Cardiovascular Risk Profile in Ménière’s Disease and Posterior Circulation Infarction: A Comparative Study
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Study Population
3.2. Ménière’s versus POCI: Risk Factors
3.3. Cardiovascular Risk Factors and Stroke Risk: A Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Espinosa-Sanchez, J.M.; Lopez-Escamez, J.A. Menière’s disease. Handb. Clin. Neurol. 2016, 137, 257–277. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Kitahara, T.; Inui, H.; Miyasaka, T.; Kichikawa, K.; Ota, I.; Nario, K.; Matsumura, Y.; Yamanaka, T. Endolymphatic space size in patients with Meniere’s disease and healthy controls. Acta Otolaryngol. 2016, 136, 879–882. [Google Scholar] [CrossRef] [PubMed]
- Salt, A.N.; Plontke, S.K. Endolymphatic hydrops: Pathophysiology and experimental models. Otolaryngol. Clin. N. Am. 2010, 43, 971–983. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, T.; Pyykkö, I.; Arroll, M.A.; Casselbrant, M.L.; Foster, C.A.; Manzoor, N.F.; Megerian, C.A.; Naganawa, S.; Young, Y.-H. Meniere’s disease. Nat. Rev. Dis. Prim. 2016, 2, 16028. [Google Scholar] [CrossRef] [PubMed]
- Attyé, A.; Eliezer, M.; Boudiaf, N.; Tropres, I.; Chechin, D.; Schmerber, S.; Dumas, G.; Krainik, A. MRI of endolymphatic hydrops in patients with Meniere’s disease: A case-controlled study with a simplified classification based on saccular morphology. Eur. Radiol. 2017, 27, 3138–3146. [Google Scholar] [CrossRef] [PubMed]
- Foster, C.A.; Breeze, R.E. The Meniere attack: An ischemia/reperfusion disorder of inner ear sensory tissues. Med. Hypotheses 2013, 81, 1108–1115. [Google Scholar] [CrossRef]
- Merchant, S.N.; Adams, J.C.; Nadol, J.B.J. Pathophysiology of Meniere’s syndrome: Are symptoms caused by endolymphatic hydrops? Otol. Neurotol. 2005, 26, 74–81. [Google Scholar] [CrossRef]
- Kimura, R.S. Experimental blockage of the endolymphatic duct and sac and its effect on the inner ear of the guinea pig. A study on endolymphatic hydrops. Ann. Otol. Rhinol. Laryngol. 1967, 76, 664–687. [Google Scholar] [CrossRef]
- Kimura, R.S. Experimental pathogenesis of hydrops. Arch. Otorhinolaryngol. 1976, 212, 263–275. [Google Scholar] [CrossRef]
