GLI1-Altered Mesenchymal Tumours in the Head and Neck: A Case Report and Literature Review
Abstract
1. Introduction
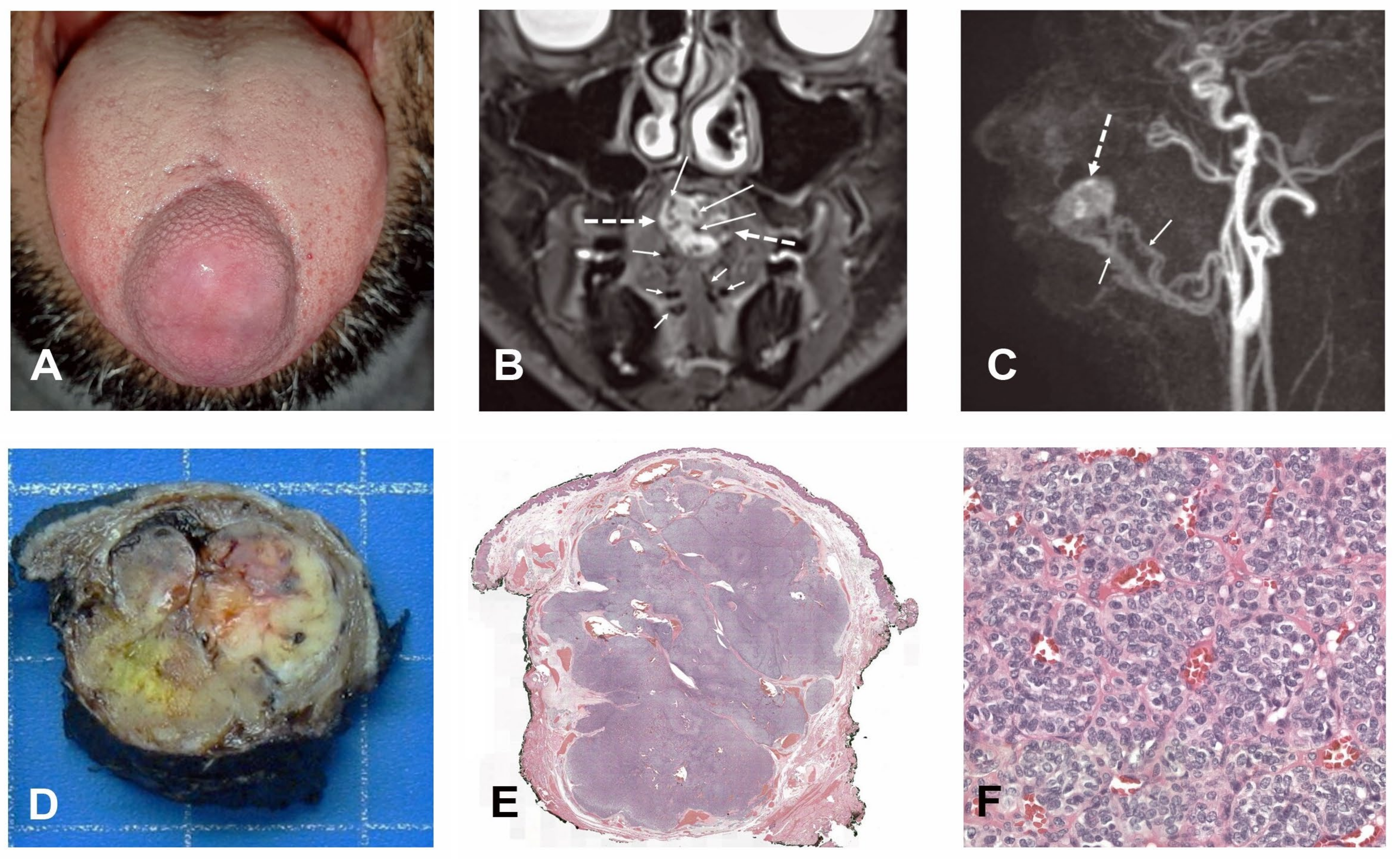
2. Case Report
2.1. Clinical History
2.2. Imaging
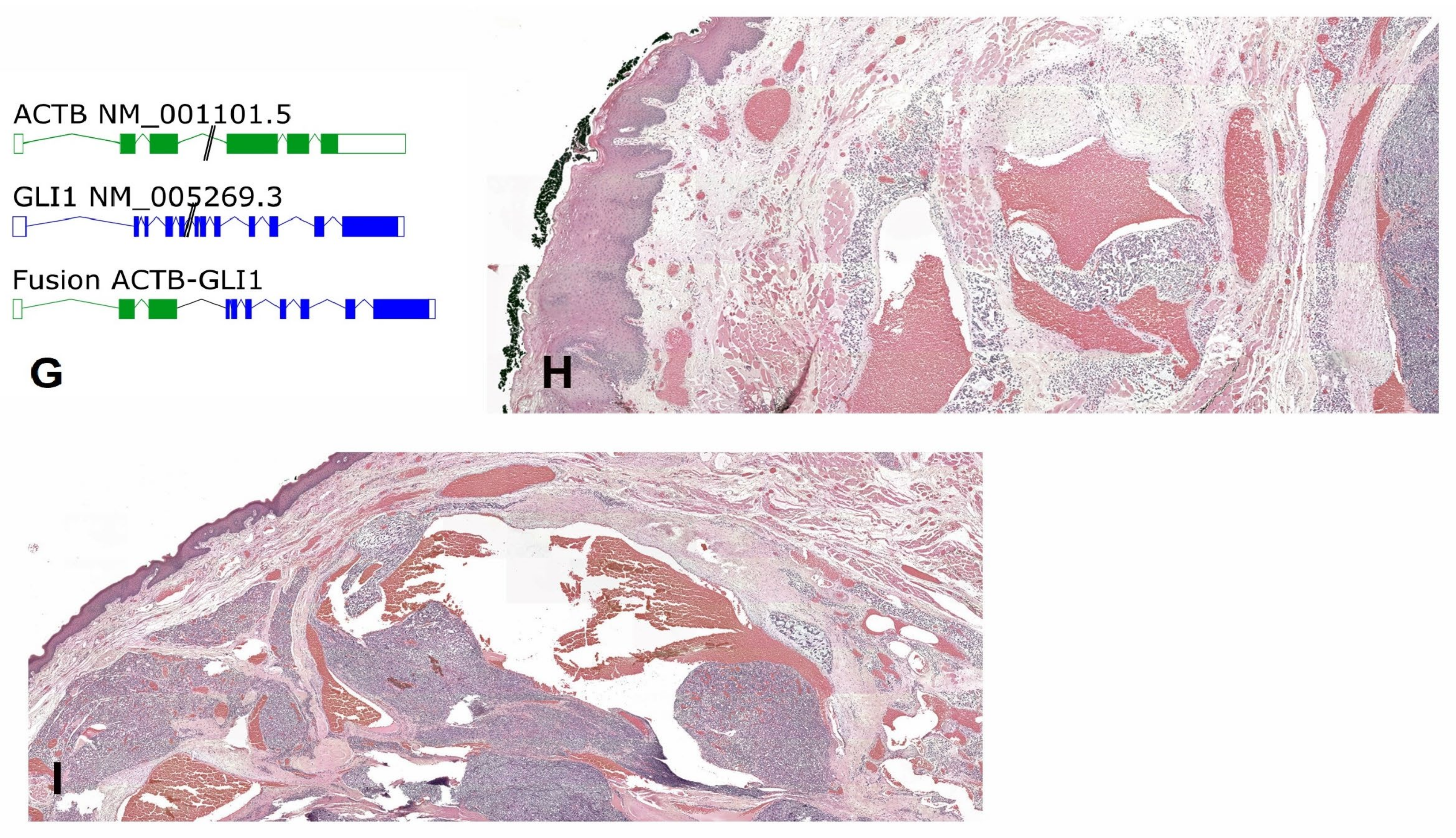
2.3. Pathology
3. Methods
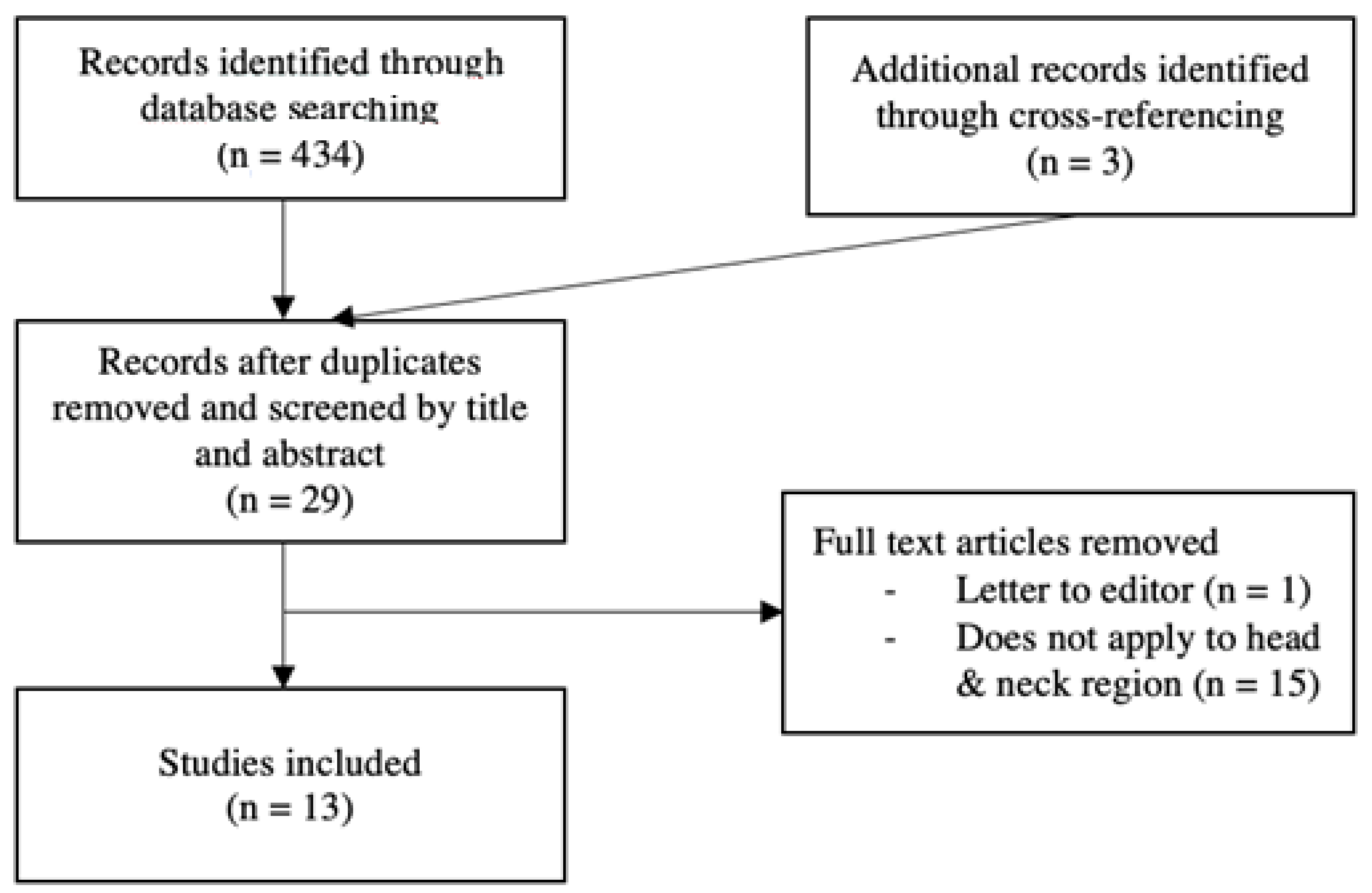
3.1. Search
3.2. Eligibility Criteria
3.3. Data Extraction
4. Results
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, B.; Chang, K.; Folpe, A.L.; Kao, Y.C.; Wey, S.L.; Huang, H.Y.; Gill, A.J.; Rooper, L.; Bishop, J.A.; Dickson, B.C.; et al. Head and Neck Mesenchymal Neoplasms With GLI1 Gene Alterations: A Pathologic Entity With Distinct Histo-logic Features and Potential for Distant Metastasis. Am. J. Surg. Pathol. 2020, 44, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Chetty, R. Gene of the month: GLI-1. J. Clin. Pathol. 2020, 73, 228–230. [Google Scholar] [CrossRef] [PubMed]
- Yeung, M.C.; Liu, A.P.; Wong, S.-I.; Loong, H.H.; Shek, T.W. GLI1-Altered Mesenchymal Tumor—Multiomic Characterization of a Case Series and Patient-Level Meta-analysis of One Hundred Sixty-Seven Cases for Risk Stratification. Mod. Pathol. 2024, 38, 100635. [Google Scholar] [CrossRef] [PubMed]
- Xu, B. Proceedings of the North American Society of Head and Neck Pathology, Los Angeles, CA, March 20, 2022. Emerging Bone and Soft Tissue Neoplasms in the Head and Neck Region. Head Neck Pathol. 2022, 16, 158–167. [Google Scholar] [CrossRef]
- Dahlén, A.; Fletcher, C.D.; Mertens, F.; Fletcher, J.A.; Perez-Atayde, A.R.; Hicks, M.J.; Debiec-Rychter, M.; Sciot, R.; Wejde, J.; Wedin, R.; et al. Activation of the GLI Oncogene through Fusion with the β-Actin Gene (ACTB) in a Group of Distinctive Pericytic Neoplasms. Am. J. Pathol. 2004, 164, 1645–1653. [Google Scholar] [CrossRef]
