DED Powder Modification for Single-Layer Coatings on High-Strength Steels
Abstract
1. Introduction
2. Materials and Methods
2.1. Determination of the Compensation Powder Composition
2.2. Methodology for the Manufacturing of the Compensated Filler Compound
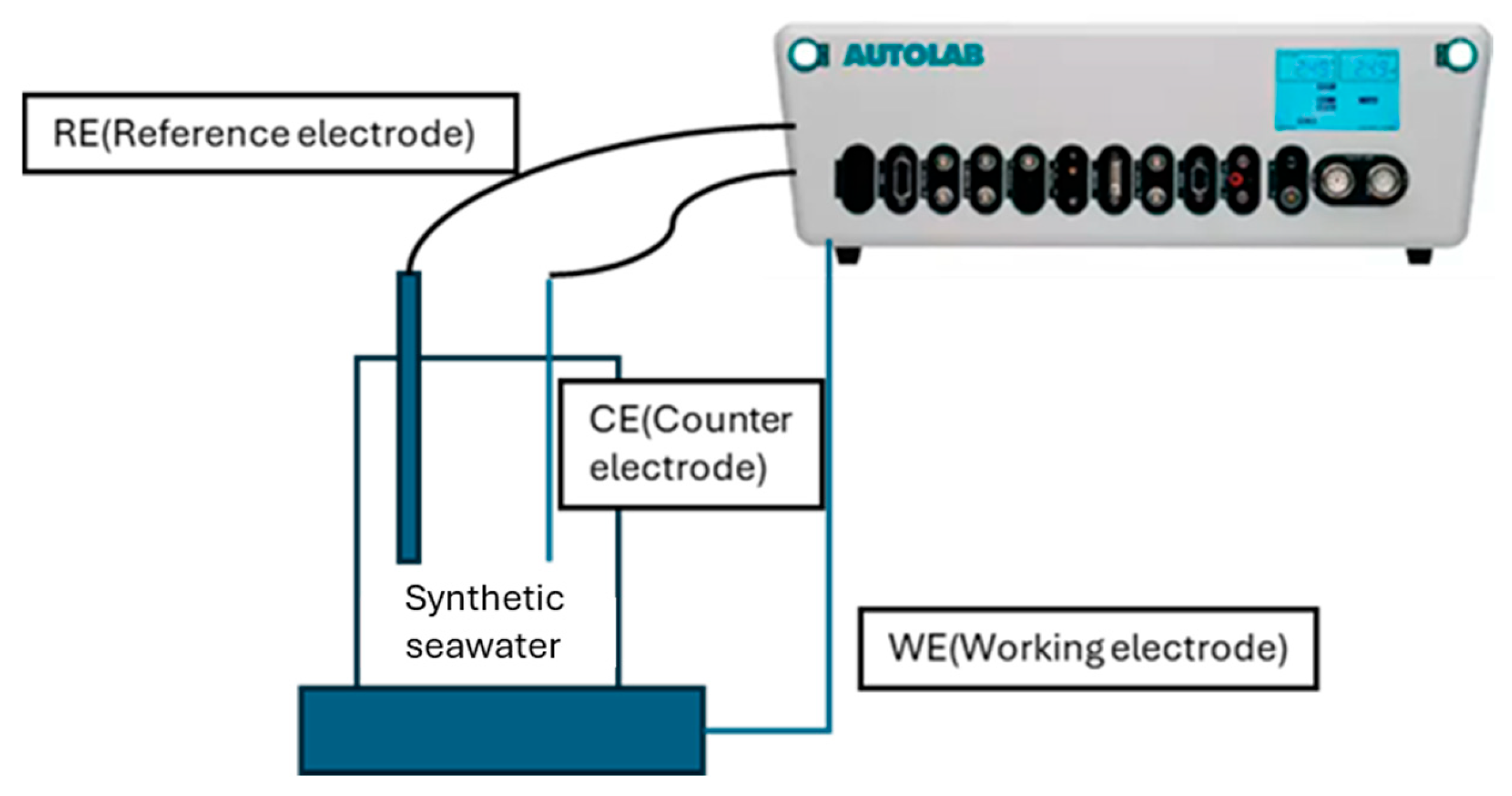
2.3. Corrosion Testing
3. Results
3.1. Chemical Composition of the L-DED Coatings With and Without Dilution Compensation
