Ag/Au Bimetallic Core–Shell Nanostructures: A Review of Synthesis and Applications
Abstract
:1. Introduction
2. Ag/Au Bimetallic Core–Shell Nanostructures with Diverse Morphologies and Applications
2.1. Nanospheres
2.1.1. Synthesis Methods of Nanospheres
2.1.2. Applications of Nanospheres
2.2. Nanocubes
2.2.1. Synthesis Methods of Nanocubes
2.2.2. Applications of Nanocubes
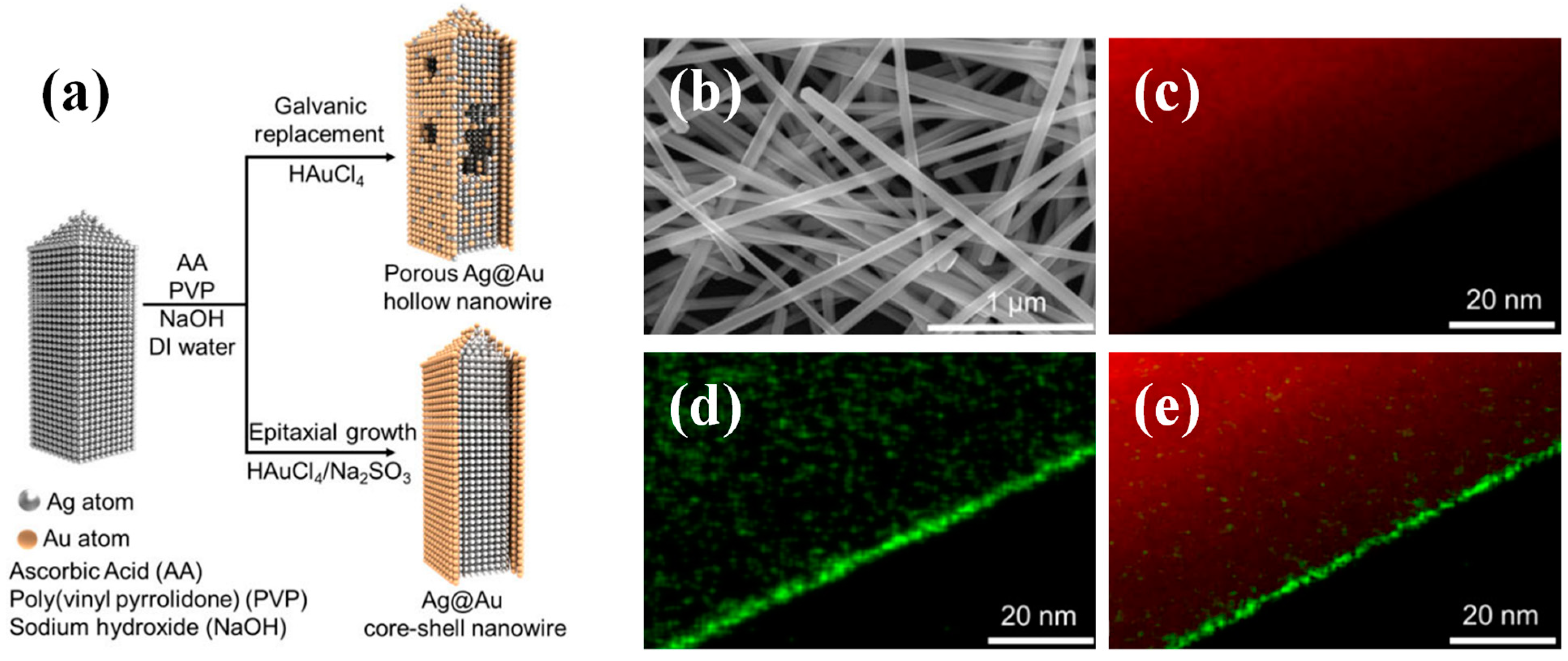
2.3. Nanowires
2.3.1. Synthesis Methods of Nanowires
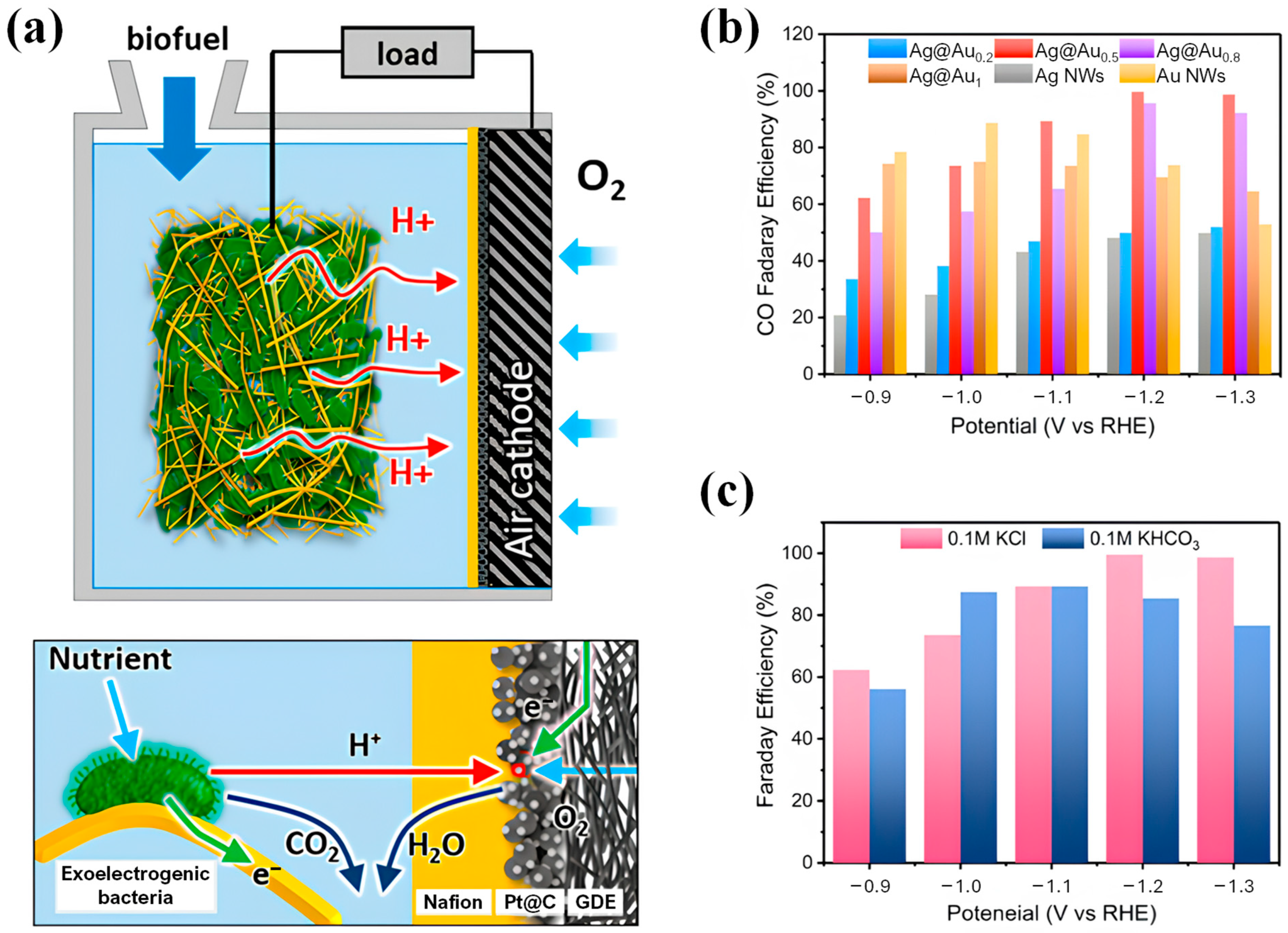
2.3.2. Applications of Nanowires
3. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Liu, K.; Chen, T.; He, S.; Robbins, J.P.; Podkolzin, S.G.; Tian, F. Observation and Identification of an Atomic Oxygen Structure on Catalytic Gold Nanoparticles. Angew. Chem. Int. Ed. 2017, 56, 12952–12957. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; He, S.; Li, L.; Liu, Y.; Huang, Z.; Liu, T.; Wu, H.; Jiang, X.; Liu, K.; Tian, F. Spectroscopically clean Au nanoparticles for catalytic decomposition of hydrogen peroxide. Sci. Rep. 2021, 11, 9709. [Google Scholar] [CrossRef] [PubMed]
- Priecel, P.; Salami, H.A.; Padilla, R.H.; Zhong, Z.; Lopez-Sanchez, J.A. Anisotropic gold nanoparticles: Preparation and applications in catalysis. Chin. J. Catal. 2016, 37, 1619–1650. [Google Scholar] [CrossRef]
- Zheng, Y.; Qi, Y.; Tang, Z.; Tan, J.; Koel, B.E.; Podkolzin, S.G. Spectroscopic observation and structure-insensitivity of hydroxyls on gold. Chem. Commun. 2022, 58, 4036–4039. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Yang, Y.; Wang, H.; Du, H. Synergistic Integration of Layer-by-Layer Assembly of Photosensitizer and Gold Nanorings for Enhanced Photodynamic Therapy in the Near Infrared. ACS Nano 2015, 9, 8744–8754. [Google Scholar] [CrossRef]
