Cochlear Implantation in a Patient with Implanted Trigeminus Stimulator—Clinical Considerations for Using Two Different Electrical Stimulators in the Same Patient and Our Results
Abstract
1. Introduction
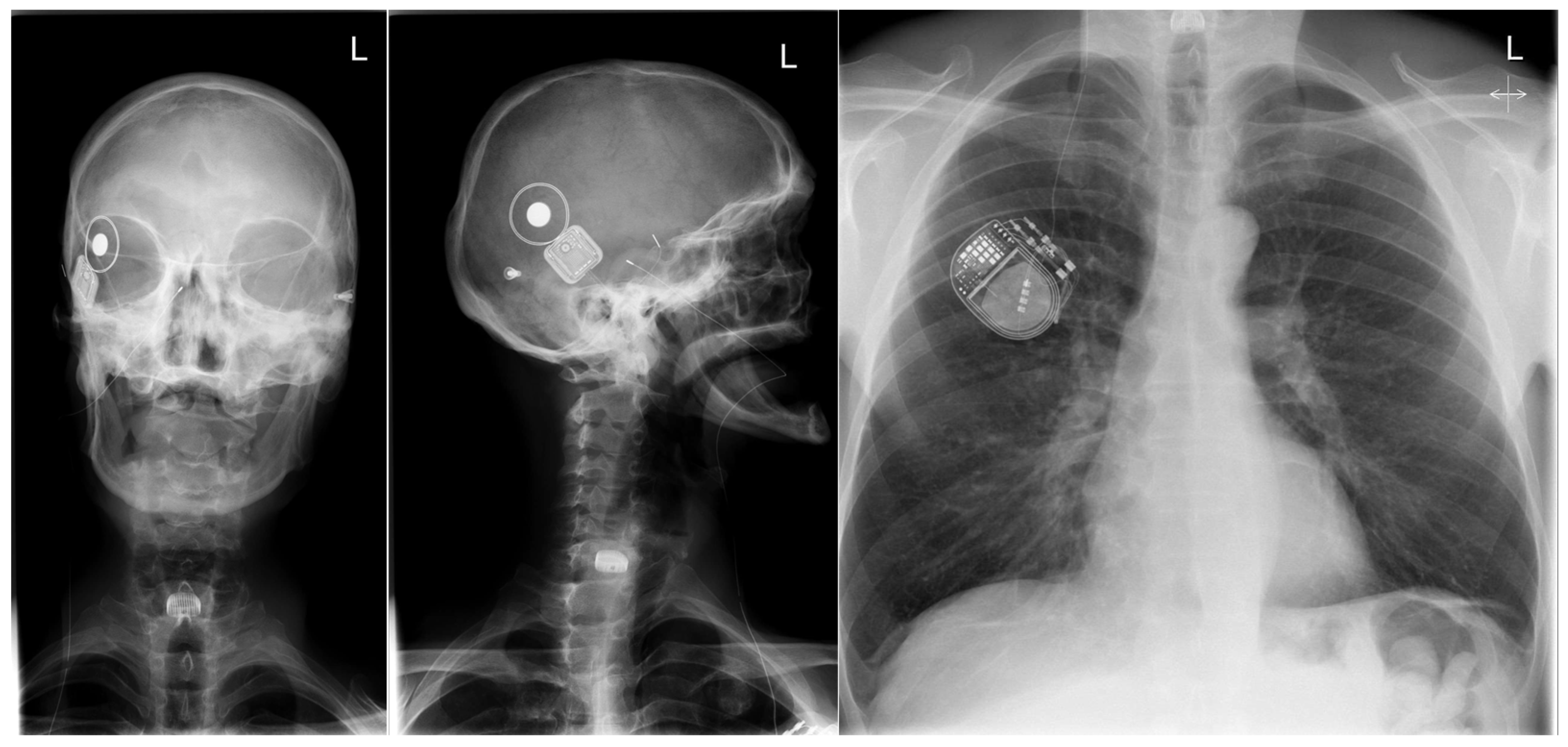
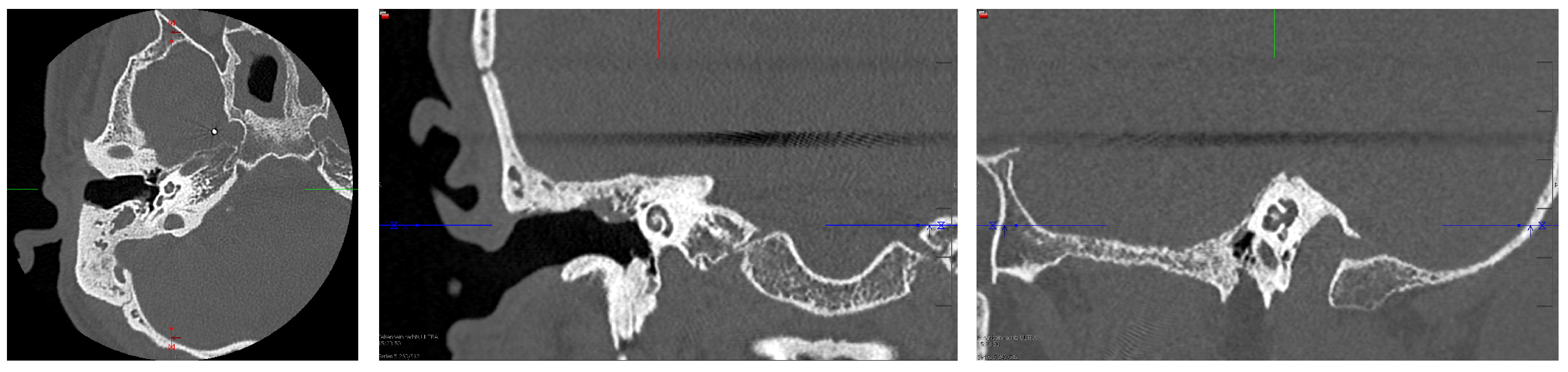
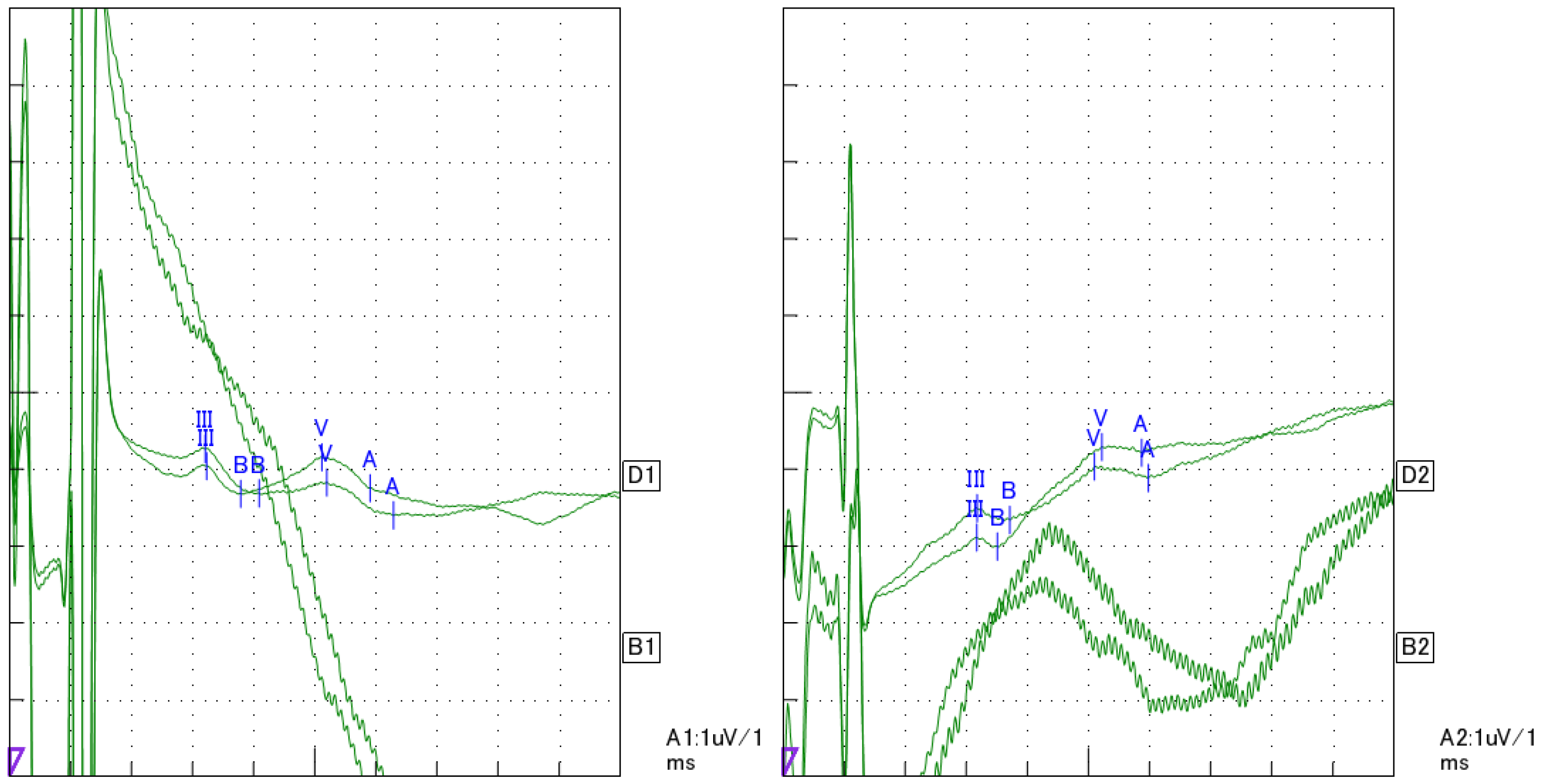
2. Case Presentation
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hochmair, I.; Nopp, P.; Jolly, C.; Schmidt, M.; Schößer, H.; Garnham, C.; Anderson, I. MED-EL Cochlear Implants: State of the Art and a Glimpse Into the Future. Trends Amplif. 2006, 10, 201–219. [Google Scholar] [CrossRef] [PubMed]
- Informal Working Group on Prevention of Deafness and Hearing Impairment Programme Planning (1991: Geneva, Switzerland); WHO Programme for Prevention of Deafness and Hearing Impairment. Report of the Informal Working Group on Prevention of Deafness and Hearing Impairment Programme Planning, Geneva, 18–21 June 1991. Switzerland. Available online: https://policycommons.net/artifacts/605652/report-of-the-informal-working-group-on-prevention-of-deafness-and-hearing-impairment-programme-planning-geneva-18-21-june-1991/1585239/ (accessed on 21 January 2024).
- Olusanya, B.O.; Davis, A.C.; Hoffman, H.J. Hearing Loss Grades and the International Classification of Functioning, Disability and Health. Bull. World Health Organ. 2019, 97, 725–728. [Google Scholar] [CrossRef]
- Zwolan, T.A.; Kileny, P.R.; Ashbaugh, C.; Telian, S.A. Patient Performance with the Cochlear Corporation “20 + 2” Implant: Bipolar versus Monopolar Activation. Am. J. Otol. 1996, 17, 717–723. [Google Scholar]
- Brown, C.J.; Abbas, P.J.; Gantz, B.J. Preliminary Experience with Neural Response Telemetry in the Nucleus CI24M Cochlear Implant. Am. J. Otol. 1998, 19, 320–327. [Google Scholar]
