphotericin B at a dose of 3 mg/kg for seven days per cycle, with the cycle repeated three times over a six-week period. This prolonged regimen was necessary given the severity and extension of mucosal involvement [7].
This clinical presentation illustrates several important diagnostic challenges frequently encountered in non-endemic regions. Firstly, MCL commonly presents with nonspecific nasal and oropharyngeal symptoms such as congestion, rhinorrhea, epistaxis, dysphagia, and ulcerations, mimicking benign or malignant conditions and complicating accurate diagnosis. Secondly, histological examination with standard hematoxylin–eosin (H&E) staining frequently reveals nonspecific chronic inflammation, requiring targeted staining methods, such as Giemsa, or advanced diagnostic modalities like isoenzyme electrophoresis and PCR for definitive identification [7,8].
Additionally, in a brief case series from Tunisia, four patients with mucosal involvement of the lips and one with endonasal symptoms were documented (Kharfi et al., 2003) [5]. This finding highlights the importance of considering MCL in patients from endemic regions [5].
Imported leishmaniasis must be considered in humans, as they are influenced by migration, travel, and conflicts that disrupt epidemiological patterns. Effective monitoring and management of these cases is crucial to prevent the spread of Leishmania into non-endemic areas [4].
It is crucial to note that the area where the patient in this study resides experienced the largest recorded community outbreak of leishmaniasis in Europe, affecting over 400 people. This outbreak, caused by “Leishmania infantum”, led to both visceral leishmaniasis and cutaneous leishmaniasis, with dogs as the primary reservoir [9,10].
Diagnosis of leishmaniasis can be challenging due to its unusual presentation. Symptoms may appear rapidly or develop slowly, complicating its diagnosis in patients. In otolaryngology, MCL can present as inflammation, edema, and ulceration of the nasal mucosa, with possible destruction of the underlying cartilage progressing to substantial tissue degradation and disfigurement. Nasal manifestations of this disease most commonly affect the cartilaginous nasal septum and the anterior portions of the nasal cavities, including the vestibule, inferior turbinates, and the floor of the nose [11]. The parasite can infiltrate cartilage and underlying tissues, leading to massive granuloma formation [1].
Other areas that can be affected include the labial region, pharynx, tongue, the floor of the mouth, and can even extend to the larynx, trachea, and bronchi [12].
Importantly, the immunologic status of the patient significantly influences the clinical manifestation and severity of MCL. Immunocompromised individuals, especially those receiving immunosuppressive therapies for autoimmune diseases or undergoing organ transplantation, are particularly susceptible. A history of immunodeficiency, such as that induced by methotrexate for rheumatoid arthritis, as in the case of our patient, is a significant predisposing comorbidity. Additionally, chronic use of inhaled or systemic steroids, smoking, and a personal history of Leishmania infection are well-documented risk factors [3,4].
The clinical relevance of MCL lies not only in its potentially severe tissue destruction and disfigurement but also in its possible misdiagnosis as a malignant or other granulomatous condition. Differential diagnosis includes carcinoma, lymphoma, midline granuloma, tuberculosis, sarcoidosis, and fungal infections. Therefore, clinicians should maintain a high index of suspicion for MCL in patients presenting with refractory mucosal lesions, particularly those with relevant epidemiological backgrounds [7,8].
Diagnosis relies heavily on detailed clinical evaluation, comprehensive physical examination, histopathological and cytological analyses, and molecular diagnostic techniques. Daneshbod et al. (2011) specifically highlighted the importance of cytological examination in diagnosing MCL, as cytology often provides rapid identification of characteristic findings such as free and intracellular Leishman–Donovan bodies, multinucleated giant cells, granulomas, and Reed–Sternberg–like cells, alongside acute and chronic inflammatory infiltrates [8]. Cytology proved particularly beneficial in cases with inconclusive biopsies or when malignancy was erroneously suspected [8].
Histopathological examinations frequently reveal granulomatous inflammation, significant lymphoplasmacytic infiltration, ulceration, and occasionally, pseudoepitheliomatous hyperplasia. Definitive identification of parasites within tissue biopsies may necessitate special staining methods (Giemsa staining), immunohistochemistry, or molecular diagnostics such as PCR, as routine histological methods may miss scarce parasites [7,8].
Furthermore, MCL is known for its unpredictable and often prolonged latency period, which can vary widely from several months to multiple decades. This latency refers to the interval between the resolution of an initial cutaneous leishmaniasis episode and the delayed onset of mucosal involvement. Such latency complicates diagnosis and clinical suspicion, especially in patients without recent travel to endemic regions or apparent risk factors. One of the most striking illustrations of this delayed presentation comes from a reported case in Peru, where a female patient who had been successfully treated for cutaneous leishmaniasis nearly thirty years earlier developed mucosal symptoms decades after her initial exposure.