- Liu, K.; Wuenschell, J.; Bera, S.; Tang, R.; Ohodnicki, P.R.; Du, H. Nanostructured sapphire optical fiber embedded with Au nanorods for high-temperature plasmonics in harsh environments. Opt. Express 2019, 27, 38125–38133. [Google Scholar] [CrossRef]
- Liu, K.; Ohodnicki, P.R.; Kong, X.; Lee, S.S.; Du, H. Plasmonic Au nanorods stabilized within anodic aluminum oxide pore channels against high-temperature treatment. Nanotechnology 2019, 30, 405704. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Sanchez, J.A.; Dimitratos, N.; Hammond, C.; Brett, G.L.; Kesavan, L.; White, S.; Miedziak, P.; Tiruvalam, R.; Jenkins, R.L.; Carley, A.F.; et al. Facile removal of stabilizer-ligands from supported gold nanoparticles. Nat. Chem. 2011, 3, 551–556. [Google Scholar] [CrossRef]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold nanoparticles in chemical and biological sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef]
- He, S.; Wang, J.; Yang, F.; Chang, T.L.; Tang, Z.; Liu, K.; Liu, S.; Tian, F.; Liang, J.F.; Du, H.; et al. Bacterial Detection and Differentiation of Staphylococcus aureus and Escherichia coli Utilizing Long-Period Fiber Gratings Functionalized with Nanoporous Coated Structures. Coatings 2023, 13, 778. [Google Scholar] [CrossRef]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver Nanoparticles as Potential Antibacterial Agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef]
- Stiufiuc, R.; Iacovita, C.; Lucaciu, C.M.; Stiufiuc, G.; Dutu, A.G.; Braescu, C.; Leopold, N. SERS-active silver colloids prepared by reduction of silver nitrate with short-chain polyethylene glycol. Nanoscale Res. Lett. 2013, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kumar, S.; Huo, T.; Du, H.; Huang, Y.-P. Photon counting Raman spectroscopy: A benchmarking study vs surface plasmon enhancement. Opt. Express 2024, 32, 16657. [Google Scholar] [CrossRef] [PubMed]