- Liu, J.; Mao, R.; Lao, I.W.; Yu, L.; Bai, Q.; Zhou, X.; Wang, J. GLI1-altered mesenchymal tumor: A clinicopathological and molecular analysis of ten additional cases of an emerging entity. Virchows Arch. 2022, 480, 1087–1099. [Google Scholar] [CrossRef]
- Zhong, H.; Xu, C.; Chen, X.; Guo, X.; Yang, S. GLI1-altered epithelioid soft tissue tumor: A newly described entity with a predilection for the tongue. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2022, 134, e14–e22. [Google Scholar] [CrossRef]
- Papke, D.J.J.; Dickson, B.C.M.; Oliveira, A.M.; Sholl, L.M.; Fletcher, C.D.M. Distinctive Nested Glomoid Neoplasm: Clinicopathologic Analysis of 20 Cases of a Mesenchymal Neoplasm With Frequent GLI1 Alterations and Indolent Behavior. Am. J. Surg. Pathol. 2022, 47, 12–24. [Google Scholar] [CrossRef]
- Klubíčková, N.; Kinkor, Z.; Michal, M.; Baněčková, M.; Hájková, V.; Michálek, J.; Pink, R.; Dvořák, Z.; Leivo, I.; Skálová, A. Epithelioid Soft Tissue Neoplasm of the Soft Palate with a PTCH1-GLI1 Fusion: A Case Report and Review of the Literature. Head Neck Pathol. 2021, 16, 621–630. [Google Scholar] [CrossRef]
- Nitta, Y.; Takeda, M.; Fujii, T.; Itami, H.; Tsukamoto, S.; Honoki, K.; Ohbayashi, C. A case of pericytic neoplasm in the shoulder with a novel DERA–GLI1 gene fusion. Histopathology 2020, 78, 466–469. [Google Scholar] [CrossRef]
- Castro, E.; Cortes-Santiago, N.; Ferguson, L.M.S.; Rao, P.H.; Venkatramani, R.; López-Terrada, D. Translocation t(7;12) as the sole chromosomal abnormality resulting in ACTB-GLI1 fusion in pediatric gastric pericytoma. Hum. Pathol. 2016, 53, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Kerr, D.A.; Pinto, A.; Subhawong, T.K.; Wilky, B.A.; Schlumbrecht, M.P.; Antonescu, C.R.; Nielsen, G.P.; Rosenberg, A.E. Pericytoma With t(7;12) and ACTB-GLI1 Fusion: Reevaluation of an Unusual Entity and its Relationship to the Spectrum of GLI1 Fusion-related Neoplasms. Am. J. Surg. Pathol. 2019, 43, 1682–1692. [Google Scholar] [CrossRef] [PubMed]
