In Vitro Characterization of 3D-Printed PLA/CPO Oxygen Releasing Scaffolds: Mechanical and Biological Properties for Bone Tissue Engineering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. PLA/CPO Filament
2.3. 3D Printing of Bone Scaffolds
2.4. Characterizations
2.4.1. Morphological Analysis
2.4.2. Osteogenic Differentiation In Vitro
Cell Culture:
RNA Extraction:
RNA Quantification:
2.4.3. Mechanical and Microstructural Properties
2.5. Statistical Analysis
3. Results and Discussion
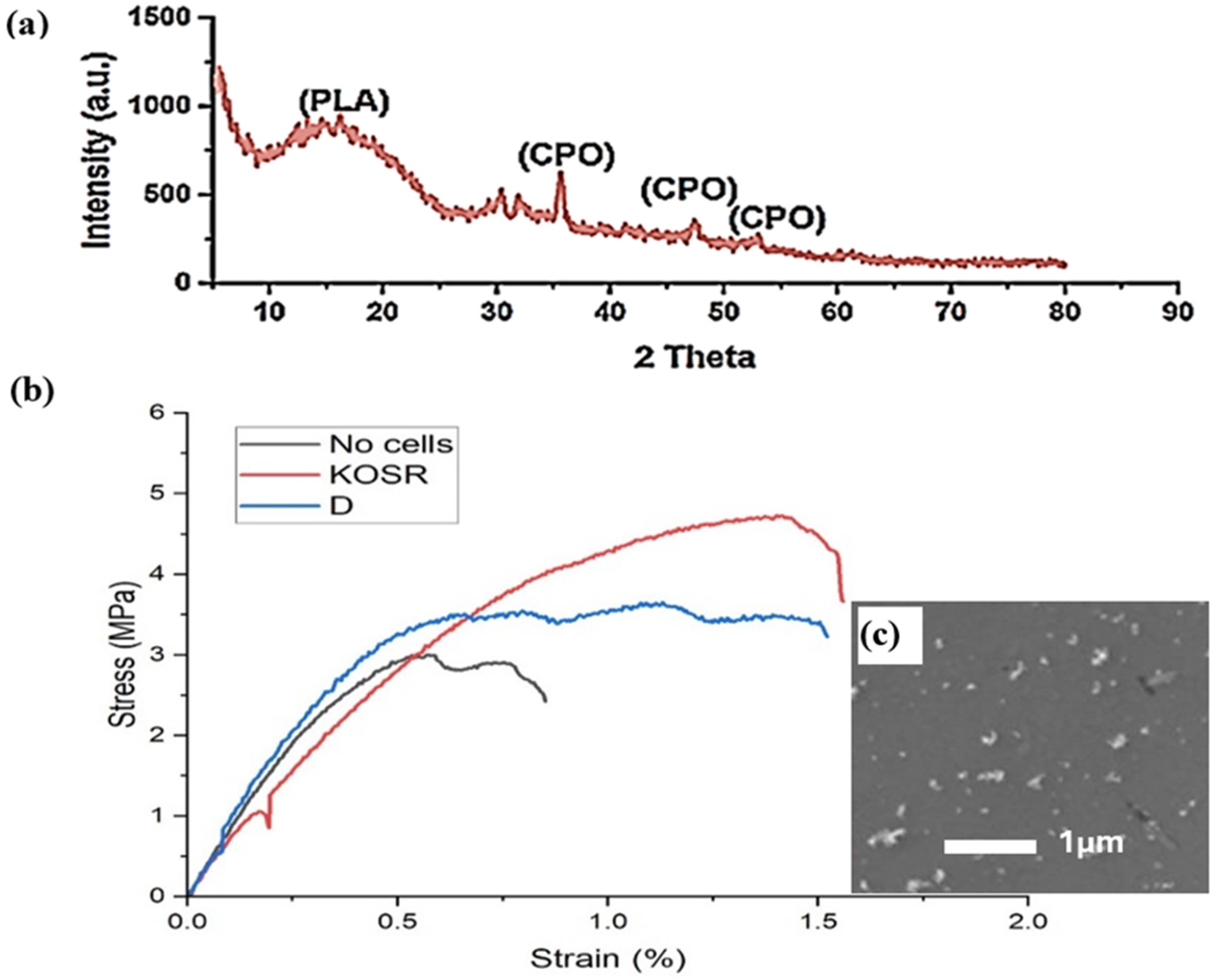
3.1. 3D Printing of Scaffolds
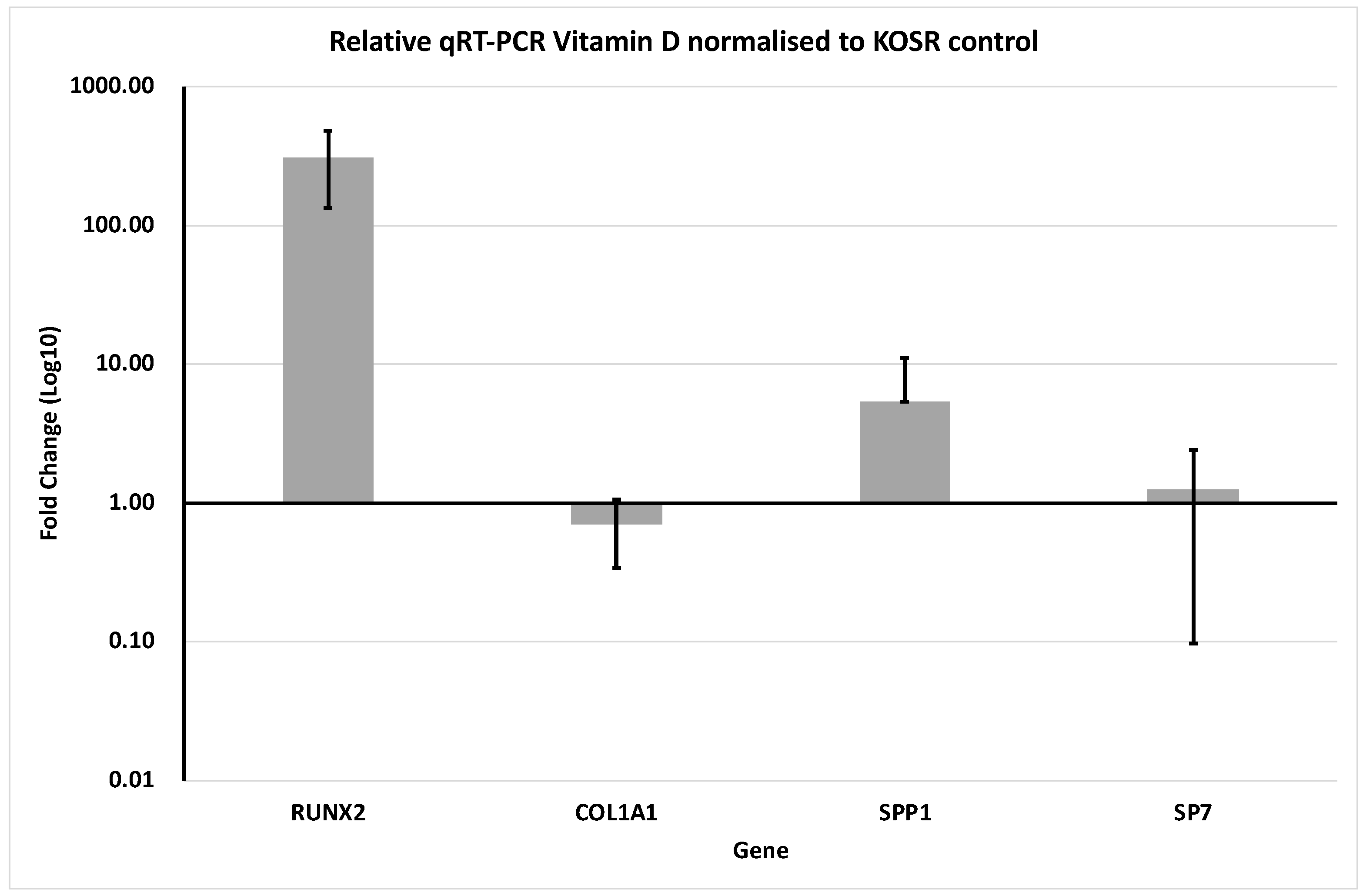
3.2. Gene Expression
3.3. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United States Department of Health And Human Services. Organ Donation Statistics. Available online: https://www.organdonor.gov/learn/organ-donation-statistics (accessed on 30 April 2025).