- Pinkhasova, P.; Chen, H.; Verhoeven, M.W.G.M.; Sukhishvili, S.; Du, H. Thermally annealed Ag nanoparticles on anodized aluminium oxide for SERS sensing. RSC Adv. 2013, 3, 17954. [Google Scholar] [CrossRef]
- Kumar, S.; Li, Y.; Huo, T.; Du, H.; Huang, Y. Raman Spectroscopy with Single Photon Counting. In Proceedings of the Frontiers in Optics + Laser Science 2023 (FiO, LS), Tacoma, DC, USA, 9–12 October 2023; p. JM7A.120. [Google Scholar]
- Tang, Z.; Chen, T.; Liu, K.; Du, H.; Podkolzin, S.G. Atomic, Molecular and Hybrid Oxygen Structures on Silver. Langmuir 2021, 37, 11603–11610. [Google Scholar] [CrossRef]
- Scoullos, E.V.; Hofman, M.S.; Zheng, Y.; Potapenko, D.V.; Tang, Z.; Podkolzin, S.G.; Koel, B.E. Guaiacol Adsorption and Decomposition on Platinum. J. Phys. Chem. C 2018, 122, 29180–29189. [Google Scholar] [CrossRef]
- Evtugyn, G.A.; Shamagsumova, R.V.; Padnya, P.V.; Stoikov, I.I.; Antipin, I.S. Cholinesterase sensor based on glassy carbon electrode modified with Ag nanoparticles decorated with macrocyclic ligands. Talanta 2014, 127, 9–17. [Google Scholar] [CrossRef]
- Gao, S.; He, S.; Zang, P.; Dang, L.; Shi, F.; Xu, H.; Liu, Z.; Lei, Z. Polyaniline Nanorods Grown on Hollow Carbon Fibers as High-Performance Supercapacitor Electrodes. ChemElectroChem 2016, 3, 1142–1149. [Google Scholar] [CrossRef]
- Robbins, J.P.; Ezeonu, L.; Tang, Z.; Yang, X.; Koel, B.E.; Podkolzin, S.G. Propane Dehydrogenation to Propylene and Propylene Adsorption on Ni and Ni-Sn Catalysts. ChemCatChem 2022, 14, e202101546. [Google Scholar] [CrossRef]
- Ezeonu, L.; Tang, Z.; Qi, Y.; Huo, F.; Zheng, Y.; Koel, B.E.; Podkolzin, S.G. Adsorption, surface reactions and hydrodeoxygenation of acetic acid on platinum and nickel catalysts. J. Catal. 2023, 418, 190–202. [Google Scholar] [CrossRef]
- Thanh, N.T.K.; Green, L.A.W. Functionalisation of nanoparticles for biomedical applications. Nano Today 2010, 5, 213–230. [Google Scholar] [CrossRef]