3.2. Microstructure Analysis
3.3. Potentiodinamic Polarization Test
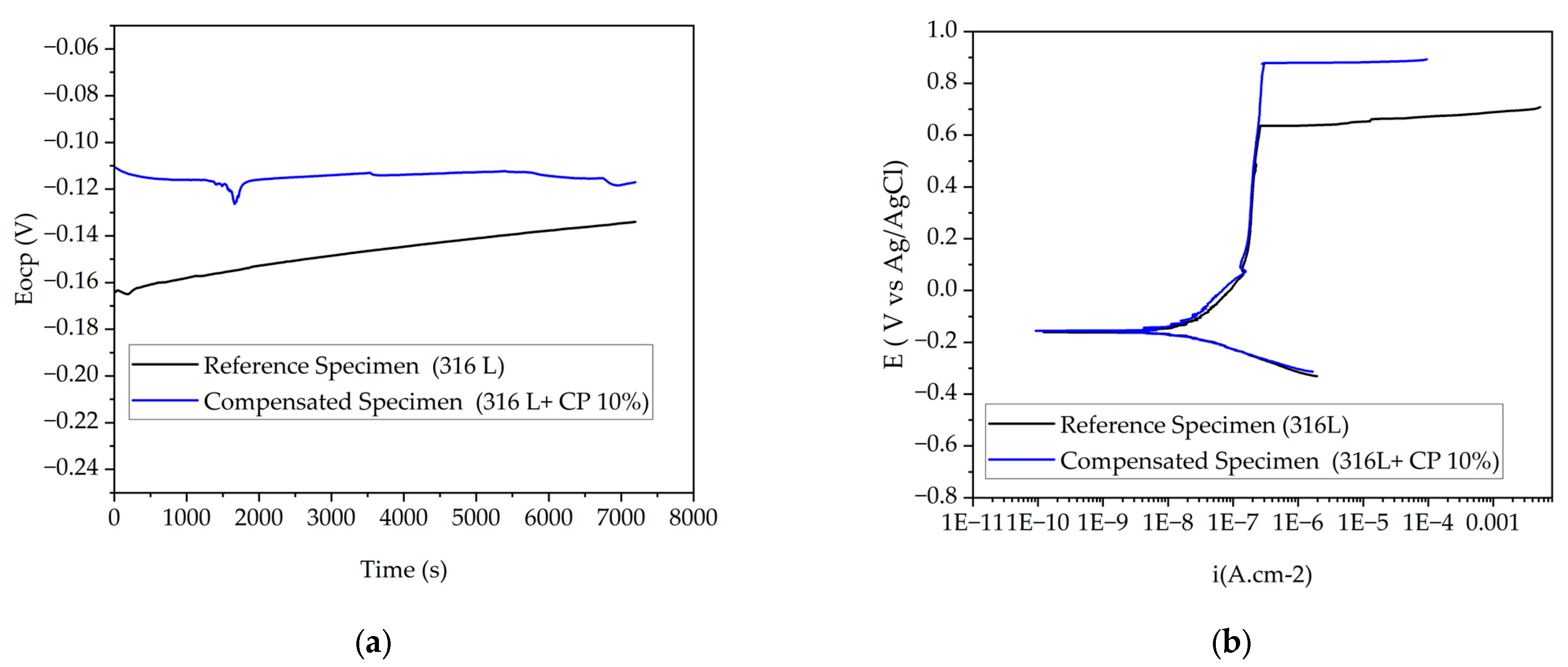
3.3.1. Open-Circuit Potential
3.3.2. Potentiodynamic Polarization
4. Discussion
5. Conclusions
- L-DED demonstrates strong potential for fabricating multifunctional surfaces by allowing flexible manipulation of feedstock compositions, enabling the rapid production of tailored cladding layers.