- Lai, W. (Ed.) An NRT Cookbook, 2nd ed.; Cochlear AG: Basel, Switzerland; Laboratory for Experimental Audiology, ENT Clinic, University Hospital of Zürich: Zürich, Switzerland, 2004; ISBN 3-9521853-0-3. [Google Scholar]
- Zhu, Z.; Tang, Q.; Zeng, F.-G.; Guan, T.; Ye, D. Cochlear-Implant Spatial Selectivity with Monopolar, Bipolar and Tripolar Stimulation. Hear. Res. 2012, 283, 45–58. [Google Scholar] [CrossRef]
- Mesnildrey, Q.; Macherey, O. Simulating the Dual-Peak Excitation Pattern Produced by Bipolar Stimulation of a Cochlear Implant: Effects on Speech Intelligibility. Hear. Res. 2015, 319, 32–47. [Google Scholar] [CrossRef]
- Wesarg, T.; Arndt, S.; Aschendorff, A.; Laszig, R.; Zirn, S. Intraoperative Audiologisch-Technische Diagnostik Bei Der Cochleaimplantatversorgung. HNO 2014, 62, 725–734. [Google Scholar] [CrossRef]
- Parreño, M.; Di Lella, F.A.; Fernandez, F.; Boccio, C.M.; Ausili, S.A. Toward Self-Measures in Cochlear Implants: Daily and “Homemade” Impedance Assessment. Front. Digit. Health 2020, 2, 582562. [Google Scholar] [CrossRef]
- Weiss, B.G.; Söchting, F.; Bertlich, M.; Busch, M.; Blum, J.; Ihler, F.; Canis, M. An Objective Method to Determine the Electrically Evoked Stapedius Reflex Threshold During Cochlea Implantation. Otol. Neurotol. 2018, 39, e5–e11. [Google Scholar] [CrossRef]
- Middlebrooks, J.C. Auditory System: Central Pathways. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 978-0-12-801238-3. [Google Scholar]
- Spiegel, J.L.; Polterauer, D.; Hempel, J.-M.; Canis, M.; Spiro, J.E.; Müller, J. Variation of the Cochlear Anatomy and Cochlea Duct Length: Analysis with a New Tablet-Based Software. Eur. Arch. Otorhinolaryngol. 2022, 279, 1851–1861. [Google Scholar] [CrossRef]