- Schuknecht, H.F. The Pathophysiology of Meniere’s Disease. Otol. Neurotol. 1984, 5, 526–527. [Google Scholar] [CrossRef]
- Chiarella, G.; Petrolo, C.; Cassandro, E. The genetics of Ménière’s disease. Appl. Clin. Genet. 2015, 8, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Long, L.; Zhao, H.; Wang, R.; Zheng, H.; Duan, M. Genetic advances in Meniere Disease. Mol. Biol. Rep. 2023, 50, 2901–2908. [Google Scholar] [CrossRef] [PubMed]
- Rego, Â.R.; Dias, D.; Pinto, A.; e Castro, S.S.; Feliciano, T.; e Sousa, C.A. The cardiovascular aspects of a Ménière’s disease population—A pilot study. J. Otol. 2019, 14, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Akagi, N.; Takumida, M.; Anniko, M. Effect of inner ear blood flow changes on the endolymphatic sac. Acta Otolaryngol. 2008, 128, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Friis, M.; Sørensen, M.S.; Qvortrup, K. The vein of the vestibular aqueduct with potential pathologic perspectives. Otol. Neurotol. 2008, 29, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Horton, W.B.; Barrett, E.J. Microvascular Dysfunction in Diabetes Mellitus and Cardiometabolic Disease. Endocr. Rev. 2021, 42, 29–55. [Google Scholar] [CrossRef] [PubMed]
- Wada, M.; Takeshima, T.; Nakamura, Y.; Nagasaka, S.; Kamesaki, T.; Kajii, E. Carotid plaque is a new risk factor for peripheral vestibular disorder: A retrospective cohort study. Medicine 2016, 95, e4510. [Google Scholar] [CrossRef]
- Ishiyama, G.; Lopez, I.A.; Ishiyama, P.; Vinters, H.V.; Ishiyama, A. The blood labyrinthine barrier in the human normal and Meniere’s disease macula utricle. Sci. Rep. 2017, 7, 253. [Google Scholar] [CrossRef] [PubMed]
- Tange, R.A. Vascular inner ear partition: A concept for some forms of sensorineural hearing loss and vertigo. ORL J. Otorhinolaryngol. Relat. Spec. 1998, 60, 78–84. [Google Scholar] [CrossRef]
- Mei, X.; Glueckert, R.; Schrott-Fischer, A.; Li, H.; Ladak, H.M.; Agrawal, S.K.; Rask-Andersen, H. Vascular Supply of the Human Spiral Ganglion: Novel ThreeDimensional Analysis Using Synchrotron Phase-Contrast Imaging and Histology. Sci. Rep. 2020, 10, 5877. [Google Scholar] [CrossRef]