- Centers for Disease Control and Prevention (CDC). Key Facts About Organ Transplant Safety. Available online: https://www.cdc.gov/transplantsafety/overview/key-facts.html (accessed on 15 September 2024).
- Kumar, P.; Vinitha, B.; Fathima, G. Bone grafts in dentistry. J. Pharm. Bioallied Sci. 2013, 5, S125–S127. [Google Scholar] [CrossRef]
- Brydone, A.S.; Meek, D.; Maclaine, S. Bone grafting, orthopaedic biomaterials, and the clinical need for bone engineering. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2010, 224, 1329–1343. (In English) [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone regeneration: Current concepts and future directions. BMC Med. 2011, 9, 66. (In English) [Google Scholar] [CrossRef]
- Barua, S.; Chattopadhyay, P.; Aidew, L.; Buragohain, A.K.; Karak, N. Infection-resistant hyperbranched epoxy nanocomposite as a scaffold for skin tissue regeneration. Polym. Int. 2015, 64, 303–311. [Google Scholar] [CrossRef]
- Suvarnapathaki, S.; Wu, X.; Lantigua, D.; Nguyen, M.A.; Camci-Unal, G. Breathing life into engineered tissues using oxygen-releasing biomaterials. NPG Asia Mater. 2019, 11, 65. [Google Scholar] [CrossRef]
- Xiao, Y.; Ahadian, S.; Radisic, M. Biochemical and Biophysical Cues in Matrix Design for Chronic and Diabetic Wound Treatment. Tissue Eng. Part B Rev. 2017, 23, 9–26. (In English) [Google Scholar] [CrossRef]
- Bose, S.; Ke, D.; Sahasrabudhe, H.; Bandyopadhyay, A. Additive manufacturing of biomaterials. Prog. Mater. Sci. 2018, 93, 45–111. (In English) [Google Scholar] [CrossRef]
