- Prologue
I hope the New Year has renewed your optimism and aspiration in your personal and professional endeavors. The beginning of the year is traditionally a good time to reflect on the past and consider the future. This Editorial aims to do just that: reflect on the past and look to the future of otolaryngology research.
The amount of information generated in otolaryngology (or otorhinolaryngology, if one wishes to be etymologically and linguistically correct [1]) is growing exponentially and is not only related to “pure” otolaryngology but also to other disciplines, such as the following:
- Psychology and psychometrics, supporting all subdisciplines of otorhinolaryngology.
- Cybernetics, which, e.g., supports advanced head and neck surgery, including cochlear implantation.
- Biotechnology, which is essential to developing new biologics used to treat head and neck tumors or allergic conditions associated with, e.g., rhinosinusitis or bronchial asthma.
- Genetics, which introduces the possibility of editing mutations that cause hearing loss and many other ORL-related diseases.
These are only a few examples, but the central claim is that the notion of an isolated or “pure” form of otolaryngology, uninfluenced by other medical or scientific disciplines, is not viable. Instead, the integration of existing achievements from numerous scientific fields, even those distant from otolaryngology, has emerged as a pivotal factor in ensuring progress within our field. Such progress is both possible and feasible in ORL’s medical, translational, and experimental domains.
- Hearing loss and dementia
An excellent example of how ORL interacts with other (far-far-away) disciplines is the identification of untreated hearing loss occurring at mid-age (45–65) as the most significant risk factor for developing dementia [2]. This finding surprised neuroscientists because the previously recognized risk factors (age and female sex, genetic traits, and vascular factors) did not include hearing loss [3]. Gill Livingston and her team published their findings in 2017 but have re-examined the issue. Their new report, published in mid-2024 [4], shows that hearing loss was still the number one risk factor for dementia, even when many other variables included in the analysis proved to be valid predictors (high LDL cholesterol, depression, traumatic brain injury, physical inactivity, smoking, diabetes, hypertension, obesity and excessive alcohol consumption in mid-age (between 45 and 65 years of age)). A tremendous growth in interest in hearing loss and dementia and the prevention or treatment of hearing loss as a means of preventing dementia can be seen after 2017 (Figure 1). Preventing and treating hearing loss, the world’s most common yet overlooked communication disorder, should be a global priority for healthy, dementia-free aging.
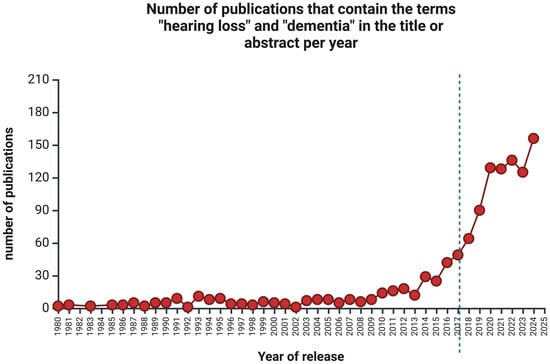
Figure 1.
The number of publications per year with the keywords “hearing loss” and “dementia” in the title or abstract retrieved from PubMed between 1980 and 2024. The vertical dotted line represents the year hearing loss was recognized as a major risk factor for developing dementia [2]. Created in https://BioRender.com (accessed on 24 January 2025).
- Hearing loss and gene therapy
Another hot topic that has emerged in otology and genetics is the successful use of gene therapy to cure a specific form of hearing loss caused by mutations in the gene encoding otoferlin (OTOF), a protein needed to properly transduce auditory signals from the hair cells to spiral ganglion neurons (forming the hearing nerve). Mutations in OTOF are associated with prelingual hearing loss, and the standard therapy involves cochlear implantation.