- Redina, E.A.; Kirichenko, O.A. Mono- and Bimetallic Nanoparticles in Catalysis. Catalysts 2024, 14, 68. [Google Scholar] [CrossRef]
- Zheng, Y.; Tang, Z.; Podkolzin, S.G. Catalytic Platinum Nanoparticles Decorated with Subnanometer Molybdenum Clusters for Biomass Processing. Chem. A Eur. J. 2020, 26, 5174–5179. [Google Scholar] [CrossRef] [PubMed]
- Blosi, M.; Ortelli, S.; Costa, A.L.; Dondi, M.; Lolli, A.; Andreoli, S.; Benito, P.; Albonetti, S. Bimetallic nanoparticles as efficient catalysts: Facile and green microwave synthesis. Materials 2016, 9, 550. [Google Scholar] [CrossRef]
- Song, D.; Li, Y.; Lu, X.; Sun, M.; Liu, H.; Yu, G.; Gao, F. Palladium-copper nanowires-based biosensor for the ultrasensitive detection of organophosphate pesticides. Anal. Chim. Acta 2017, 982, 168–175. [Google Scholar] [CrossRef]
- Larrañaga-Tapia, M.; Betancourt-Tovar, B.; Videa, M.; Antunes-Ricardo, M.; Cholula-Díaz, J.L. Green synthesis trends and potential applications of bimetallic nanoparticles towards the sustainable development goals 2030. Nanoscale Adv. 2023, 6, 51–71. [Google Scholar] [CrossRef]
- Liu, X.; Liang, X.; Yu, J.; Xu, K.; Shen, J.W.; Duan, W.; Zeng, J. Recent development of noble metal-based bimetallic nanoparticles for colorimetric sensing. TrAC Trends Anal. Chem. 2023, 169, 117386. [Google Scholar] [CrossRef]
- Dobrynin, D.; Zlotver, I.; Polishchuk, I.; Kauffmann, Y.; Suharenko, S.; Koifman, R.; Kuhrts, L.; Katsman, A.; Sosnik, A.; Pokroy, B. Controlled Synthesis of Bimetallic Gold-Silver Nanostars: Atomic Insights and Predictive Formation Model. Small 2025, 2410152. [Google Scholar] [CrossRef]
- Zheng, Y.; Qi, Y.; Tang, Z.; Hanke, F.; Podkolzin, S.G. Kinetics and Reaction Mechanisms of Acetic Acid Hydrodeoxygenation over Pt and Pt-Mo Catalysts. ACS Sustain. Chem. Eng. 2022, 10, 5212–5224. [Google Scholar] [CrossRef]