This patient, without any known new exposure to the parasite, began experiencing recurrent episodes of epistaxis, which persisted for approximately three years before an accurate diagnosis was reached. Even after receiving treatment for mucosal leishmaniasis, she continued to test positive for Leishmania DNA through sensitive molecular diagnostics like PCR for up to sixteen months following therapy. This prolonged presence of parasite genetic material—despite clinical management—highlights the chronic, relapsing nature of MCL and the potential for parasite persistence in mucosal tissues long after initial infection and even long after apparent treatment success [13].
In Colombia, Osorio et al. highlighted that mucosal lesions due to L. panamensis frequently and concomitantly presented with active cutaneous lesions, which are usually less severe and destructive compared to L. braziliensis-related lesions. Such cases highlight the necessity for systematic examination of mucosal tissues in patients presenting with active cutaneous lesions, allowing for early diagnosis and improved management outcomes [6].
For the diagnosis of mucocutaneous leishmaniasis, a thorough clinical history, physical examination, and histopathological study of the lesion are essential. Additionally, immunohistochemical evaluation and the Montenegro skin test can aid in diagnosis and monitoring. The Montenegro test indicates exposure to the parasite but does not differentiate whether the disease is active or treated [10].
To confirm the diagnosis, the rK39 test and PCR are highly effective diagnostic tools. The rK39 test detects antibodies against a specific parasite antigen, while PCR allows for the detection of the parasite’s DNA in biological samples. These tests are especially useful when the clinical presentation is atypical or when the patient presents with factors complicating the diagnosis [10].
Treatment for MCL includes pentavalent antimonials such as sodium stibogluconate, liposomal amphotericin B, and oral medications such as azoles, miltefosine, and paromomycin [10].
Di Lella et al. (2006) notably described successful treatment using liposomal amphotericin B, which resulted in significant lesion resolution [7]. However, they also highlighted important potential toxicities, such as cardiac side effects including hypotension and bradycardia, which necessitated close clinical monitoring and occasionally treatment discontinuation [7].
In the systematic review conducted by Dr. Fischer on treatment options for mucocutaneous leishmaniasis, various previously mentioned therapies are included. However, the choice of treatment should be individualized in each case, depending on local availability and the patient’s comorbidities [14]. Given that our patient was immunosuppressed and elderly, a more potent and safe treatment was required. Liposomal amphotericin B was considered a suitable option, providing an improved safety profile compared to conventional amphotericin B, especially in patients with conditions affecting immunity [3].
4. Conclusions
Nasal leishmaniasis is an uncommon entity that should be part of the differential diagnosis of patients presenting with difficult-to-manage nasal lesions, especially those from endemic areas. The patients’ travel history needs to be carefully reviewed.
Once mucosal leishmaniasis is identified, prompt and appropriate treatment becomes critical. Liposomal amphotericin B remains the gold standard therapy, and treatment should be maintained until there is evident and sustained clinical improvement. Ultimately, maintaining a high index of suspicion and proactively including leishmaniasis in the diagnostic work-up is vital to avoid delays in diagnosis and ensure the patient receives timely and effective care. Doing so can significantly influence long-term health outcomes and help prevent irreversible complications associated with the disease.
In conclusion, MCL presents a series of complex challenges related to clinical presentation, diagnosis, and treatment.
The diagnostic process benefits greatly from cytological examination supported by histopathological findings and molecular methods such as PCR, all of which enhance diagnostic precision. Effective therapeutic management requires a nuanced approach, taking into account the patient’s underlying health conditions, the toxicity profiles of antiparasitic agents, and the need for close post-treatment monitoring to detect potential relapses. Broader public health efforts should emphasize early identification, timely intervention, and long-term prevention through sustained vector control measures in order to reduce the impact and burden of this potentially severe disease.
Author Contributions
Conceptualization, A.A. and G.P.; methodology, A.A.; validation, A.A., A.N. and G.B.; investigation, A.A. and C.M.M.; data curation, A.A., A.N. and G.B.; writing—original draft preparation, A.A.; writing—review and editing, A.A., G.B. and G.P.; supervision, G.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to local regulations on case reports.