- Lopez-Escamez, J.A.; Carey, J.; Chung, W.-H.; Goebel, J.A.; Magnusson, M.; Mandalà, M.; Newman-Toker, D.E.; Strupp, M.; Suzuki, M.; Trabalzini, F.; et al. Diagnostic criteria for Menière’s disease. Consensus document of the Bárány Society, the Japan Society for Equilibrium Research, the European Academy of Otology and Neurotology (EAONO), the American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS) and the Korean Balance Society. Acta Otorrinolaringol. Esp. 2016, 67, 1–7. [Google Scholar] [CrossRef] [PubMed]
- González-Marrero, I.; Castañeyra-Ruiz, L.; González-Toledo, J.M.; Castañeyra-Ruiz, A.; de Paz-Carmona, H.; Castro, R.; Hernandez-Fernaud, J.R.; Castañeyra-Perdomo, A.; Carmona-Calero, E.M. High Blood Pressure Effects on the Blood to Cerebrospinal Fluid Barrier and Cerebrospinal Fluid Protein Composition: A Two-Dimensional Electrophoresis Study in Spontaneously Hypertensive Rats. Int. J. Hypertens. 2013, 2013, 164653. [Google Scholar] [CrossRef] [PubMed]
- Nowroozpoor, A.; Gutterman, D.; Safdar, B. Is microvascular dysfunction a systemic disorder with common biomarkers found in the heart, brain, and kidneys?—A scoping review. Microvasc. Res. 2021, 134, 104123. [Google Scholar] [CrossRef] [PubMed]
- Cabrera DeBuc, D.; Somfai, G.M.; Koller, A. Retinal microvascular network alterations: Potential biomarkers of cerebrovascular and neural diseases. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Diao, T.; Han, L.; Tao, Y.; Yu, L. Association of Meniere’s disease and retinal vascular calibre: A prospective observational study in China. BMJ Open 2018, 8, 022069. [Google Scholar] [CrossRef] [PubMed]
- Martín-Sierra, C.; Gallego-Martinez, A.; Requena, T.; Frejo, L.; Batuecas-Caletrío, A.; Lopez-Escamez, J.A. Variable expressivity and genetic heterogeneity involving DPT and SEMA3D genes in autosomal dominant familial Meniere’s disease. Eur. J. Hum. Genet. 2017, 25, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Wangemann, P.; Liu, J.; Shimozono, M.; Schimanski, S.; Scofield, M.A. K+ secretion in strial marginal cells is stimulated via beta 1-adrenergic receptors but not via beta 2-adrenergic or vasopressin receptors. J. Membr. Biol. 2000, 175, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Wangemann, P. K+ cycling and its regulation in the cochlea and the vestibular labyrinth. Audiol. Neurotol. 