- Müller, J.; Schön, F. Lautheitsskalierung bei Cochlear-Implant-Patienten im Rahmen der präoperativen Austestung. Laryngorhinootologie 1994, 73, 128–131. [Google Scholar] [CrossRef]
- Irwin, C. NIC v2 Software Interface Specification E11318RD (Technical Report); Cochlear Ltd.: Lane Cove, NSW, Australia, 2006. [Google Scholar]
- Polterauer, D.; Mandruzzato, G.; Neuling, M.; Polak, M.; Müller, J.; Hempel, J. Evaluation of Auditory Pathway Excitability Using a Pre-Operative Trans-Tympanic Electrically Evoked Auditory Brainstem Response under Local Anesthesia in Cochlear Implant Candidates. Int. J. Audiol. 2023, 62, 1176–1186. [Google Scholar] [CrossRef]
- Polterauer, D. Intraoperative and Postoperative Measurement of Brainstem Responses through Electrical Stimulation of the Auditory Nerve via Implantable Neurostimulators; LMU Klinikum: München, Germany, 2020. [Google Scholar]
- Leake, P.A.; Hradek, G.T.; Bonham, B.H.; Snyder, R.L. Topography of Auditory Nerve Projections to the Cochlear Nucleus in Cats after Neonatal Deafness and Electrical Stimulation by a Cochlear Implant. J. Assoc. Res. Otolaryngol. 2008, 9, 349–372. [Google Scholar] [CrossRef] [PubMed]
- Lammers, M.J.; Van Eijl, R.H.; Van Zanten, G.A.; Grolman, W.; Versnel, H. Delayed Auditory Brainstem Responses in Prelingually Deaf and Late Implanted Cochlear Implant Users. J. Assoc. Res. Otolaryngol. 2015, 16, 669–678. [Google Scholar] [CrossRef]
- Minami, S.B.; Takegoshi, H.; Shinjo, Y.; Enomoto, C.; Kaga, K. Usefulness of Measuring Electrically Evoked Auditory Brainstem Responses in Children with Inner Ear Malformations during Cochlear Implantation. Acta Oto-Laryngol. 2015, 135, 1007–1015. [Google Scholar] [CrossRef]