In 2024, three nearly concurrent reports validated the potential for local OTOF gene transfer utilizing adenovirus-associated virus (AAV) (Figure 2). A tremendous success was reported in 2024 by a team from Shanghai Refreshgene Therapeutics in China, which was established in 2020 and became part of OBiO Technology (Shanghai) Corp. in October 2024. Of six prelingually deafened children unilaterally treated with the OTOF construct, five showed hearing recovery on the treated side [5]. A few months later, the same group reported another successful outcome in five patients treated bilaterally [6]. Other biotech companies exploring this technology include Akouos, which Lilly acquired in 2022 (clinical trial number NCT05821959), and Decibel Therapeutics [7], acquired by Regeneron in 2023. Despite gene therapy being a “hot topic” in hearing loss therapy, this subject presents significant ethical challenges, as genetic manipulation in human medicine is prohibited in multiple countries. We will keep an eye on the situation as it develops.
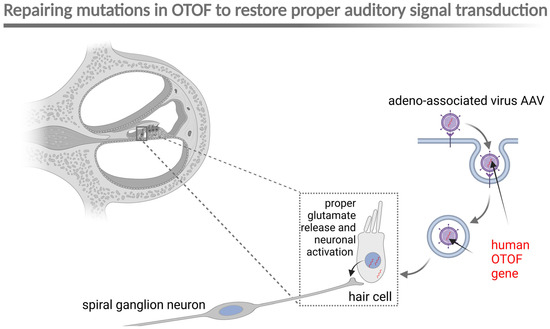
Figure 2.
Schematic representation of AAV-mediated gene therapy for OTOF mutations. AAV, adeno-associated virus; OTOF, otoferlin. Created in BioRender. Szczepek, A. (2025) https://BioRender.com/u55a782 (accessed on 24 January 2025).
- Treatment of head and neck tumors
The treatment of head and neck cancer remains a significant yet challenging issue. Considerable hope has been placed on alternative agents for patients who are ineligible or unable to receive cisplatin due to organ dysfunction, advanced age, or comorbidities. The final report of a multicenter, open-label, randomized phase 2 trial published in December 2024 dashed hopes that radiation therapy combined with durvalumab (human immunoglobulin G1 kappa monoclonal antibody that blocks the interaction of programmed cell death ligand 1 (PD-L1)) would be more effective than radiation therapy combined with cetuximab (a chimeric mouse/human monoclonal antibody which binds to and inhibits epidermal growth factor receptor (EGFR)) for patients with advanced squamous cell carcinoma of the head and neck (SCCHN). This trial was halted, and the findings were published in Lancet Oncology [8], underscoring the importance of sharing all results, even when they do not meet expectations.
In 2021, when Malaysian researchers from the University of Malaya in Kuala Lumpur, using the PRISM dataset, identified 36 drugs that, upon repurposing, could potentially be used for the treatment of head and neck squamous carcinoma [9], PD-L1 inhibitors were not on their list. A remarkable total of twenty-one EGFR inhibitors were on the list, demonstrating promising killing activity against SCCHN (gefitinib, erlotinib, lapatinib, afatinib, osimertinib, neratinib, dacomitinib, vandetanib, AZD8931, poziotinib, BIBU-1361, icotinib, pelitinib, ARRY-334543, canertinib, PD-153035, PD-168393, BMS-599626, AEE788, BMS-690514, and XL-647)—the first eight are FDA-approved for other cancers. The list also included one heat shock protein 90 (HSP90) inhibitor (SNX-5422), one Janus kinase/signal transducer and activator of transcription (JAK) inhibitor (CEP-33779), three MEK inhibitors (AZD8330, trametinib, cobimetinib), one PI3 kinase inhibitor (taselisib), one dual inhibitor of EGFR and ErbB2 tyrosine kinases (AV-412), one selective Src kinase inhibitor (saracatinib), one telomerase inhibitor (costunolide), and two vitamin D receptor agonists (tacalcitol and alfacalcidol). Time will tell if the identified drugs can indeed be repurposed for the successful treatment of SCCHN, especially its advanced forms.