- Koh, N.W.C.; Seow, W.Y.; Lee, Y.T.; Lam, J.C.M.; Lian, D.W.Q. Pericytoma With t(7;12): The First Ovarian Case Reported and a Review of the Literature. Int. J. Gynecol. Pathol. 2019, 38, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Prall, O.W.J.; McEvoy, C.R.E.; Byrne, D.J.; Iravani, A.; Browning, J.; Choong, D.Y.-H.; Yellapu, B.; O’haire, S.; Smith, K.; Luen, S.J.; et al. A Malignant Neoplasm From the Jejunum With a MALAT1-GLI1 Fusion and 26-Year Survival History. Int. J. Surg. Pathol. 2020, 28, 553–562. [Google Scholar] [CrossRef]
- Zeng, Y.; Yao, H.; Jiang, X.; Tang, X.; Wang, X. GLI1-altered Mesenchymal Tumor Involving the Duodenum: Case Report and Literature Review. Int. J. Surg. Pathol. 2023, 31, 1538–1547. [Google Scholar] [CrossRef]
- Argani, P.; Boyraz, B.; Oliva, E.; Matoso, A.; Gross, J.; Fridman, E.; Zhang, L.; Dickson, B.C.; Antonescu, C.R. GLI1 Gene Alterations in Neoplasms of the Genitourinary and Gynecologic Tract. Am. J. Surg. Pathol. 2021, 46, 677–687. [Google Scholar] [CrossRef]
- Bridge, J.A.; Sanders, K.; Huang, D.; Nelson, M.; Neff, J.R.; Muirhead, D.; Walker, C.; Seemayer, T.A.; Sumegi, J. Pericytoma with t(7;12) and ACTB-GLI1 fusion arising in bone. Hum. Pathol. 2012, 43, 1524–1529. [Google Scholar] [CrossRef]
- Rollins, B.T.; Cassarino, D.S.; Lindberg, M. Primary cutaneous epithelioid mesenchymal neoplasm with ACTB-GLI1 fusion: A case report. J. Cutan. Pathol. 2021, 49, 284–287. [Google Scholar] [CrossRef]