- Firszt, J.B.; Chambers And, R.D.; Kraus, N. Neurophysiology of Cochlear Implant Users II: Comparison among Speech Perception, Dynamic Range, and Physiological Measures. Ear Hear. 2002, 23, 516–531. [Google Scholar] [CrossRef]
- Baljić, I.; Müller, A.; Fröhlich, L.; Polterauer, D.; Dziemba, O. Elektrisch Evozierte Potentiale Des Auditorischen Systems—Teil 2. Z. Für Audiol. (Audiol. Acoust.) 2021, 60, 71–75. [Google Scholar]
- Polterauer, D.; Mandruzzato, G.; Neuling, M.; Polak, M.; Müller, J.; Hempel, J. LA-TT-EALR/PromCERA: Comparison of Preoperatively Performed Electrically Evoked Auditory Potentials at the Brainstem and Cortical Level during Local Anesthesia. Curr. Dir. Biomed. Eng. 2022, 8, 233–236. [Google Scholar] [CrossRef]
- Eddelman, D.; Wewel, J.; Wiet, R.M.; Metman, L.V.; Sani, S. Deep Brain Stimulation with a Pre-Existing Cochlear Implant: Surgical Technique and Outcome. Surg. Neurol. Int. 2017, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Buell, T.J.; Ksendzovsky, A.; Shah, B.B.; Kesser, B.W.; Elias, W.J. Deep Brain Stimulation in the Setting of Cochlear Implants: Case Report and Literature Review. Stereotact. Funct. Neurosurg. 2015, 93, 245–249. [Google Scholar] [CrossRef] [PubMed]
- St. Martin, M.B.; Hirsch, B.E. Cochlear Implantation in a Patient With Bilateral Deep Brain Stimulators. Laryngoscope 2007, 117, 183–185. [Google Scholar] [CrossRef] [PubMed]
- Reyes, K.D.L.; Chandrasekhar, S.S.; Tagliati, M.; Alterman, R. Successful Implantation of a Deep Brain Stimulator for Essential Tremor in a Patient With a Preexisting Cochlear Implant: Surgical Technique: Technical Case Report. Oper. Neurosurg. 2010, 66, onsE372. [Google Scholar] [CrossRef]
- Cif, L.; Gonzalez, V.; Garcia-Ptacek, S.; James, S.; Boetto, J.; Seychelles, A.; Roujeau, T.; Moura De Ribeiro, A.M.; Sillon, M.; Mondain, M.; et al. Progressive Dystonia in Mohr-Tranebjaerg Syndrome With Cochlear Implant and Deep Brain Stimulation. Mov. Disord. 2013, 28, 737–738. [Google Scholar] [CrossRef] [PubMed]