2002, 7, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Ishiyama, G.; Lopez, I.A.; Acuna, D.; Ishiyama, A. Investigations of the Microvasculature of the Human Macula Utricle in Meniere’s Disease. Front. Cell. Neurosci. 2019, 13, 445. [Google Scholar] [CrossRef]
- Lopez, I.A.; Ishiyama, G.; Hosokawa, S.; Hosokawa, K.; Acuna, D.; Linthicum, F.H.; Ishiyama, A. Immunohistochemical techniques for the human inner ear. Histochem. Cell Biol. 2016, 146, 367–387. [Google Scholar] [CrossRef]
- Shi, X. Physiopathology of the cochlear microcirculation. Hear. Res. 2011, 282, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Shi, X. Pathophysiology of the cochlear intrastrial fluid-blood barrier (review). Hear. Res. 2016, 338, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Hirose, K.; Hartsock, J.J.; Johnson, S.; Santi, P.; Salt, A.N. Systemic lipopolysaccharide compromises the bloodlabyrinth barrier and increases entry of serum fluorescein into the perilymph. J. Assoc. Res. Otolaryngol. 2014, 15, 707–719. [Google Scholar] [CrossRef]
- Takumida, M.; Akagi, N.; Anniko, M. A new animal model for Ménière’s disease. Acta Otolaryngol. 2008, 128, 263–271. [Google Scholar] [CrossRef]

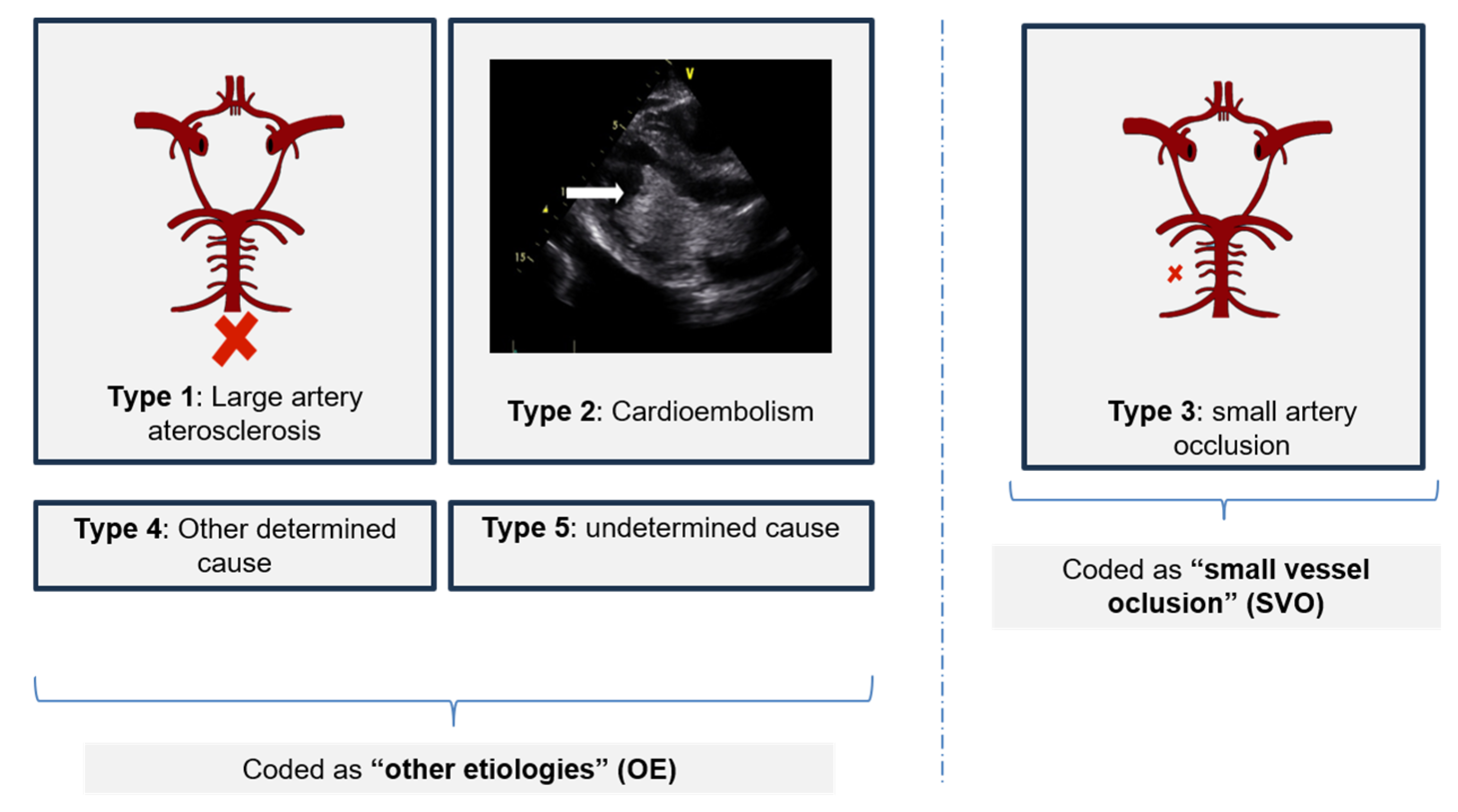

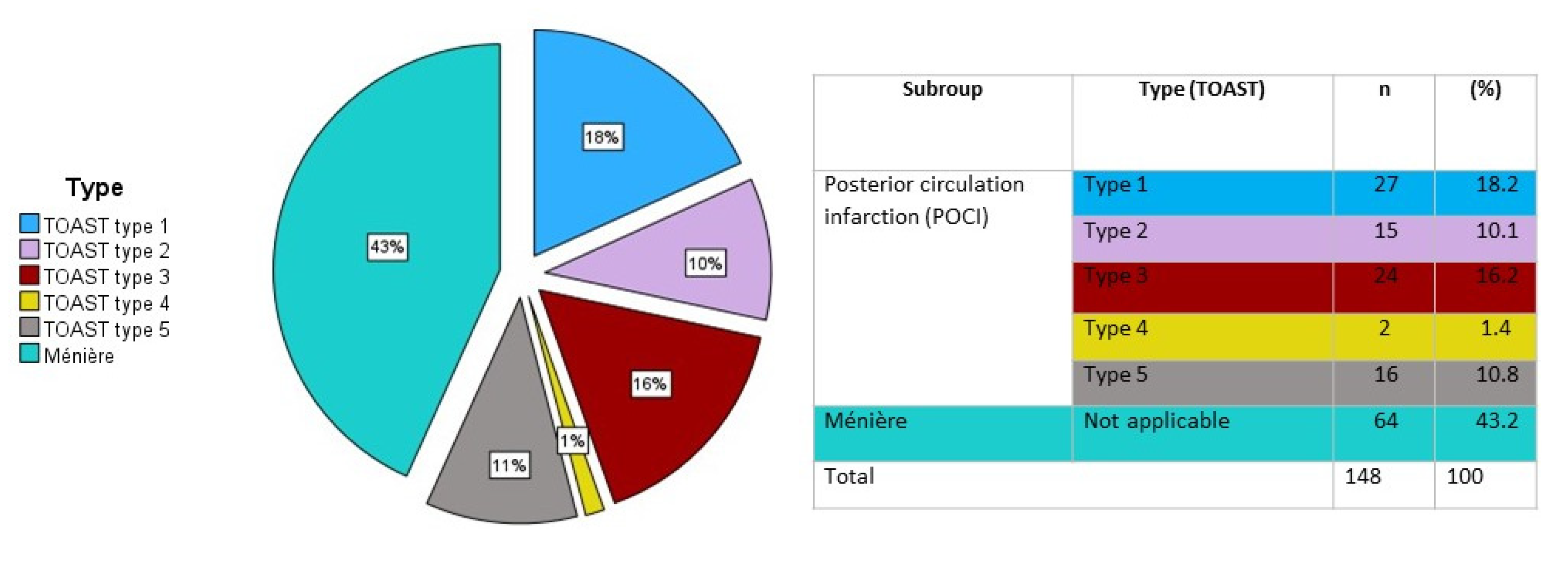
| Continuous Variables | Mean (±Standard Deviation) | p-Value | Categorical Variables | Frequency (%) | p-Value | ||
|---|---|---|---|---|---|---|---|
| Ménière | POCI | Ménière | POCI | ||||
| Age (years) 1 | 56.2 ± 12.9 | 61.7 ± 13.8 | 0.014 | Age (categories) | |||
| <45 years | 18.8 | 14.3 | 0.465 | ||||
| 45–55 years | 34.4 | 10.7 | <0.001 | ||||
| 55–65 years | 20.3 | 35.7 | 0.041 | ||||
| 65–75 years | 17.2 | 20.2 | 0.639 | ||||
| >75 years | 9.4 | 19 | 0.101 | ||||
| Sex (male) | 40.6 | 73.8 | <0.001 | ||||
| Group | Total | |||||
|---|---|---|---|---|---|---|
| Ménière | POCI SVO | POCI OE | ||||
| Number of patients | Age category (years) | <45 | 12 | 2 | 10 | 24 |
| 45–55 | 22 | 3 | 6 | 31 | ||
| 55–65 | 13 | 14 | 16 | 43 | ||
| 65–75 | 11 | 2 | 15 | 28 | ||
| >75 | 6 | 3 | 13 | 22 | ||
| Total | 64 | 24 | 60 | 148 | ||