- Wang, C.; Shi, Y.; Qin, D.; Xia, Y. Bimetallic Core−Shell Nanocrystals: Opportunities and Challenges. Nanoscale Horiz. 2023, 8, 1194–1204. [Google Scholar] [CrossRef]
- He, S.; Wu, D.; Chen, S.; Liu, K.; Yang, E.-H.; Tian, F.; Du, H. Au-on-Ag nanostructure for in-situ SERS monitoring of catalytic reactions. Nanotechnology 2022, 33, 155701. [Google Scholar] [CrossRef]
- He, S.; Tang, Z. Fabrication and Control of Porous Structures Via Layer-By-Layer Assembly on PAH/PAA Polyelectrolyte Coatings. Biomed. J. Sci. Tech. Res. 2023, 51, 43118–43121. [Google Scholar] [CrossRef]
- Choi, J.; Min, J.K.; Kim, D.; Kim, J.; Kim, J.; Yoon, H.; Lee, J.; Jeong, Y.; Kim, C.Y.; Ko, S.H. Hierarchical 3D Percolation Network of Ag-Au Core-Shell Nanowire-Hydrogel Composite for Efficient Biohybride Electrodes. ACS Nano 2023, 17, 17966–17978. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; McLellan, J.M.; Siekkinen, A.; Xiong, Y.; Li, Z.Y.; Xia, Y. Facile synthesis of gold-silver nanocages with controllable pores on the surface. J. Am. Chem. Soc. 2006, 128, 14776–14777. [Google Scholar] [CrossRef]
- Da Silva, A.G.M.; Rodrigues, T.S.; Haigh, S.J.; Camargo, P.H.C. Galvanic replacement reaction: Recent developments for engineering metal nanostructures towards catalytic applications. Chem. Commun. 2017, 53, 7135–7148. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, J.; Fu, Z.W.; Qin, D. Galvanic replacement-free deposition of au on ag for core-shell nanocubes with enhanced chemical stability and SERS activity. J. Am. Chem. Soc. 2014, 136, 8153–8156. [Google Scholar] [CrossRef]
- Hou, P.; Liu, H.; Li, J.; Yang, J. One-pot synthesis of noble metal nanoparticles with a core-shell construction. CrystEngComm 2015, 17, 1826–1832. [Google Scholar] [CrossRef]