- Agaram, N.P.; Zhang, L.; Sung, Y.-S.; Singer, S.; Stevens, T.; Prieto-Granada, C.N.; Bishop, J.A.; Wood, B.A.; Swanson, D.; Dickson, B.C.; et al. GLI1-amplifications expand the spectrum of soft tissue neoplasms defined by GLI1 gene fusions. Mod. Pathol. 2019, 32, 1617–1626. [Google Scholar] [CrossRef]
- Antonescu, C.R.; Agaram, N.P.; Sung, Y.-S.M.; Zhang, L.; Swanson, D.B.; Dickson, B.C. A Distinct Malignant Epithelioid Neoplasm With GLI1 Gene Rearrangements, Frequent S100 Protein Expression, and Metastatic Potential: Expanding the Spectrum of Pathologic Entities With ACTB/MALAT1/PTCH1-GLI1 Fusions. Am. J. Surg. Pathol. 2018, 42, 553–560. [Google Scholar] [CrossRef]
- Pettus, J.R.; Kerr, D.A.; Stan, R.V.; Tse, J.Y.; Sverrisson, E.F.; Bridge, J.A.; Linos, K. Primary myxoid and epithelioid mesenchymal tumor of the kidney with a novel GLI1-FOXO4 fusion. Genes Chromosom. Cancer 2021, 60, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Nunez, O.; Surrey, L.F.; Alaggio, R.; Herradura, A.; McGough, R.L.; John, I. Novel APOD–GLI1 rearrangement in a sarcoma of unknown lineage. Histopathology 2021, 78, 338–340. [Google Scholar] [CrossRef] [PubMed]
- Agaimy, A. What is new in epithelioid soft tissue tumors? Virchows Arch. 2020, 476, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Palsgrove, D.N.; Rooper, L.M.; Stevens, T.M.; Shin, C.; Damm, D.D.; Gagan, J.; Bridge, J.A.; Thompson, L.D.R.; Koduru, P.R.; Bishop, J.A. GLI1-Altered Soft Tissue Tumors of the Head and Neck: Frequent Oropharyngeal Involvement, p16 Immunoreactivity, and Detectable Alterations by DDIT3 Break Apart FISH. Head Neck Pathol. 2022, 16, 1146–1156. [Google Scholar] [CrossRef]
| Study (N° of Cases) | Body Site | Age/Sex | Metastasis (Localization, Months Before Relapse) | Local Recurrence (Months Before Relapse) | Outcome (Follow-Up in Month) | Treatment | GLI Alteration | Size (cm) | Necrosis |
