Abstract
Isolated lateral semicircular canal dysplasia (LSCCD) is one of the most frequent malformations of the bony labyrinth. The aim of this study is to depict morphology and size of the vestibular endolymphatic space in patients with isolated LSCCD using a dedicated 3D high resolution MR sequence called 3D inversion recovery with REAL reconstruction (3D-REAL-IR). From January 2018 to February 2020, we reviewed 281 CT and 241 MR temporal bone studies, and 103 MR studies performed for the evaluation of endolymphatic hydrops (EH). Five patients with LSCCD were found, one of them with bilateral malformation. Three patients (four affected ears) underwent specific MR examination for the evaluation of EH, consisting of a heavily T2-weighed cisternography sequence (T2 SPACE) and a 3D inversion-recovery with REAL reconstruction. The endolymphatic volumetric ratio (ELR) was calculated as the total endolymphatic volume divided by the total lymph (vestibular) volume multiplied by 100. Hydrops MR imaging was available in four of the affected ears. ELR ranged from 22% to 81%. Both extremes were present in the same patient, corresponding to a patient with right unilateral Ménière’s syndrome but with bilateral LSCCD. A patient affected with hearing loss had an ELR of 33% and the last patient with unilateral probable Ménière’s syndrome showed an ELR of 42%. Endolymphatic hydrops imaging is feasible and can be performed on patients with inner ear malformations like LSCCD. The endolymphatic volumetric ratio could be a useful and reproducible tool in daily clinical practice.
1. Introduction
Congenital malformations of the inner ear are characterized by small structural changes and variations in anatomical development and are responsible of 20% of congenital sensorineural hearing loss [1]. Minor malformations may not cause symptoms (or only in adulthood) and are usually found incidentally on imaging, especially temporal bone CT [2]. Among them, one of the most frequent malformations of the bony labyrinth is the one affecting the lateral semicircular canal (LSCC) [3,4].
Isolated LSCC dysplasia (LSCCD), cystoid enlargement, or cystic LSCC (as is also mentioned) shows no other associated abnormalities at the cochlea or dilated vestibular aqueduct. In those cases, the relationship with hearing loss or vestibular symptoms is somehow controversial. Some authors found no correlation between sensorineural hearing loss and isolated LSCCD [5]. Other series found that hearing function in isolated LSCCD is usually, but not always, impaired. However, bilateral LSCCD/aplasia when comorbid with other inner ear anomalies may present as profound bilateral hearing loss and vestibulopathy [6].
By definition, the diagnosis of LSCCD can be made when the area of the central bony island measured by magnetic resonance (MR) or computed tomography (CT) is less than 7 mm2 [3] or completely absent. In recent years, the evaluation of this anomaly has progressed to better characterization due to the possibility of delineating the endolymphatic and perilymphatic compartments. This has been achieved by using special MR imaging (MRI) [7] given the fact that gadolinium (Gd) only diffuses to the perilymphatic compartment, through the blood–perilymph barrier, and not to the endolymphatic space. This causes a negative contrast with the non-enhancing endolymphatic space [8]. There are few papers assessing morphology and size of the vestibular endolymphatic space in isolated LSCCD [9,10].
The aim of this study is to review this finding in our series of patients undergoing imaging of the temporal bone in a short period of time. Also, to report the findings of a more detailed evaluation of the inner ear and, in particular, of endolymphatic and perilymphatic spaces.
2. Materials and Methods
From January 2018 to February 2020, we reviewed from our hospital’s database 281 CT and 241 MR temporal bone studies as well as 103 MR studies performed with dedicated hydrops sequences. Among the total 625 temporal bone studies, we found 5 patients with LSCCD, one of these patients with bilateral disease. One of them has been the subject of a previous communication [11]. Therefore, a total number of 6 diseased ears were recorded. All affected ears lacked -both on CT or MRI T2 cisternography sequence- the central bony island, representing the form of complete LSCCD. Three patients (4 affected ears) underwent specific MR examination for the evaluation of hydropic ear disease.