- Sukma, F.O.R.; Hanif, M.A.; Masruroh; Santjojo, D.J.D.H.; Apsari, R.; Susanto, H.; Tazi, I. Effects of thickness and roughness on plasmonic characteristics of gold thin films deposited on polished optical fiber. Mater. Res. Express 2024, 11, 016201. [Google Scholar] [CrossRef]
- Wenxin, N.; Ling, Z.; Guobao, X. Seed-mediated growth method for high-quality noble metal nanocrystals. Sci. China Chem. 2012, 55, 2311–2317. [Google Scholar] [CrossRef]
- Chen, J.; Wiley, B.; Li, Z.Y.; Campbell, D.; Saeki, F.; Cang, H.; Au, L.; Lee, J.; Li, X.; Xia, Y. Gold nanocages: Engineering their structure for biomedical applications. Adv. Mater. 2005, 17, 2255–2261. [Google Scholar] [CrossRef]
- Xia, Y.; Xiong, Y.; Lim, B.; Skrabalak, S.E. Shape-controlled synthesis of metal nanocrystals: Simple chemistry meets complex physics? Angew. Chem. Int. Ed. 2009, 48, 60–103. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yang, Y.; Luo, Y.; Yang, X.; Li, M.; Song, Q. Double Detection of Mycotoxins Based on SERS Labels Embedded Ag@Au Core-Shell Nanoparticles. ACS Appl. Mater. Interfaces 2015, 7, 21780–21786. [Google Scholar] [CrossRef]
- Zhang, H.; Okuni, J.; Toshima, N. One-pot synthesis of Ag-Au bimetallic nanoparticles with Au shell and their high catalytic activity for aerobic glucose oxidation. J. Colloid Interface Sci. 2011, 354, 131–138. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Q.; Zhou, X.; Wen, C.Y.; Yu, J.; Han, X.; Li, X.; Yan, Z.F.; Zeng, J. A convenient colorimetric method for sensitive and specific detection of cyanide using Ag@Au core–shell nanoparticles. Sens. Actuators B Chem. 2016, 228, 366–372. [Google Scholar] [CrossRef]