|---|---|---|---|---|---|---|---|---|---|
| Xu et al., Antonescu et al., Agaram et al. [11] | |||||||||
| Neck | 39/M | Y (Lungs, 6) | Y (6) | AWD (36) | Amp. | Y | |||
| Tongue | 38/M | N | N | NED (2) | PTCH1::GLI1 | NA | N | ||
| Tongue | 46/F | N | N | NED (3) | Amp. | N | |||
| Tongue | 65/M | NA | Amp. | N | |||||
| Tongue | 60/M | Amp. | N | ||||||
| Subm. gland | 32/F | Y (Lymph nodes and lungs, 83) | Y (83) | AWD (104) | NA | PTCH1::GLI1 | Y | ||
| Neck | 37/M | N | N | NED (30) | ACTB::GLI1 | N | |||
| Tongue | 1/M | N | N | NED (2) | ACTB::GLI1 | N | |||
| Tongue | 28/M | N | N | ACTB::GLI1 | N | ||||
| Tongue | 14/M | NA | ACTB::GLI1 | N | |||||
| Tongue | 56/F | MALAT1::GLI1 | N | ||||||
| Dahlen et al. [3] | |||||||||
| Tongue | 27/F | N | N | NED (60) | ChT + Surgery * | ACTB::GLI1 | 0.8 | N | |
| Tongue | 11/M | N | N | NED (22) | ChT + Surgery * | ACTB::GLI1 | 5 | N | |
| Tongue | 12/M | N | N | NED (120) | Surgery | ACTB::GLI1 | 2.4 | N | |
| Liu et al. [5] | |||||||||
| Tongue base | 27/M | N | N | NED (16) | Surgery + RT/ChT | ACTB::GLI1 | 3.5 | N | |
| Tongue | 4/M | N | N | NED (11) | Surgery | Amp. | 3 | N | |
| Tongue | 17/M | N | N | NED (5) | Surgery | Amp. | 1 | N | |
| Mouth floor | 8/M | N | Y # | AWD (40) | Surgery + RT/ChT | ACTB::GLI1 | 2 | N | |
| Mouth floor | 34/M | NA | Surgery | MALAT1::GLI1 | NA | N | |||
| Palsgrove et al. [5] | |||||||||
| Palatine tonsil | 35/M | Recent case | Surgery | Amp. | 1.5 | N | |||
| Tongue base | 84/M | Recent case | Surgery | ACTB::GLI1 | NA | N | |||
| Oropharynx | 53/M | Recent case | Surgery | ACTB::GLI1 | 6 | N | |||
| Tongue | 41/M | NA | Surgery | ACTB::GLI1 | NA | N | |||
| Tongue | 65/M | Surgery | Amp. | 2 | N | ||||
| Zhong et al. [1] | |||||||||