Informed Consent Statement
Verbal informed consent has been obtained from the patient to publish this paper rather than written because the patient has left the district.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We would like to extend our gratitude to the Departments of Otolaryngology, Pathology, and Internal Medicine at Fuenlabrada Hospital for their excellent collaboration and active involvement in the management of this case. We also wish to express our sincere appreciation to Carmen Bernardino, an exceptionally skilled biochemist, for her invaluable assistance in the preparation of this manuscript. Her meticulous work in translating key sections from Spanish to English significantly enhanced the clarity and precision of the text.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CT scan | Computed Tomography |
| PCR | Polymerase Chain Reaction |
| CPA | Circulating Parasite Antigen |
| HE | Hematoxylin and Eosin |
| MCL | Mucocutaneous Form of Leishmaniasis |
References
- Shareef, M.M.; Trotter, M.I.; Cullen, R.J. Leishmaniasis of the nasal cavity: A case report. J. Laryngol. Otol. 2005, 119, 1015–1017. [Google Scholar] [CrossRef] [PubMed]
- Harrison, N.; Walochnik, J.; Ramsebner, R.; Veletzky, L.; Lagler, H.; Ramharter, M. Progressive perforation of the nasal septum due to Leishmania major: A case of mucosal leishmaniasis in a traveler. Am. J. Trop. Med. Hyg. 2017, 96, 653–655. [Google Scholar] [CrossRef] [PubMed]
- Grech, P.; Vella, S.M.; Piscopo, T. Leishmania donovani mucosal leishmaniasis in Malta. BMJ Case Rep. 2020, 13, e237687. [Google Scholar] [CrossRef] [PubMed]
- Suqati, A.A.; Pudszuhn, A.; Hofmann, V.M. Mucocutaneous leishmaniasis: Case report and literature review of a rare endonasal infection. Pan Afr. Med. J. 2020, 36, 292. [Google Scholar] [CrossRef] [PubMed]
- Kharfi, M.; Fazaa, B.; Chaker, E.; Kamoun, M.R. Mucosal localization of leishmaniasis in Tunisia: Five cases. Ann. Dermatol. Vénéréol. 2003, 130, 27–30. [Google Scholar] [PubMed]
- Osorio, L.E.; Castillo, C.M.; Ochoa, M.T. Mucosal leishmaniasis due to Leishmania (Viannia) panamensis in Colombia: Clinical characteristics. Am. J. Trop. Med. Hyg. 1998, 59, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Di Lella, F.; Vincenti, V.; Zennaro, D.; Bacciu, A.; Bacciu, S.; Di Lella, G. Mucocutaneous leishmaniasis: Report of a case with massive involvement of nasal, pharyngeal and laryngeal mucosa. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2006, 102, 870–872. [Google Scholar] [CrossRef] [PubMed]
- Daneshbod, Y.; Oryan, A.; Davarmanesh, M.; Shirian, S.; Negahban, S.; Aledavood, A.; Davarpanah, M.A.; Soleimanpoor, H.; Daneshbod, K. Clinical, histopathologic, and cytologic diagnosis of mucosal leishmaniasis and literature review. Arch. Pathol. Lab. Med. 2011, 135, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Horrillo, L.; San Martín, J.V.; Molina, L. Atypical presentation in adults in the largest community outbreak of leishmaniasis in Europe (Fuenlabrada, Spain). Clin. Microbiol. Infect. 2015, 21, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.G.; Navarro, A.; Coello, M.M.R.; Miranda, E.; Plaza, G.; Arbía, A. Laryngeal leishmaniasis: Still a challenging diagnosis. J. Clin. Images Med. Case Rep. 2022, 3, 2194. [Google Scholar] [CrossRef]
- Marra, F.; Chiappetta, M.C.; Vincenti, V. Ear, nose and throat manifestations of mucocutaneous leishmaniasis: A literature review. Acta Biomed. 2014, 85, 3–7. [Google Scholar] [PubMed]
- De Vries, H.J.C.; Schallig, H.D. Cutaneous leishmaniasis: A 2022 updated narrative review into diagnosis and management developments. Am. J. Clin. Dermatol. 2022, 23, 823–840. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Jaimes, J.; Frischtak, H.L.; Arenas, J.; Lescano, A.G. Mucosal leishmaniasis presenting with nasal septum perforation after almost thirty years. Am. J. Trop. Med. Hyg. 2018, 99, 327–330. [Google Scholar] [CrossRef]
- Fischer, T.; Fischer, M.; Schliemann, S.; Elsner, P. Treatment of mucocutaneous leishmaniasis—A systematic review. J. Dtsch. Dermatol. Ges. 2024, 22, 763–773. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).