2.1. MR Imaging
All MR imaging was performed using a 3-Tesla scanner (MAGNETOM Vida, Siemens Healthineers, Erlangen, Germany) using a 20-channel phase-array head coil. Images were obtained 4 h after a single dose of intravenous gadolinium (Gd) administration (Gadovist®; Bayer-Schering Pharma, Berlin, Germany; 1.0 mmol/mL at a dose of 0.1 mmol/kg). Informed consent was obtained from all individual participants included in the study.
According to the imaging protocol of our institution, we obtained:
- −
- A heavily T2 weighed sequence 3D SPACE (“Sampling Perfection with Application optimized Contrasts using different flip angle Evolution”), also called cisternography sequence, with the following parameters: section thickness, 0.5 mm; TR, 1400 ms; TE, 152 ms; flip angle, 120°; bandwidth, 289 Hz/pixel; voxel size, 0.5 × 0.5 × 0.5; and scan time, 5 min.
- −
- A 3D-REAL-IR: section thickness, 0.8 mm; TR, 16,000 ms; TE, 551 ms; TI: 2700 ms; flip angle, 140°; bandwidth, 434 Hz/pixel; voxel size, 0.5 × 0.5 × 0.8; and scan time, 11 min, as described by Naganawa [7].
2.2. Image Analysis
On a dedicated workstation with Syngo.via software (Siemens Healthineers, Erlangen, Germany, Client version 5.1), we used the MR cisternography sequence (T2 3D SPACE) to measure the total volume of vestibular lymph fluid (bright signal) on diseased ears defined as bright signal within the cystoid cavity formed by the union of the vestibule and the aplastic LSCC in a semi-automatic fashion. An experienced radiologist manually delineated the boundaries of the vestibule in all slices, and the software automatically calculated the total volume. As no central bony islands were depicted, no subtractions to this total volume were needed. The total volume of endolymph within the cystoid cavity was also calculated semi-automatically using the 3D-IR with REAL reconstruction sequence manually tracking the low signal in the vestibule. The endolymphatic volumetric ratio (ELR) [9] was calculated as the total vestibular endolymphatic volume divided by the total vestibular cavity volume multiplied by 100. The radiologist was not aware of the clinical information during the process of volume segmentation (Figure 1).
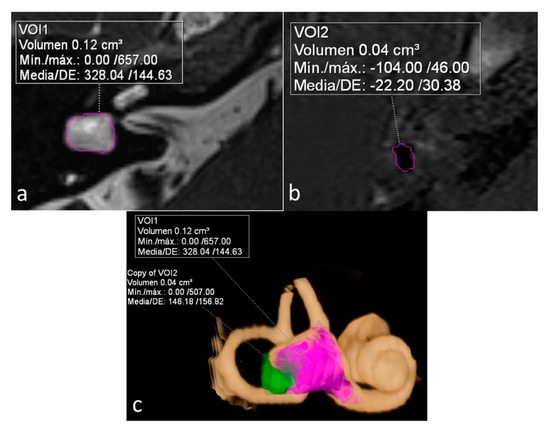
Figure 1.
Sequences used for the calculation and depiction of endolymphatic and total vestibular volumes and endolymphatic ratio (ELR). (a) Heavily T2 weighed cisternography sequence depicts lymph within a right cystoid lateral semicircular canal (LSCC). A semi-automatic calculation of total vestibular lymph volume is shown. (b) 3D-IR-REAL hydrops sequence depicts low signal (dark) vestibular endolymphatic volume. (c) 3D volume-rendered image of the inner ear based on the cisternography sequence with color coded overlay of both vestibular and endolymphatic hydrops volumes.
3. Results
Table 1 summarizes the findings in all six ears affected by a LSCCD, as well as clinical symptoms. MR hydrops imaging with a dedicated protocol including 3D-IR sequence was available in four ears.