- The addition of compensation powder to AISI 316L results in a laser-clad layer that closely matches the theoretical composition of standard AISI 316L stainless steel.
- A more homogeneous distribution of alloying elements, particularly C and Cr, within the matrix effectively delays the initiation of galvanic corrosion and pitting phenomena.
- Potentiodynamic polarization tests indicate that the pitting corrosion resistance of the deposited layer improves with increased elemental homogeneity, especially in terms of C and Cr dispersion.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BS | Substrate steel |
| CE | Counter electrode |
| CFC | Compensated filler compound (powder mixture of AISI 316L and CP) |
| CP | Compensation powder |
| EDS | Energy-dispersive spectroscopy |
| Ecorr | Corrosion potential |
| Eocp | OCP potential |
| Ep | Pitting potential |
| HWTIG | Hot wire tungsten inert gas |
| ICP-OES | Inductively coupled plasma optical emission spectroscopy |
| L-DED | Laser-directed energy deposition |
| MIG | Metal inert gas |
| MMA | Manual metal arc |
| OCP | Open-circuit potential |
| R[%X] | Dilution coefficient |
| RE | Reference electrode |
| SEM | Scanning electron microscope |
| SS | Stainless steel |
| WE | Working wlectrode |
| WPS | Welding procedure specification |
| Z | Proportion of CP in the CFC expressed as wt.% |
| %X | Mass percent of the element X |
References
- The American Society of Mechanical Engineers. 2023 ASME Boiler & Pressure Vessel Code Section IX; The American Society of Mechanical Engineers: New York, NY, USA, 2023; pp. 32, 208–213. [Google Scholar]
- The American Society of Mechanical Engineers. 2023 ASME Boiler & Pressure Vessel Code Section III Part C; The American Society of Mechanical Engineers: New York, NY, USA, 2023; pp. 67, 307, 312, 687, 922. [Google Scholar]
- Kumar, V.; Lee, C.; Verhaeghe, G.; Raghunathan, S. CRA Weld Overlay—Influence of Welding Process and Parameters on Dilution and Corrosion Resistance. In Proceedings of the Stainless Steel World America 2010, Houston, TX, USA, 5–7 October 2010. [Google Scholar]
- Arrizubieta, J.I.; Ostolaza, M.; Muro, M.; Andonegi, H.; Lamikiz, A. L-DED numerical model for sensor embedding. Int. J. Heat Mass Transf. 2023, 201, 123639. [Google Scholar] [CrossRef]
- Sethi, A.; Mohanty, I.; Misra, S.; Chakraborty, R.; Saha, P. A study integrating in-process thermal signatures, microstructure, and corrosion behaviour of AISI 316L coating on low carbon steel substrate deposited by laser-directed energy deposition (L-DED). J. Surf. Coat. 2024, 493, 131268. [Google Scholar] [CrossRef]
- Gittos, M.F.; Gooch, T.G. Effects of iron dilution on corrosion resistance of Ni-Cr-Mo alloy cladding. Br. Corros. J. 1996, 31, 309–314. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, D.; Liu, G.; Qian, Y.; Xu, Y.; Xiang, D. Surface Modification of 42CrMo Steels: A Review from Wear and Corrosion Resistance. Coatings 2024, 14, 337. [Google Scholar] [CrossRef]
- Liu, Y.; Xiang, D.; Wang, K.; Yu, T. Corrosion of Laser Cladding High-Entropy Alloy Coatings: A Review. Coatings 2022, 12, 1669. [Google Scholar] [CrossRef]