| Comorbidity | Age in Years (Category) | Prevalence | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Ménière (1) | POCI SVO (2) | POCI OE (3) | 1 vs. 2 | 2 vs. 3 | 1 vs. 3 | ||
| Hypertension | <45 | 25% | 40% | 50% | 0.119 | 0.882 | 0.029 |
| 45–55 | 13.6% | 40% | 83.3% | 0.091 | 0.571 | <0.001 | |
| 55–65 | 46.2% | 71.4% | 68.75% | 0.182 | 0.873 | 0.219 | |
| 65–75 | 54.5% | 66.7% | 86.7% | 0.224 | 0.582 | 0.068 | |
| >75 | 66.7% | 100% | 92.3% | 0.257 | 0.620 | 0.154 | |
| Diabetes Mellitus | <45 | 8.3% | 5.2% | 10% | 0.672 | 0.593 | 0.350 |
| 45–55 | 4.5% | 0% | 33.3% | 0.706 | 0.257 | 0.043 | |
| 55–65 | 50% | 37.5% | 23.1% | 0.148 | 0.491 | 0.404 | |
| 65–75 | 18.2% | 50% | 33.3% | 0.326 | 0.643 | 0.390 | |
| >75 | 33.3% | 0% | 23.1% | 0.257 | 0.356 | 0.637 | |
| Dyslipidemia | <45 | 20% | 40% | 30% | 0.119 | 0.584 | 0.190 |
| 45–55 | 31.8% | 66.7% | 50% | 0.239 | 0.687 | 0.410 | |
| 55–65 | 38.5% | 92.9% | 75% | 0.003 | 0.190 | 0.047 | |
| 65–75 | 45.5% | 50% | 86.7% | 0.906 | 0.201 | 0.024 | |
| >75 | 50% | 66.7% | 53.8% | 0.635 | 0.687 | 0.876 | |
| Obesity | <45 | 16.7% | 0% | 30% | 0.533 | 0.371 | 0.457 |
| 45–55 | 9.1% | 33% | 16.7% | 0.225 | 0.571 | 0.595 | |
| 55–65 | 7.7% | 28.6% | 37.5% | 0.163 | 0.605 | 0.062 | |
| 65–75 | 18.2% | 50% | 6.7% | 0.326 | 0.074 | 0.364 | |
| >75 | 0% | 0% | 23.1% | NC | 0.356 | 0.200 | |
| Cardiac Disease | <45 | 8.3% | 0% | 0% | 0.350 | 0.462 | 0.350 |
| 45–55 | 0% | 0% | 0% | NC | NC | NC | |
| 55–65 | 7.7% | 0% | 18.8% | 0.290 | 0.088 | 0.390 | |
| 65–75 | 0% | 0% | 20% | NC | 0.486 | 0.115 | |
| >75 | 33.3% | 33.3% | 38.5% | 1 | 0.869 | 0.829 | |
| Smoking | <45 | 16.7% | 40% | 70% | 0.119 | 0.371 | 0.011 |
| 45–55 | 0% | 16.7% | 66.7% | 0.529 | 0.134 | 0.048 | |
| 55–65 | 0% | 28.6% | 37.5% | 0.057 | 0.605 | 0.013 | |
| 65–75 | 0% | 0% | 20% | NC | 0.486 | 0.115 | |
| >75 | 16.7% | 33.3% | 15.4% | 0.571 | 0.473 | 0.943 | |
| Predictor | χ2 | df | p-Value |
|---|---|---|---|
| Hypertension | 16.146 | 2 | <0.001 |
| Diabetes Mellitus | 1.140 | 2 | 0.566 |
| Dyslipidemia | 8.766 | 2 | 0.012 |
| Obesity | 0.589 | 2 | 0.745 |
| Cardiac Disease | 4.625 | 2 | 0.099 |
| Smoking | 29.522 | 2 | <0.001 |