- Mohammed, A.; Jiménez, A.; Bidare, P.; Elshaer, A.; Memic, A.; Hassanin, H.; Essa, K. Review on Engineering of Bone Scaffolds Using Conventional and Additive Manufacturing Technologies. 3D Print. Addit. Manuf. 2024, 11, 1418–1440. [Google Scholar] [CrossRef]
- Ferrari, A.; Baumann, M.; Coenen, C.; Frank, D.; Hennen, L.; Moniz, A.; Torgersen, H.; Torgersen, J.; van Bodegom, L.; van Duijne, F.; et al. Additive Bio-Manufacturing: 3D Printing for Medical Recovery and Human Enhancement; European Parliament: Strasbourg, France, 2018.
- Felfel, R.M.; Poocza, L.; Gimeno-Fabra, M.; Milde, T.; Hildebrand, G.; Ahmed, I.; Scotchford, C.; Sottile, V.; Grant, D.M.; Liefeith, K. In vitro degradation and mechanical properties of PLA-PCL copolymer unit cell scaffolds generated by two-photon polymerization. Biomed. Mater. 2016, 11, 015011. [Google Scholar] [CrossRef]
- Chia, H.N.; Wu, B.M. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015, 9, 4. [Google Scholar] [CrossRef]
- Raeisdasteh Hokmabad, V.; Davaran, S.; Ramazani, A.; Salehi, R. Design and fabrication of porous biodegradable scaffolds: A strategy for tissue engineering. J. Biomater. Sci. Polym. Ed. 2017, 28, 1797–1825. [Google Scholar] [CrossRef] [PubMed]
- Armentano, I.; Bitinis, N.; Fortunati, E.; Mattioli, S.; Rescignano, N.; Verdejo, R.; Lopez-Manchado, M.; Kenny, J. Multifunctional nanostructured PLA materials for packaging and tissue engineering. Prog. Polym. Sci. 2013, 38, 1720–1747. [Google Scholar] [CrossRef]