- Biologics in treatment of rhinosinusitis
The next “hot” topic I will cover is biologics in rhinology. With roughly every tenth person globally affected [10], chronic rhinosinusitis (CR) burdens society and the healthcare system worldwide. The most severe type of chronic rhinosinusitis (CR) is linked to nasal polyps (CRwNP) and may be accompanied by bronchial asthma and aspirin sensitivity. Recently, biologics targeting the signaling pathway leading to CRwNP have been used for treatment. These biologics include omalizumab (a monoclonal antibody that specifically binds to free and membrane-bound human immunoglobulin E), dupilumab (a monoclonal antibody that attaches to the alpha subunit of the interleukin-4 receptor (IL-4Rα), which is also part of the IL-13 receptor, thus blocking IL-4 and IL-13 signaling), and mepolizumab (a monoclonal antibody that strongly binds to IL-5). In addition to these three drugs, new biologics such as benralizumab (a monoclonal antibody directed against the alpha chain of the interleukin-5 receptor—IL5Rα) and reslizumab (a monoclonal antibody aimed at IL-5) have been employed in the treatment of CRwNP [11]. Long-term studies determined the high efficacy of the treatment [11,12]. All of the biologics target elements of Type 2 inflammation, which predominantly drives the CRSwNP [13] (Figure 3).
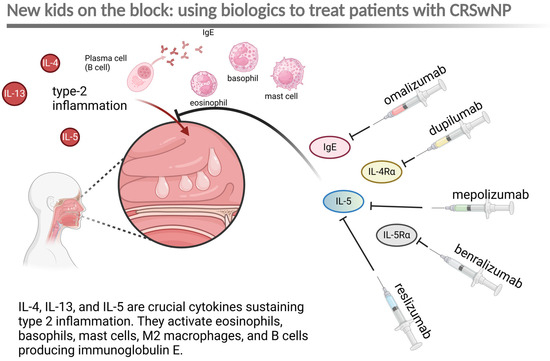
Figure 3.
Schematic representation of the primary mechanism driving chronic rhinosinusitis with nasal polyps (CRSwNP), characterized by type 2 inflammation, and the main targets of biologics used for treating CRSwNP. IgE—immunoglobulin E; IL-4a—alpha chain of interleukin-4 receptor. Created in BioRender. Szczepek, A. (2025) https://BioRender.com/j57t524 (accessed on 24 January 2025).
Unfortunately, these therapies are not without side effects; a recently published meta-analysis indicated a significant rise in rheumatic adverse events among CRwNP patients treated with biologics [14]. Future research in 2025 should concentrate on long-term side effects, possible signs of resistance, and the challenge of limited access to biologics in underserved countries or populations.
- Side effects of old and new drugs
The responsibility falls on therapists who use pharmacotherapy to educate themselves about the possible adverse effects of medications. A SIDER (Side Effect Resource) database has been created to gather relevant information in this area [15]. The database can be searched using various criteria, such as the name of the drug, the condition it is meant to treat, or the symptoms it aims to relieve. A recent study using this database found that many medications can cause tinnitus [16]. It is worrying that the most recent update to SIDER was a decade ago, with no signs of continued development for this project, which is regrettable. Still, gathering safety pharmacology information on drugs that may potentially induce otorhinolaryngological symptoms or conditions should remain a priority in our specialty.
- Epilogue
These brief analyses of contemporary topics in otolaryngology are anticipated to stimulate further research, clinical observations, and the development of clinical or foundational research studies. Readers are invited to contribute their observations and findings to the pages of JOHBM this year and in the years to come.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ramoutsaki, I.A.; Bizakis, J.G.; Ramoutsakis, J.A.; Bizakis, A.J.; Helidonis, E.S. Otorhinolaryngology or otolaryngology? An etymological approach. Otolaryngol. Head Neck Surg. 2004, 131, 765–766. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Sommerlad, A.; Orgeta, V.; Costafreda, S.G.; Huntley, J.; Ames, D.; Ballard, C.; Banerjee, S.; Burns, A.; Cohen-Mansfield, J.; et al. Dementia prevention, intervention, and care. Lancet 2017, 390, 2673–2734. [Google Scholar] [CrossRef] [PubMed]