Table 1.
Clinical and imaging findings in affected ears.
ELR ranged from 22% to 81%. It´s interesting to note that both extremes were present in the same patient, corresponding to a patient with right unilateral Ménière’s syndrome but with bilateral LSCCD (patient 4). On the dysplastic clinically affected side, ELR was 81% (Figure 2), whereas on the dysplastic clinically asymptomatic ear, ELR was barely 22% (Figure 3). In patients with unilateral LSCCD and available hydrops MR imaging (patients 3 and 5), the contralateral “normal anatomy” inner ear showed no signs of endolymphatic hydrops with endolymphatic ratios of less than 25% (not shown). The contralateral “normal anatomy” inner ear was asymptomatic.
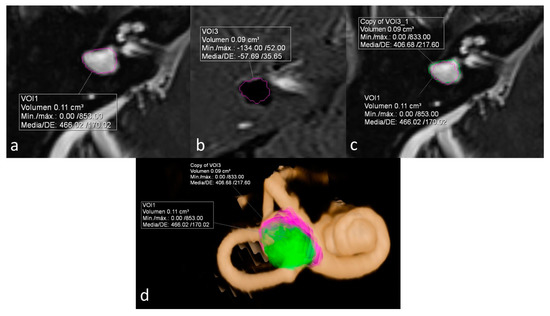
Figure 2.
Patient with bilateral LSCCD. Right clinically affected ear. (a) Heavily T2 weighed cisternography sequence with total vestibular lymph volume. (b) 3D-IR-REAL hydrops sequence shows vestibular endolymphatic volume (dark signal). Note also cochlear hydrops. (c) Overlay of both volume values in the cisternography sequence for the calculation of the endolymphatic ratio (ELR). (d) 3D volume-rendered image of the inner ear with color coded overlay of both cisternal and vestibular hydrops volumes. ELR of 81% suggesting severe vestibular hydrops.
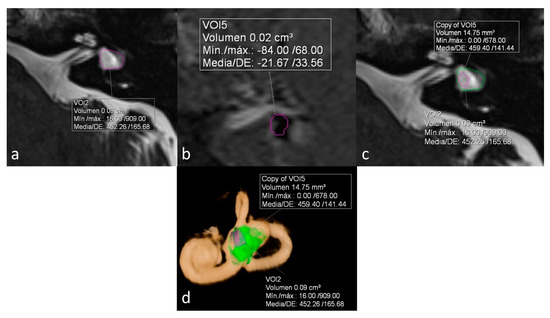
Figure 3.
Same patient as in Figure 2. Left ear. No Ménièriform symptoms on this side. (a) Cisternography sequence showing LSCCD with volumetric measurement of total vestibular cavity volume. (b) 3D-IR-REAL sequence depicts volume of vestibular endolymph. (c) Overlay of both volumetric measurements in the cisternography image (d) 3D volume-rendered with color-coded volumes, obtaining an ELR of 22%, consistent with the lack of symptoms on this ear.
The other two patients, both with unilateral LSCCD, showed intermediate percentages. One patient was suffering from hearing loss without vestibular symptoms (patient 3) and showed an ELR of 33% (shown on Figure 1). The other patient had symptoms of probable MD (patient 6) and showed an ELR of 42% (not shown).
4. Discussion
LSCCD is one of the most frequent inner ear malformations and can be called so when no other malformations of the inner ear are present [4,12]. Superior semicircular canal is the first to develop at five weeks of gestation, followed by the posterior semicircular canal. LSCC is the last one to develop and probably because of this, could occur as an isolated anomaly. In cases of complete LSCC dysplasia (LSCCD), the vestibule and lateral semicircular canal form a single fluid-filled cavity. Aplasia, defined as the absence of LSCC, is the most severe form and may manifest as part of several broader syndromes, such as CHARGE syndrome [13].