- Yang, J.; Bing, B.; Ke, H.; Cui, Z.; Zhou, Z.; Xu, H.; Xiao, J.; Liu, Q.; Li, H. Effect of metallurgical behavior on microstructure and properties of FeCrMoMn coatings prepared by high-speed laser cladding. Opt. Laser Technol. 2021, 144, 107431. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Q.; Han, B.; Li, M.; Hu, C.; Wang, J. Comparative studies on microstructure and properties of CoCrFeMnNi high entropy alloy coatings fabricated by high-speed laser cladding and normal laser cladding. J. Alloys Compd. 2023, 947, 169517. [Google Scholar] [CrossRef]
- Vogt, S.; Göbel, M.; Fu, E. Perspectives for Conventional Coating Processes Using High-Speed Laser Cladding. J. Manuf. Sci. Eng. 2022, 144, 044501. [Google Scholar] [CrossRef]
- Sommer, N.; Stredak, F.; Böhm, S. High-Speed Laser Cladding on Thin-Sheer-Substrates-Influence of Process Parameters on Clad Geometry and Dilution. Coatings 2021, 11, 952. [Google Scholar] [CrossRef]
- Gui, W.; Zhong, C.; Gu, J.; Ding, Y.; Wang, X.; Wu, T.; Liang, Y.; Qin, J.; Qu, Y.; Lin, J. Laser-clad Inconel 625 coating on Q245R structure steel: Microstructure, wear and corrosion resistance. npj Mater. Degrad. 2022, 6, 37. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Peng, P.; Guo, S.; Ynag, L.; Guo, C. Wear and Corrosion Resistance of 304SS Coating Using High Speed Laser Cladding. Int. J. Precis. Eng. Manuf. 2025. [Google Scholar] [CrossRef]
- Garate, U.; Mardaras, E.; González, R.; Arrizubieta, J.I.; Artola, G.; Aldazabal, J. Influence of the Laser Deposited 316L Single Layers on Corrosion in Physiological Media. Metals 2022, 12, 1047. [Google Scholar] [CrossRef]
- Ünal, E.; Karaca, F.; Sarsilmaz, F. Investigation of interface microstructure properties of AISI 316L/AISI 4140 steel couple welded by friction welding process. J. Fac. Eng. Arch. Gazi Univ. 2019, 34, 701–708. [Google Scholar] [CrossRef]
- Mamphekgo, T.C.; Maledi, N.; Tshabalala, L.C.; Hagedorn-Hansen, D. The effects of laser wire directed energy deposition processing parameters on the properties of 309L stainless steel. In Proceedings of the 2023 RAPDASA-RobMech-PRASA-AMI Conference, Pretoria, South Africa, 30 October–2 November 2023. [Google Scholar] [CrossRef]
- Bozeman, S.C.; Burkan Isgor, O.; Tucker, J.D. Effects of processing conditions on the solidification and heat-affected zone of 309L stainless steel claddings on carbon steel using wire-directed energy deposition. J. Surf. Coat. 2022, 444, 128698. [Google Scholar] [CrossRef]
- Muro, M.; Artola, G.; Leunda, J.; Soriano, C.; Angulo, C. Compositional Modification of Tool Steel to Improve Its Wear Resistance. Metall. Mater. Trans. A 2018, 50, 3912–3921. [Google Scholar] [CrossRef]
- Rousson, C.; Deligiannis, S.; Ioannidou, D.; Papapamps, G.; Tsakiridis, P.E. Microstructural and Mechanical Properties of Laser Cladding-Deposited AISI 1060 Steel with a Mixture of 410L Alloy and 4140 Alloy Powders. J. Mater. Eng. Perform. 2024, 33, 11944–11956. [Google Scholar] [CrossRef]
- ASTM D1141-98; Standard Practice for the Preparation of Substitute Ocean Water (Reapproved 2003). ASTM International: West Conshohocken, PA, USA, 2003.
- ASTM A262-15; Standard Practice for Detection of Sensitization in Austenitic Steels. ASTM International: West Conshohocken, PA, USA, 2015.