| Tongue base Ω | 56/M | Y (Lymph node and bone, 27) | N | NED (36) | Surgery | « GLI1 rearrangement » | 6 | N | |
| Klubíćková et al. [1] | |||||||||
| Soft palate | 34/F | N | N | NED (4) | Surgery | PTCH1::GLI1 | ca. 3 | N | |
| Papke et al. [5] | |||||||||
| Neck | 12/M | N | N | NA | Surgery | ACTB::GLI1 | 1.5 | N | |
| Tongue | 46/F | N | N | NED (18) | Surgery | Amp. | 5.8 | N | |
| Tongue | 10/F | NA | Surgery | ACTB::GLI1 | 2 | N | |||
| Tongue | 0/M | Recent case | Surgery | NA | 0.9 | N | |||
| Tongue | 1/M | Surgery | Amp. | NA | N | ||||
| Current study [1] | Tongue | 39/M | N | N | NED (24) | Surgery | ACTB::GLI1 | 2.5 | N |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janjic, O.; De Vito, C.; Lobrinus, J.A.; Becker, M.; Dulguerov, N. GLI1-Altered Mesenchymal Tumours in the Head and Neck: A Case Report and Literature Review. J. Otorhinolaryngol. Hear. Balance Med. 2025, 6, 2. https://doi.org/10.3390/ohbm6010002
Janjic O, De Vito C, Lobrinus JA, Becker M, Dulguerov N. GLI1-Altered Mesenchymal Tumours in the Head and Neck: A Case Report and Literature Review. Journal of Otorhinolaryngology, Hearing and Balance Medicine. 2025; 6(1):2. https://doi.org/10.3390/ohbm6010002
Chicago/Turabian StyleJanjic, Olivier, Claudio De Vito, Johannes Alexander Lobrinus, Minerva Becker, and Nicolas Dulguerov. 2025. "GLI1-Altered Mesenchymal Tumours in the Head and Neck: A Case Report and Literature Review" Journal of Otorhinolaryngology, Hearing and Balance Medicine 6, no. 1: 2. https://doi.org/10.3390/ohbm6010002
APA StyleJanjic, O., De Vito, C., Lobrinus, J. A., Becker, M., & Dulguerov, N. (2025). GLI1-Altered Mesenchymal Tumours in the Head and Neck: A Case Report and Literature Review. Journal of Otorhinolaryngology, Hearing and Balance Medicine, 6(1), 2. https://doi.org/10.3390/ohbm6010002