- Omar, G.; Abd Ellah, R.G.; Elzayat, M.M.Y.; Afifi, G.; Imam, H. Superior removal of hazardous dye using Ag/Au core–shell nanoparticles prepared by laser ablation. Opt. Laser Technol. 2024, 168, 109868. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Y.; Wang, Y.; Li, X.; Qi, K.; Wang, J.; Xu, H. Black Silver Nanocubes@Amino Acid-Encoded Highly Branched Gold Shells with Efficient Photothermal Conversion for Tumor Therapy. ACS Appl. Mater. Interfaces 2023, 15, 236–248. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Jiang, H.; Jiang, H.; Zhou, X.; Li, Y.; Li, C. Ag@Au Core-Shell Nanowires for Nearly 100% CO2-to-CO Electroreduction. Chem. Asian J. 2020, 15, 425–431. [Google Scholar] [CrossRef]
- Xia, X.; Wang, Y.; Ruditskiy, A.; Xia, Y. 25th Anniversary Article: Galvanic Replacement: A Simple and Versatile Route to Hollow Nanostructures with Tunable and Well-Controlled Properties. Adv. Mater. 2013, 25, 6313–6333. [Google Scholar] [CrossRef]
- Lee, P.C.; Meisel, D. Adsorption and surface-enhanced Raman of dyes on silver and gold sols. J. Phys. Chem. 1982, 86, 3391–3395. [Google Scholar] [CrossRef]
- Luty-Błocho, M.; Pacławski, K.; Wojnicki, M.; Fitzner, K. The kinetics of redox reaction of gold(III) chloride complex ions with l-ascorbic acid. Inorganica Chim. Acta 2013, 395, 189–196. [Google Scholar] [CrossRef]
- Personick, M.L.; Mirkin, C.A. Making sense of the mayhem behind shape control in the synthesis of gold nanoparticles. J. Am. Chem. Soc. 2013, 135, 18238–18247. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Kim, J.; Gilroy, K.D.; Liu, J.; König, T.A.F.; Qin, D. Gold-Based Cubic Nanoboxes with Well-Defined Openings at the Corners and Ultrathin Walls Less Than Two Nanometers Thick. ACS Nano 2016, 10, 8019–8025. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Ahn, J.; Wang, X.; Qin, D. Defect-Assisted Deposition of Au on Ag for the Fabrication of Core-Shell Nanocubes with Outstanding Chemical and Thermal Stability. Chem. Mater. 2019, 31, 1057–1065. [Google Scholar] [CrossRef]