Patients in this study were seen after an incidental finding in 1 case (patient #2), as a part of the diagnostic procedure for MD in 2 cases (patients #4 and #5) both of whom fulfilled diagnostic criteria according to recent guidelines [14], and because of BPPV and mixed hearing loss (patients #1 and #3).
In this paper, we have shown a wide spectrum of results when imaging of the temporal bone enables a more detailed identification of the different compartments of the inner ear: in particular, the endolymphatic and perilymphatic compartments. To our knowledge, enlargement of the endolymphatic space in LSCCD has only been addressed in the literature by two papers [9,10].
Different grading systems for endolymphatic hydrops severity have been proposed in the literature, based on morphological diagnosis [15,16,17]. But none of them are currently established for patients with inner ear malformations.
As shown here, the ELR has promising results to better characterize LSCCD as we can see how this value progresses from the lowest in the less symptomatic case (patient #4, ear #5), increasing in the case with just hearing loss (patient #3, ear #3), then the probable (patient #5, ear #6) and finally the definite (patient #4, ear #4) MD cases. The anatomic counterpart of is spectrum is likely due to an increasing abnormality in the area where the missing bony island can remain from partially formed to non-existing, becoming part of the total volume of the vestibule, and then being “invaded” by the bulging utricle.
One of the limitations of this pictorial essay is the low number of diseased ears and, to establish criteria of severity, further studies with a larger number of patients are needed. In addition, a correlation between the quantitative hydrops and clinical symptoms could be interesting.
5. Conclusions
Endolymphatic hydrops imaging is feasible and can be performed in patients with inner ear malformations like LSCCD. The endolymphatic volumetric ratio could be a useful and easily applicable tool in daily clinical practice.
Author Contributions
Conceptualization, V.M.S.-V., P.D. and N.P.-F.; methodology, V.M.S.-V.; software, V.M.S.-V.; validation, V.M.S.-V., P.D. and N.P.-F.; investigation, V.M.S.-V.; resources, V.M.S.-V.; data curation, V.M.S.-V.; writing—original draft preparation, V.M.S.-V.; writing—review and editing, V.M.S.-V., P.D. and N.P.-F.; visualization, V.M.S.-V.; supervision, V.M.S.-V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cross, N.C.; Stephens, S.D.G.; Francis, M.; Hourihan, M.D.; Reardon, W. Computed tomography evaluation of the inner ear as a diagnostic, counselling and management strategy in patients with congenital sensorineural hearing impairment. Clin. Otolaryngol. Allied Sci. 1999, 24, 235–238. Available online: https://pubmed.ncbi.nlm.nih.gov/10384853/ (accessed on 4 January 2021). [CrossRef] [PubMed]
- Pont, E.; Mazón, M.; Montesinos, P.; Sánchez, M.Á.; Más-Estellés, F. Imaging Diagnostics: Congenital Malformations and Acquired Lesions of the Inner Ear. Acta Otorrinolaringol. 2015, 66, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Sennaroglu, L.; Saatci, I. A new classification for cochleovestibular malformations. Laryngoscope 2002, 112, 2230–2241. [Google Scholar] [CrossRef] [PubMed]
- Casselman, J.W.; Offeciers, E.F.; De Foer, B.; Govaerts, P.; Kuhweide, R.; Somers, T. CT and MR imaging of congential abnormalities of the inner ear and internal auditory canal. Eur. J. Radiol. 2001, 40, 94–104. [Google Scholar] [CrossRef]