- Das, T.; Mukherjee, M.; Chatterjee, D.; Samanata, S.K.; Lohar, A.K. A comparative evaluation of the microstructural characteristics of L-DED and W-DED processed 316L stainless steel. CIRP J. Manuf. Sci. Technol. 2023, 40, 114–128. [Google Scholar] [CrossRef]
- Kerr, H.W.; Kurz, W. Solidification of peritectic alloys. Int. Mater. Rev. 1996, 41, 129–164. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Dobara, K.; Todoroki, H.; Nam, C.; Morishita, K.; Yasuda, H. In-situ measurements of solute partition coefficients between solid and liquid phases in Fe–Cr–Ni–Mo–Cu alloys during solidification. ISIJ Int. 2020, 60, 276–285. [Google Scholar] [CrossRef]
- Schaeffler, A.L. Constitution diagram for stainless steel weld metal. Met. Prog. 1949, 56, 680. [Google Scholar]
- Osorio, W.R.; Freitas, E.S.; Spinelli, J.E.; Garcia, A. Electrochemical behavior of a lead-free Sn-Cu solder alloy in NaCl solution. Corros. Sci. 2014, 80, 71–81. [Google Scholar] [CrossRef]
- Liu, J.; Liu, H.; Tian, X.; Yang, H.; Hao, J. Microstructural evolution and corrosion properties of Ni-based alloy coatings fabricated by multi-layer laser cladding on cast iron. J. Alloys Compd. 2020, 822, 153708. [Google Scholar] [CrossRef]
- Trelewicz, J.R.; Halada, G.P.; Donaldson, O.K.; Manogharan, G. Microstructure and Corrosion Resistance of Laser Additively Manufactured 316L Stainless Steel. JOM 2016, 68, 850–859. [Google Scholar] [CrossRef]
- Lodhi, M.J.K.; Iams, A.D.; Sikore, E.; Palmer, T.A. Microstructural features contributing to macroscopic corrosion: The role of oxide inclusions on the corrosion properties of additively manufactured 316L stainless steel. Corros. Sci. 2022, 203, 110354. [Google Scholar] [CrossRef]
- Hong, H.U.; Nam, S.W. The occurrence of grain boundary serration and its effects on the M23C6 carbide characteristics in an AISI 316 stainless steel. Mater. Sci. Eng. A 2002, 332, 255–261. [Google Scholar] [CrossRef]
- Liu, T.; Cui, Y.; Zheng, K.; Yin, F.; Luo, Z. Synergistic effect of grain size and second-phase particle on the oxidation behaviour of a high-manganese austenitic heat-resistant steel. Corros. Sci. 2023, 215, 11054. [Google Scholar] [CrossRef]





| Material | Chemical Composition (wt.%) | ||||||
|---|---|---|---|---|---|---|---|
| C | Si | Mn | Cr | Ni | Mo | Fe | |
| AISI 316L powder | 0.01 | 2.30 | 1.49 | 17.00 | 12.00 | 2.95 | Bal. |
| AISI 4140 substrate | 0.42 | 0.20 | 0.75 | 1.10 | 0.00 | 0.22 | Bal. |
| Coating on reference specimen | 0.07 | 1.94 | 1.40 | 14.9 | 11.0 | 2.80 | Bal. |
| Spot (mm) | Input Energy (kJ/m) | Powder Mass Flow (g/min) | Shielding/Carrier Gas |
|---|---|---|---|
| 4.0 | 200 | 6.5 | Ar/Ar |
| Material | Dilution Coefficient (R[%X]) | ||||||
| CP (compensation powder) | C | Si | Mn | Cr | Ni | Mo | Fe |
| 12.63 | 9.50 | 2.52 | 7.37 | 9.00 | 7.53 | 7.80 | |
| Chemical Composition (wt.%) | |||||||
| C | Si | Mn | Cr | Ni | Mo | Fe | |
| 0.00 | 4.50 | 4.09 | 38.40 | 25.10 | 6.41 | 21.50 | |
| Atomization Platform | Frequency (kHz) | Argon Flow (L/min) | Arc Intensity (A) | Ultrasound Amplitude |