- Lasprilla, A.J.R.; Martinez, G.A.R.; Lunelli, B.H.; Jardini, A.L.; Filho, R.M. Poly-lactic acid synthesis for application in biomedical devices—A review. Biotechnol. Adv. 2012, 30, 321–328. [Google Scholar] [CrossRef]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef]
- Gholipourmalekabadi, M.; Zhao, S.; Harrison, B.S.; Mozafari, M.; Seifalian, A.M. Oxygen-Generating Biomaterials: A New, Viable Paradigm for Tissue Engineering? Trends Biotechnol. 2016, 34, 1010–1021. [Google Scholar] [CrossRef]
- Oh, S.H.; Ward, C.L.; Atala, A.; Yoo, J.J.; Harrison, B.S. Oxygen generating scaffolds for enhancing engineered tissue survival. Biomaterials 2009, 30, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Camci-Unal, G.; Alemdar, N.; Annabi, N.; Khademhosseini, A. Oxygen Releasing Biomaterials for Tissue Engineering. Polym. Int. 2013, 62, 843–848. (In English) [Google Scholar] [CrossRef]
- Pedraza, E.; Coronel, M.M.; Fraker, C.A.; Ricordi, C.; Stabler, C.L. Preventing hypoxia-induced cell death in beta cells and islets via hydrolytically activated, oxygen-generating biomaterials. Proc. Natl. Acad. Sci. USA 2012, 109, 4245–4250. (In English) [Google Scholar] [CrossRef]
- Cassidy, D.P.; Irvine, R.L. Use of calcium peroxide to provide oxygen for contaminant biodegradation in a saturated soil. J. Hazard. Mater. 1999, 69, 25–39. [Google Scholar] [CrossRef]
- Waite, A.J.; Bonner, J.S.; Autenrieth, R. Kinetics and Stoichiometry of Oxygen Release from Solid Peroxides. Environ. Eng. Sci. 1999, 16, 187–199. [Google Scholar] [CrossRef]