- Van der Flier, W.M.; Scheltens, P. Epidemiology and risk factors of dementia. J. Neurol. Neurosurg. Psychiatry 2005, 76, v2–v7. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Huntley, J.; Liu, K.Y.; Costafreda, S.G.; Selbæk, G.; Alladi, S.; Ames, D.; Banerjee, S.; Burns, A.; Brayne, C.; et al. Dementia prevention, intervention, and care: 2024 report of the lancet standing commission. Lancet 2024, 404, 572–628. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Wang, H.; Cheng, X.; Chen, Y.; Wang, D.; Zhang, L.; Cao, Q.; Tang, H.; Hu, S.; Gao, K.; et al. Aav1-hotof gene therapy for autosomal recessive deafness 9: A single-arm trial. Lancet 2024, 403, 2317–2325. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, Y.; Lv, J.; Cheng, X.; Cao, Q.; Wang, D.; Zhang, L.; Zhu, B.; Shen, M.; Xu, C.; et al. Bilateral gene therapy in children with autosomal recessive deafness 9: Single-arm trial results. Nat. Med. 2024, 30, 1898–1904. [Google Scholar] [CrossRef]
- Sellon, J.B.; So, K.S.; D’Arcangelo, A.; Cancelarich, S.; Drummond, M.C.; Slade, P.G.; Pan, N.; Gibson, T.M.; Yang, T.; Burns, J.C.; et al. Recovery kinetics of dual aav-mediated human otoferlin expression. Front. Mol. Neurosci. 2024, 17, 1376128. [Google Scholar] [CrossRef]
- Mell, L.K.; Torres-Saavedra, P.A.; Wong, S.J.; Kish, J.A.; Chang, S.S.; Jordan, R.C.; Liu, T.; Truong, M.T.; Winquist, E.W.; Takiar, V.; et al. Radiotherapy with cetuximab or durvalumab for locoregionally advanced head and neck cancer in patients with a contraindication to cisplatin (nrg-hn004): An open-label, multicentre, parallel-group, randomised, phase 2/3 trial. Lancet Oncol. 2024, 25, 1576–1588. [Google Scholar] [CrossRef]
- Chai, A.W.Y.; Tan, A.C.; Cheong, S.C. Uncovering drug repurposing candidates for head and neck cancers: Insights from systematic pharmacogenomics data analysis. Sci. Rep. 2021, 11, 23933. [Google Scholar] [CrossRef] [PubMed]
- Min, H.K.; Lee, S.; Kim, S.; Son, Y.; Park, J.; Kim, H.J.; Lee, J.; Lee, H.; Smith, L.; Rahmati, M.; et al. Global incidence and prevalence of chronic rhinosinusitis: A systematic review. Clin. Exp. Allergy 2025, 55, 52–66. [Google Scholar] [CrossRef] [PubMed]
- Koski, R.R.; Hill, L.; Taavola, K. Efficacy and safety of biologics for chronic rhinosinusitis with nasal polyps. J. Pharm. Technol. 2022, 38, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Frankenberger, H.; Wiebringhaus, R.; Paul, B.; Huber, P.; Haubner, F.; Gröger, M.; Stihl, C. The use of biologics in patients suffering from chronic rhinosinusitis with nasal polyps—A 4-year real life observation. Eur. Arch. Oto-Rhino-Laryngol. 2024, 281, 5773–5782. [Google Scholar] [CrossRef] [PubMed]
- Bachert, C.; Hicks, A.; Gane, S.; Peters, A.T.; Gevaert, P.; Nash, S.; Horowitz, J.E.; Sacks, H.; Jacob-Nara, J.A. The interleukin-4/interleukin-13 pathway in type 2 inflammation in chronic rhinosinusitis with nasal polyps. Front. Immunol. 2024, 15, 1356298. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.B.; Hsiao, H.; Khalil, C.; Lee, J.M. Rheumatic adverse events associated with biologic therapy for chronic rhinosinusitis: A systematic review and meta-analysis. Int. Forum Allergy Rhinol. 2024, 14, 1618–1633. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, M.; Letunic, I.; Jensen, L.J.; Bork, P. The sider database of drugs and side effects. Nucleic Acids Res. 2016, 44, D1075–D1079. [Google Scholar] [CrossRef] [PubMed]
- Szczepek, A.J. Safety pharmacology and tinnitus. In Drug Discovery and Evaluation: Safety and Pharmacokinetic Assays; Hock, F.J., Pugsley, M.K., Eds.; Springer International Publishing: Cham, Switzerland, 2024; pp. 801–823. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).