|---|---|---|---|---|
| W | 35 | 15 | 110 | 75% |
| Compound | Concentration (g/L) |
|---|---|
| NaCl | 24.53 |
| MgCl2 | 5.20 |
| Na2SO4 | 4.09 |
| CaCl2 | 1.16 |
| KCl | 0.695 |
| NaHCO3 | 0.201 |
| KBr | 0.101 |
| H3BO3 | 0.027 |
| SrCl2 | 0.025 |
| NaF | 0.003 |
| Ba(NO3)2 | 0.0000994 |
| Mn(NO2)2 | 0.0000340 |
| Cu(NO3)2 | 0.0000308 |
| Zn(NO3)2 | 0.0000096 |
| Pb(NO3)2 | 0.0000066 |
| AgNO3 | 0.00000049 |
| Material | C | Si | Mn | Cr | Ni | Mo | Fe |
|---|---|---|---|---|---|---|---|
| Standard for AISI 316L | <0.03 | <1.0 | <2.0 | 17–19 | 12.5–15.0 | 2.5–3.0 | Bal. |
| Reference specimen (316L) | 0.07 | 1.94 | 1.40 | 14.9 | 11.0 | 2.80 | Bal. |
| Compensated specimen (316L + CP 10%) | 0.07 | 1.65 | 1.30 | 16.70 | 13.10 | 2.70 | Bal. |
| Material | TSolidus (°C) | TLiquidus (°C) | L (J/g) |
|---|---|---|---|
| AISI 316 L | 1383 | 1427 | 170 |
| AISI 4140 | 1443 | 1493 | 177 |
| CP (compensation powder) | 1157 | 1311 | 200 |
| CFC (compensated filler compound) | 1362 | 1415 | 162 |
| Material | Zone | Cr | Mo | Ni |
|---|---|---|---|---|
| Coating on reference specimen (316L) | Cell | 12.8 | 1.5 | 8.4 |
| Cell boundary | 15.6 | 3.7 | 8.8 | |
| Coating on compensated specimen (316L + CP 10%) | Cell | 16.1 | 2.0 | 10.9 |
| Cell boundary | 17.7 | 4.3 | 11.7 |
| Material | Eocp (mV) | Ecorr (mV) | Ep (mV) | Icorr (µA·cm−2) |
|---|---|---|---|---|
| Reference specimen (316L) | −136 ± 3 | −137 ± 7 | 725 ± 6 | 2.45 ± 0.03 |
| Compensated specimen (316L + CP 10%) | −128 ± 9 | −168 ± 4 | 890 ± 9 | 2.11 ± 0.03 |
| Coating | KCr (Exp.) | KCr (TC) ferrite | KCr (TC) austenite | KMo (Exp.) | KMo (TC) ferrite | KMo (TC) austenite | KNi (Exp.) | KNi (TC) ferrite | KNi (TC) austenite |
|---|---|---|---|---|---|---|---|---|---|
| Reference specimen | 0.82 | 1.06 | 0.89 | 0.41 | 1.23 | 0.77 | 0.98 | 0.62 | 0.94 |
| Compensated specimen | 0.91 | 0.95 | 0.46 | 0.77 | 0.93 | 0.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garate, U.; Mardaras, E.; Arruabarrena, J.; Artola, G.; Lamikiz, A.; Lacalle, L.N.L.d. DED Powder Modification for Single-Layer Coatings on High-Strength Steels. J. Manuf. Mater. Process. 2025, 9, 152. https://doi.org/10.3390/jmmp9050152
Garate U, Mardaras E, Arruabarrena J, Artola G, Lamikiz A, Lacalle LNLd. DED Powder Modification for Single-Layer Coatings on High-Strength Steels. Journal of Manufacturing and Materials Processing. 2025; 9(5):152. https://doi.org/10.3390/jmmp9050152
Chicago/Turabian StyleGarate, Unai, Enara Mardaras, Jon Arruabarrena, Garikoitz Artola, Aitzol Lamikiz, and Luis Norberto López de Lacalle. 2025. "DED Powder Modification for Single-Layer Coatings on High-Strength Steels" Journal of Manufacturing and Materials Processing 9, no. 5: 152. https://doi.org/10.3390/jmmp9050152
APA StyleGarate, U., Mardaras, E., Arruabarrena, J., Artola, G., Lamikiz, A., & Lacalle, L. N. L. d. (2025). DED Powder Modification for Single-Layer Coatings on High-Strength Steels. Journal of Manufacturing and Materials Processing, 9(5), 152. https://doi.org/10.3390/jmmp9050152