- Mohammed, A.H.; Kovacev, N.; Elshaer, A.; Melaibari, A.A.; Iqbal, J.; Hassanin, H.; Memić, A. Preparation of Polylactic Acid/Calcium Peroxide Composite Filaments for Fused Deposition Modelling. Polymers 2023, 15, 2229. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.; Saeed, A.; Elshaer, A.; Melaibari, A.A.; Memić, A.; Hassanin, H.; Essa, K. Fabrication and Characterization of Oxygen-Generating Polylactic Acid/Calcium Peroxide Composite Filaments for Bone Scaffolds. Pharmaceuticals 2023, 16, 627. [Google Scholar] [CrossRef]
- Mota, C.; Puppi, D.; Chiellini, F.; Chiellini, E. Additive manufacturing techniques for the production of tissue engineering constructs. J. Tissue Eng. Regen. Med. 2015, 9, 174–190. [Google Scholar] [CrossRef]
- Li, C.; Zhang, J.; Han, J.; Yao, B. A numerical solution to the effects of surface roughness on water–coal contact angle. Sci. Rep. 2021, 11, 459. [Google Scholar] [CrossRef]
- Yu, W.; Shi, J.; Sun, L.; Lei, W. Effects of Printing Parameters on Properties of FDM 3D Printed Residue of Astragalus/Polylactic Acid Biomass Composites. Molecules 2022, 27, 7373. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kwon, S.H.; Chin, I.-J.; Choi, H.J. Toughness and rheological characteristics of poly(lactic acid)/acrylic core–shell rubber blends. Polym. Bull. 2019, 76, 5483–5497. [Google Scholar] [CrossRef]
- Breeland, G.; Sinkler, M.A.; Menezes, R.G. Embryology, bone ossification. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Feng, X. Chemical and Biochemical Basis of Cell-Bone Matrix Interaction in Health and Disease. Curr. Chem. Biol. 2009, 3, 189–196. (In English) [Google Scholar] [CrossRef] [PubMed]