- Arafat, T.; Miron, G.; Strauss, I.; Fahoum, F. Electrodiagnostic Artifacts Due to Neurostimulation Devices for Drug Resistant Epilepsy. Epilepsy Behav. Rep. 2022, 20, 100566. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polterauer, D.; Neuling, M.; Stoecklein, S.; Mueller, J. Cochlear Implantation in a Patient with Implanted Trigeminus Stimulator—Clinical Considerations for Using Two Different Electrical Stimulators in the Same Patient and Our Results. J. Otorhinolaryngol. Hear. Balance Med. 2024, 5, 2. https://doi.org/10.3390/ohbm5010002
Polterauer D, Neuling M, Stoecklein S, Mueller J. Cochlear Implantation in a Patient with Implanted Trigeminus Stimulator—Clinical Considerations for Using Two Different Electrical Stimulators in the Same Patient and Our Results. Journal of Otorhinolaryngology, Hearing and Balance Medicine. 2024; 5(1):2. https://doi.org/10.3390/ohbm5010002
Chicago/Turabian StylePolterauer, Daniel, Maike Neuling, Sophia Stoecklein, and Joachim Mueller. 2024. "Cochlear Implantation in a Patient with Implanted Trigeminus Stimulator—Clinical Considerations for Using Two Different Electrical Stimulators in the Same Patient and Our Results" Journal of Otorhinolaryngology, Hearing and Balance Medicine 5, no. 1: 2. https://doi.org/10.3390/ohbm5010002
APA StylePolterauer, D., Neuling, M., Stoecklein, S., & Mueller, J. (2024). Cochlear Implantation in a Patient with Implanted Trigeminus Stimulator—Clinical Considerations for Using Two Different Electrical Stimulators in the Same Patient and Our Results. Journal of Otorhinolaryngology, Hearing and Balance Medicine, 5(1), 2. https://doi.org/10.3390/ohbm5010002