- Yamashita, K.; Yoshiura, T.; Hiwatashi, A.; Tuvshinjargal, D.; Kamano, H.; Inoguchi, T.; Honda, H. Sensorineural hearing loss: There is no correlation with isolated dysplasia of the lateral semi-circular canal on temporal bone CT. Acta Radiol. 2011, 52, 229–233. Available online: http://www.ncbi.nlm.nih.gov/pubmed/21498353 (accessed on 29 February 2020). [CrossRef] [PubMed]
- Kwak, S.H.; Kim, M.K.; Kim, S.H.; Jung, J. Audiological and Vestibular Functions in Patients With Lateral Semicircular Canal Dysplasia and Aplasia. Clin. Exp. Otorhinolaryngol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Naganawa, S.; Kawai, H.; Taoka, T.; Sone, M. Improved 3D-real inversion recovery: A robust imaging technique for endolymphatic hydrops after intravenous administration of gadolinium. Magn. Reson. Med. Sci. 2019, 18, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Bernaerts, A.; De Foer, B. Imaging of Ménière Disease. Neuroimaging Clin. N. Am. 2019, 29, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Naganawa, S.; Kawai, H.; Sone, M.; Ikeda, M. Ratio of vestibular endolymph in patients with isolated lateral semicircular canal dysplasia. Magn. Reson. Med. Sci. 2015, 14, 203–210. Available online: http://www.ncbi.nlm.nih.gov/pubmed/25833266 (accessed on 29 February 2020). [CrossRef] [PubMed]
- Higashi-Shingai, K.; Imai, T.; Takimoto, Y.; Okumura, T.; Ohta, Y.; Morihana, T.; Uno, A.; Watanabe, Y.; Horii, A.; Inohara, H. Gadolinium contrast-enhanced MRI reveals cystic lateral semicircular canal contents. Acta Otolaryngol. 2015, 135, 1000–1006. [Google Scholar] [CrossRef] [PubMed]
- Wegmann-Vicuña, R.; Garaycochea, O.; Domínguez-Echavarri, P.; Guajardo-Vergara, C.; García-Eulate, R.; Pérez-Fernández, N. Dissociated responses to caloric and head impulse stimulation in a case of isolated vestibule-lateral semicircular canal dysplasia. Acta Oto-Laryngol. Case Rep. 2018, 3, 5–10. [Google Scholar] [CrossRef]
- Telian, B.S.M. Congenital Aplasia of the Semicircular Canals. Otol Neurotol. 2003, 24, 437–446. [Google Scholar]
- Wineland, A.; Menezes, M.D.; Shimony, J.S.; Shinawi, M.S.; Hullar, T.E.; Hirose, K. Prevalence of semicircular canal hypoplasia in patients with charge syndrome 3c syndrome. JAMA Otolaryngol. Head Neck Surg. 2017, 143, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Escamez, J.A.; Carey, J.; Chung, W.-H.; Goebel, J.A.; Magnusson, M.; Mandalà, M.; Newman-Toker, D.E.; Strupp, M.; Suzuki, M.; Trabalzini, F.; et al. Diagnostic criteria for Menière’s disease. J. Vestib. Res. Equilib. Orientat. 2015, 25, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, T.; Naganawa, S.; Pyykkö, I.; Gibson, W.P.R.; Sone, M.; Nakata, S.; Teranishi, M. Grading of endolymphatic hydrops using magnetic resonance imaging. Acta Oto-Laryngologica. 2009, 129, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Baráth, K.; Schuknecht, B.; Monge Naldi, A.; Schrepfer, T.; Bockisch, C.J.; Hegemann, S.C.A. Detection and grading of endolymphatic hydrops in Menière disease using MR imaging. Am. J. Neuroradiol. 2014, 35, 1387–1392. [Google Scholar] [CrossRef] [PubMed]
- Bernaerts, A.; Vanspauwen, R.; Blaivie, C.; van Dinther, J.; Zarowski, A.; Wuyts, F.L.; Bossche, S.V.; Offeciers, E.; Casselman, J.W.; De Foer, B. The value of four stage vestibular hydrops grading and asymmetric perilymphatic enhancement in the diagnosis of Menière’s disease on MRI. Neuroradiology 2019, 61, 421–429. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).