| Age (years) | 1.049 | 2 | 0.592 |
| Predictor | POCI OE vs. | B | OR | p-Value |
|---|---|---|---|---|
| Hypertension | Méniére’s disease | −2.070 | 0.126 | <0.001 |
| POCI SVO | −0.406 | 0.666 | 0.556 | |
| Diabetes Mellitus | Ménière’s disease | −0.173 | 0.841 | 0.750 |
| POCI SVO | 0.507 | 1.660 | 0.371 | |
| Dyslipidemia | Ménière’s disease | −0.398 | 0.672 | 0.413 |
| POCI SVO | 1.506 | 4.509 | 0.024 | |
| Obesity | Ménière’s disease | −0.455 | 0.635 | 0.446 |
| POCI SVO | −0.145 | 0.865 | 0.815 | |
| Cardiac Disease | Ménière’s disease | −0.345 | 0.708 | 0.645 |
| POCI SVO | −2.008 | 0.134 | 0.074 | |
| Smoking | Ménière’s disease | −3.198 | 0.041 | <0.001 |
| POCI SVO | 0.285 | 1.330 | 0.630 | |
| Age | Ménière’s disease | −0.020 | 0.980 | 0.315 |
| POCI SVO | −0.003 | 0.997 | 0.913 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Sousa, F.A.; Tarrio, J.; Rodrigues, R.; Alves, C.S.; Santos, M.; Pinto, A.N.; Meireles, L.; Rego, Â.R. Cardiovascular Risk Profile in Ménière’s Disease and Posterior Circulation Infarction: A Comparative Study. J. Otorhinolaryngol. Hear. Balance Med. 2024, 5, 10. https://doi.org/10.3390/ohbm5020010
de Sousa FA, Tarrio J, Rodrigues R, Alves CS, Santos M, Pinto AN, Meireles L, Rego ÂR. Cardiovascular Risk Profile in Ménière’s Disease and Posterior Circulation Infarction: A Comparative Study. Journal of Otorhinolaryngology, Hearing and Balance Medicine. 2024; 5(2):10. https://doi.org/10.3390/ohbm5020010
Chicago/Turabian Stylede Sousa, Francisco Alves, João Tarrio, Rita Rodrigues, Clara Serdoura Alves, Mariline Santos, Ana Nóbrega Pinto, Luís Meireles, and Ângela Reis Rego. 2024. "Cardiovascular Risk Profile in Ménière’s Disease and Posterior Circulation Infarction: A Comparative Study" Journal of Otorhinolaryngology, Hearing and Balance Medicine 5, no. 2: 10. https://doi.org/10.3390/ohbm5020010
APA Stylede Sousa, F. A., Tarrio, J., Rodrigues, R., Alves, C. S., Santos, M., Pinto, A. N., Meireles, L., & Rego, Â. R. (2024). Cardiovascular Risk Profile in Ménière’s Disease and Posterior Circulation Infarction: A Comparative Study. Journal of Otorhinolaryngology, Hearing and Balance Medicine, 5(2), 10. https://doi.org/10.3390/ohbm5020010