- Boraschi-Diaz, I.; Wang, J.; Mort, J.S.; Komarova, S.V. Collagen Type I as a Ligand for Receptor-Mediated Signaling. Front. Phys. 2017, 5, 12. (In English) [Google Scholar] [CrossRef]
- Komori, T. Regulation of osteoblast differentiation by Runx2. Adv. Exp. Med. Biol. 2010, 658, 43–49. (In English) [Google Scholar] [CrossRef]
- Choi, J.U.A.; Kijas, A.W.; Lauko, J.; Rowan, A.E. The Mechanosensory Role of Osteocytes and Implications for Bone Health and Disease States. Front. Cell Dev. Biol. 2022, 9, 770143. (In English) [Google Scholar] [CrossRef]
- Liu, Q.; Li, M.; Wang, S.; Xiao, Z.; Xiong, Y.; Wang, G. Recent Advances of Osterix Transcription Factor in Osteoblast Differentiation and Bone Formation. Front. Cell Dev. Biol. 2020, 8, 601224. (In English) [Google Scholar] [CrossRef] [PubMed]
- De Luca, A.; Vitrano, I.; Costa, V.; Raimondi, L.; Carina, V.; Bellavia, D.; Conoscenti, G.; Di Falco, R.; Pavia, F.C.; La Carrubba, V.; et al. Improvement of osteogenic differentiation of human mesenchymal stem cells on composite poly l-lactic acid/nano-hydroxyapatite scaffolds for bone defect repair. J. Biosci. Bioeng. 2019, 129, 250–257. [Google Scholar] [CrossRef] [PubMed]
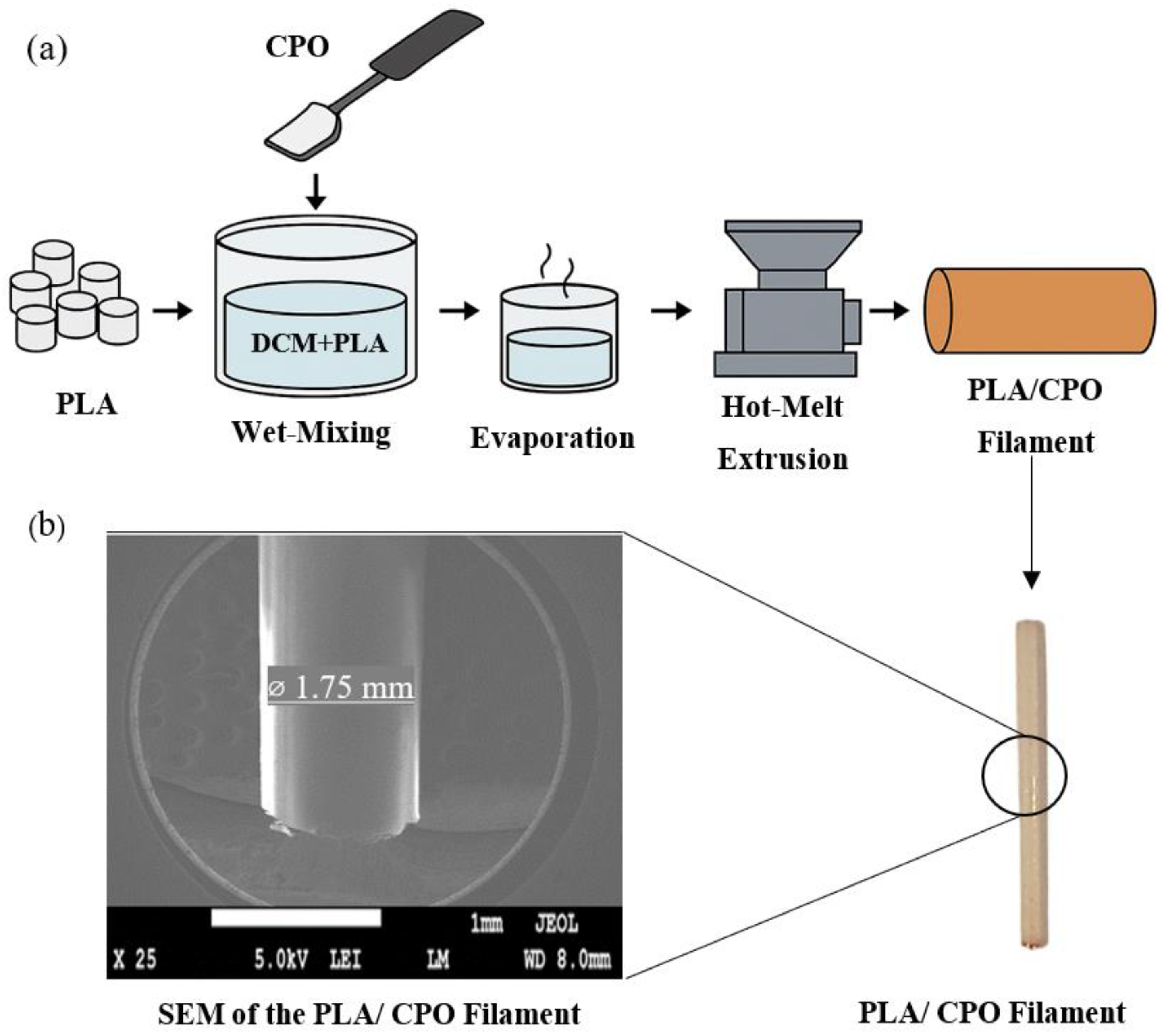
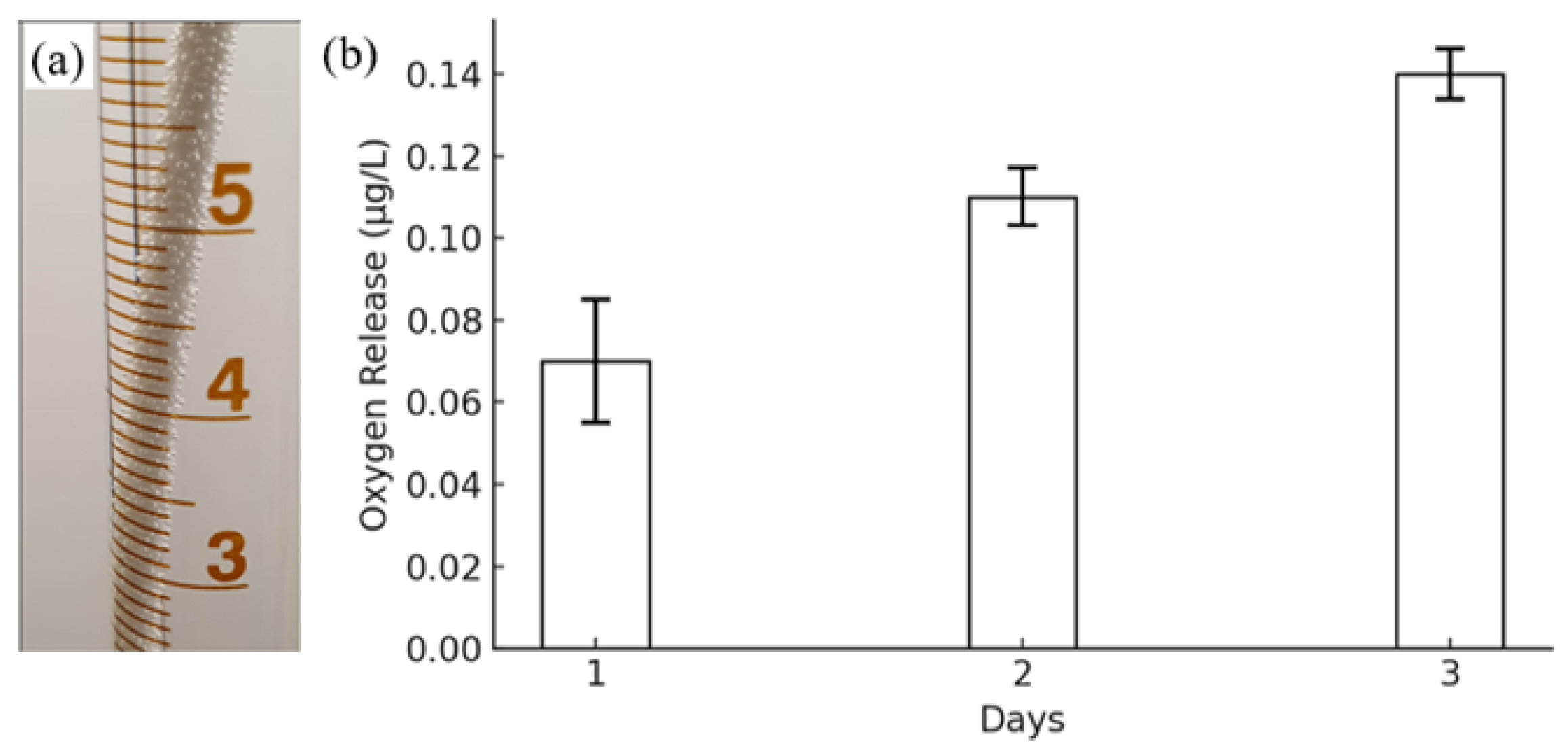
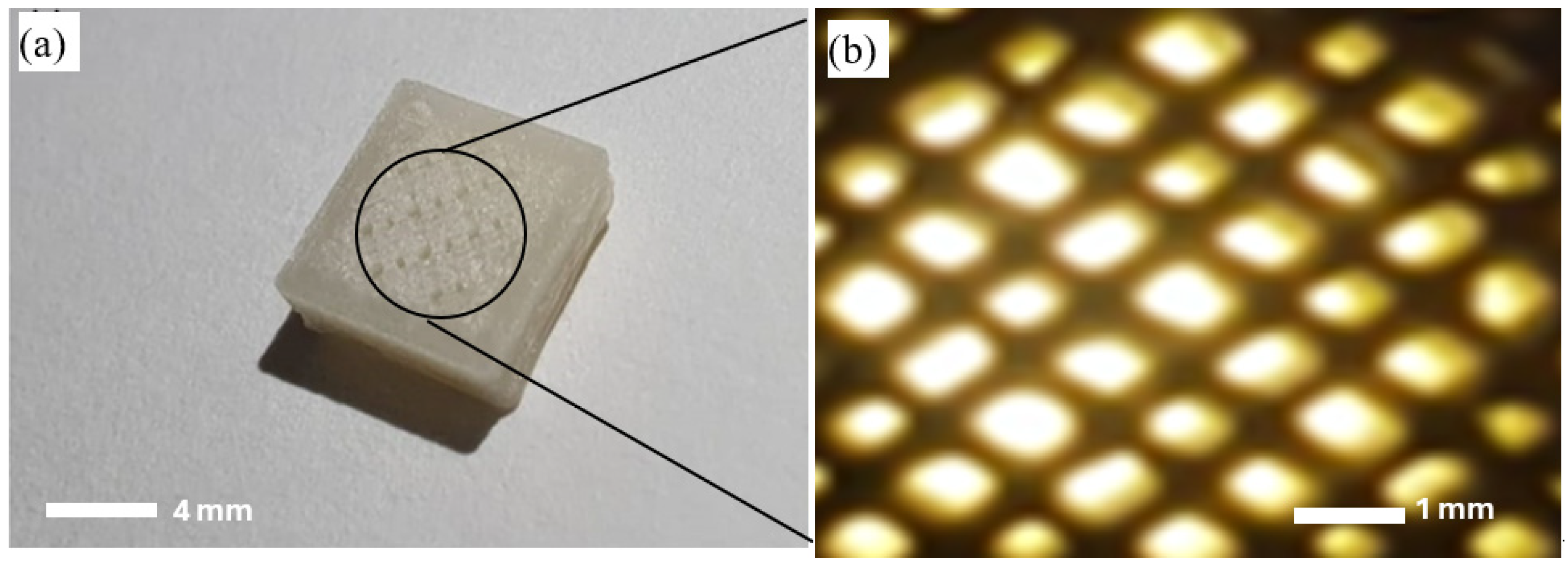
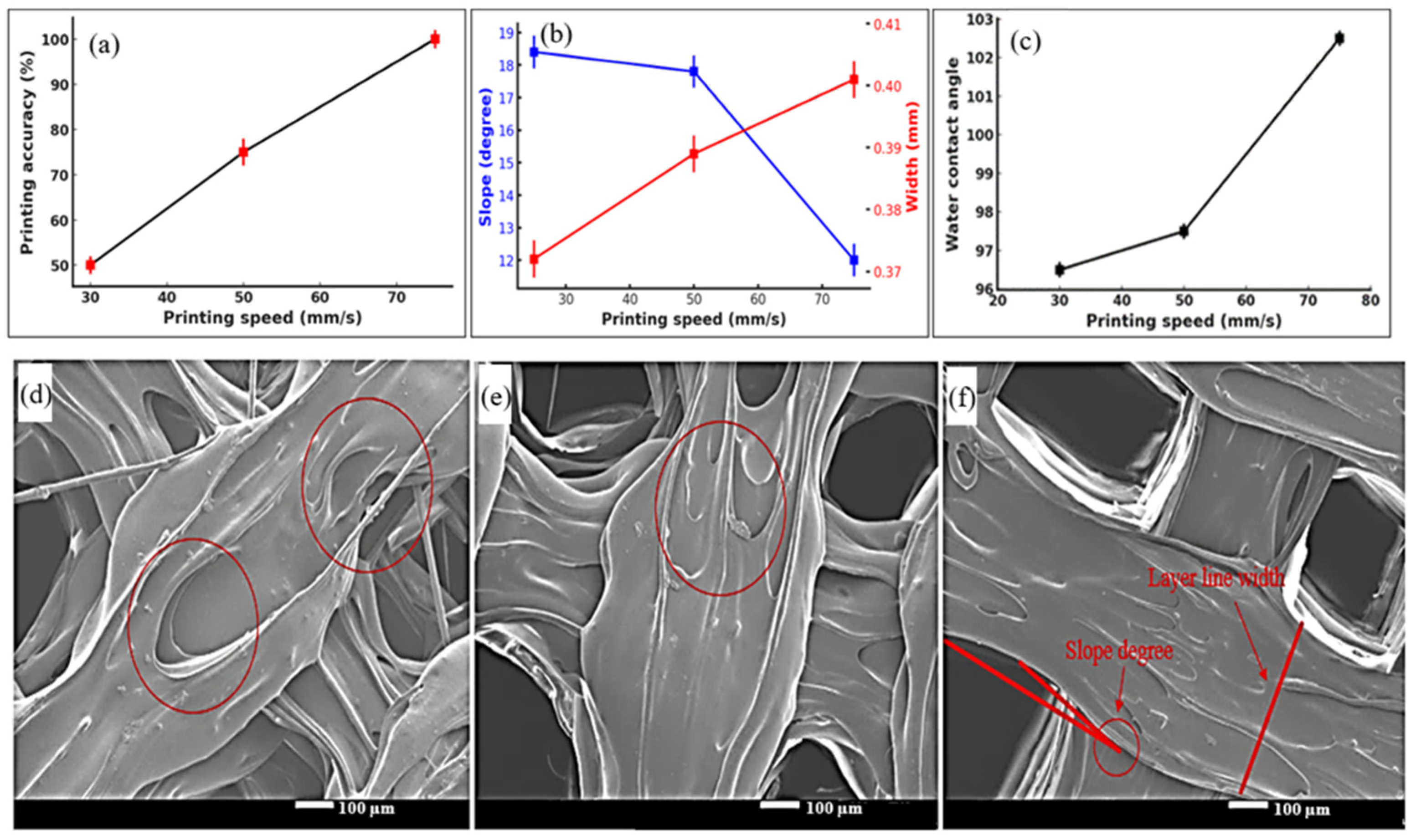
| Sample No. | Printing Temperature (°C) | Building Platform Temperature (°C) | Printing Speed (mm/s) |
|---|---|---|---|
| 1 | 200 | 70 | 25 |
| 2 | 200 | 70 | 50 |
| 3 | 200 | 70 | 75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammed, A.; Tirnoveanu, A.; Webb, W.R.; Melaibari, A.A.; Memić, A.; Aslam, M.; Elshaer, A.; Hassanin, H.; Essa, K. In Vitro Characterization of 3D-Printed PLA/CPO Oxygen Releasing Scaffolds: Mechanical and Biological Properties for Bone Tissue Engineering. J. Manuf. Mater. Process. 2025, 9, 149. https://doi.org/10.3390/jmmp9050149
Mohammed A, Tirnoveanu A, Webb WR, Melaibari AA, Memić A, Aslam M, Elshaer A, Hassanin H, Essa K. In Vitro Characterization of 3D-Printed PLA/CPO Oxygen Releasing Scaffolds: Mechanical and Biological Properties for Bone Tissue Engineering. Journal of Manufacturing and Materials Processing. 2025; 9(5):149. https://doi.org/10.3390/jmmp9050149
Chicago/Turabian StyleMohammed, Abdullah, Alice Tirnoveanu, William Richard Webb, Ammar A. Melaibari, Adnan Memić, Mohammad Aslam, Amr Elshaer, Hany Hassanin, and Khamis Essa. 2025. "In Vitro Characterization of 3D-Printed PLA/CPO Oxygen Releasing Scaffolds: Mechanical and Biological Properties for Bone Tissue Engineering" Journal of Manufacturing and Materials Processing 9, no. 5: 149. https://doi.org/10.3390/jmmp9050149
APA StyleMohammed, A., Tirnoveanu, A., Webb, W. R., Melaibari, A. A., Memić, A., Aslam, M., Elshaer, A., Hassanin, H., & Essa, K. (2025). In Vitro Characterization of 3D-Printed PLA/CPO Oxygen Releasing Scaffolds: Mechanical and Biological Properties for Bone Tissue Engineering. Journal of Manufacturing and Materials Processing, 9(5), 149. https://doi.org/10.3390/jmmp9050149