- Ren, W.; Yan, Y.; Zeng, L.; Shi, Z.; Gong, A.; Schaaf, P.; Wang, D.; Zhao, J.; Zou, B.; Yu, H.; et al. A Near Infrared Light Triggered Hydrogenated Black TiO2 for Cancer Photothermal Therapy. Adv. Healthc. Mater. 2015, 4, 1526–1536. [Google Scholar] [CrossRef]
- Wang, X.; Sun, X.; Bu, T.; Wang, Q.; Jia, P.; Dong, M.; Wang, L. In situ fabrication of metal-organic framework derived hybrid nanozymes for enhanced nanozyme-photothermal therapy of bacteria-infected wounds. Compos. Part B Eng. 2022, 229, 109465. [Google Scholar] [CrossRef]
- Araki, T.; Yoshida, F.; Uemura, T.; Noda, Y.; Yoshimoto, S.; Kaiju, T.; Suzuki, T.; Hamanaka, H.; Baba, K.; Hayakawa, H.; et al. Long-Term Implantable, Flexible, and Transparent Neural Interface Based on Ag/Au Core–Shell Nanowires. Adv. Healthc. Mater. 2019, 8, 1900130. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Kim, S.; Ma, X.; Byrley, P.; Yu, N.; Liu, Q.; Sun, X.; Xu, D.; Peng, S.; Hartel, M.C.; et al. Ultrathin-shell epitaxial Ag@Au core-shell nanowires for high-performance and chemically-stable electronic, optical, and mechanical devices. Nano Res. 2021, 14, 4294–4303. [Google Scholar] [CrossRef]
- Zhu, D.D.; Liu, J.L.; Qiao, S.Z. Recent Advances in Inorganic Heterogeneous Electrocatalysts for Reduction of Carbon Dioxide. Adv. Mater. 2016, 28, 3423–3452. [Google Scholar] [CrossRef]
- Dey, G.R.; Young, H.L.; Teklu, S.; Soliman, S.S.; Schaak, R.E. Influence of Nanoparticle Seeds on the Formation and Growth of High Entropy Alloys during Core@Shell Nanoparticle Synthesis. ACS Nano 2025, 19, 8826–8841. [Google Scholar] [CrossRef]
- Kar, N.; McCoy, M.; Wolfe, J.; Bueno, S.L.A.; Shafei, I.H.; Skrabalak, S.E. Retrosynthetic design of core–shell nanoparticles for thermal conversion to monodisperse high-entropy alloy nanoparticles. Nat. Synth. 2024, 3, 175–184. [Google Scholar] [CrossRef]

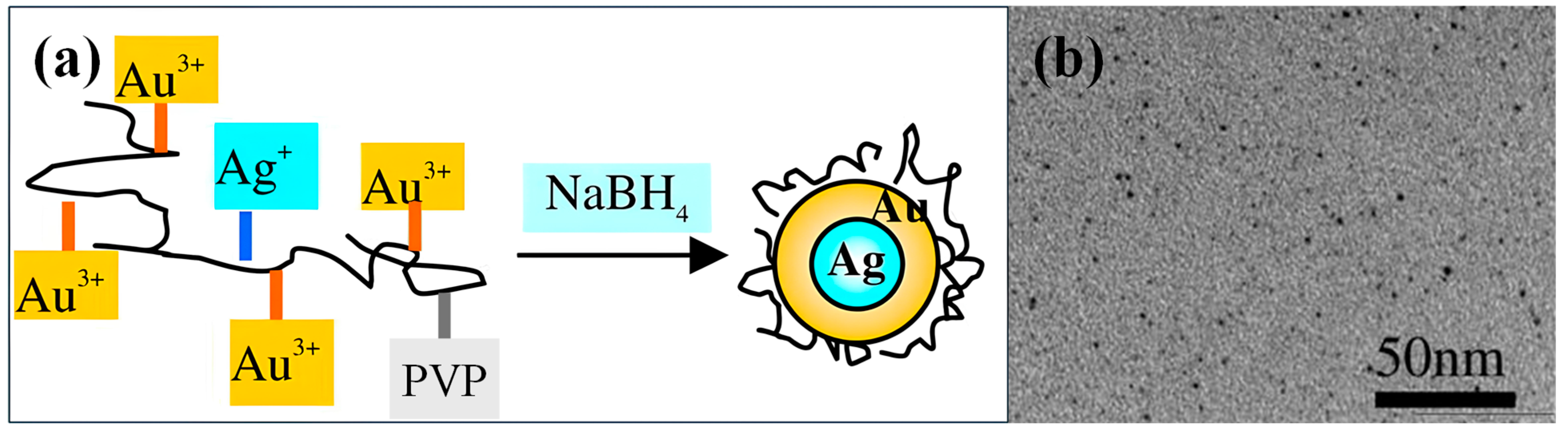

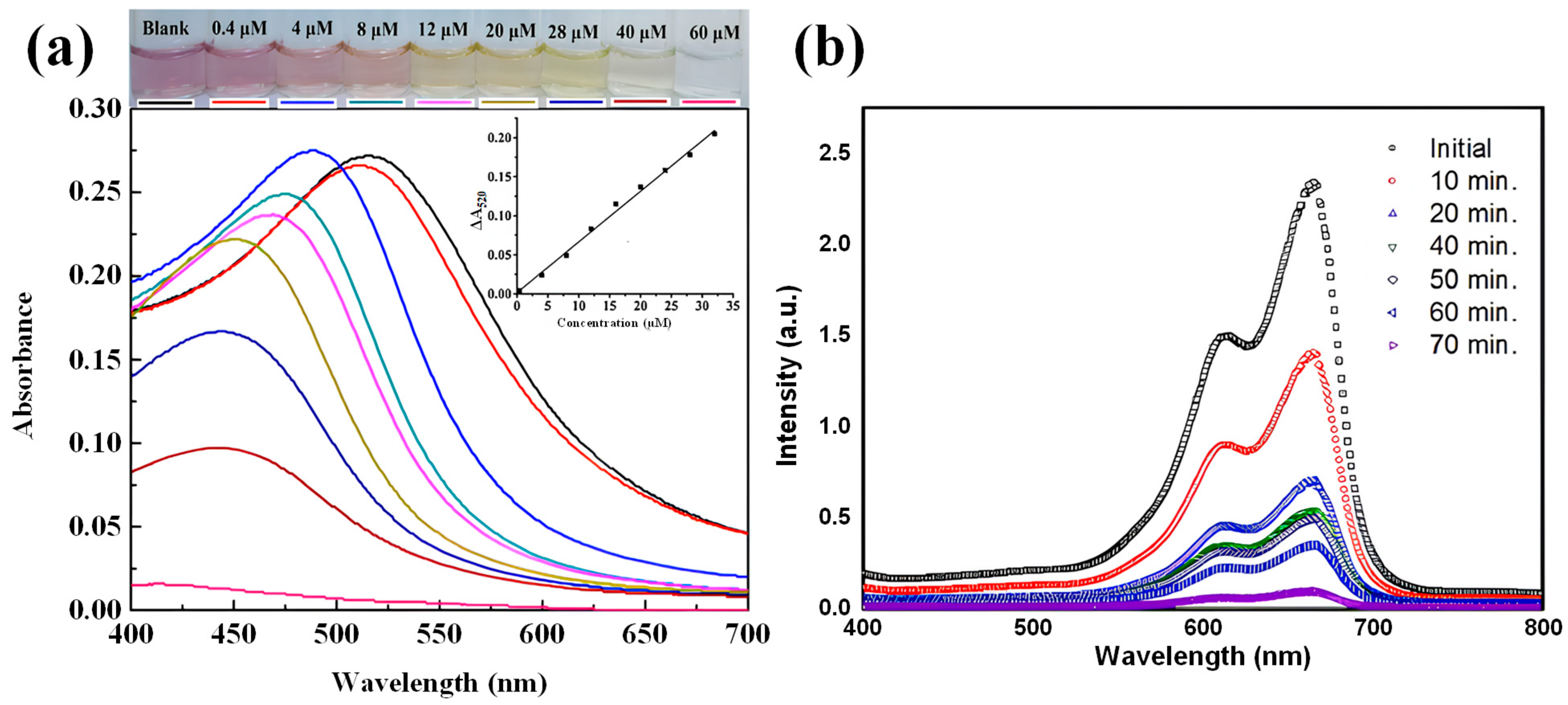
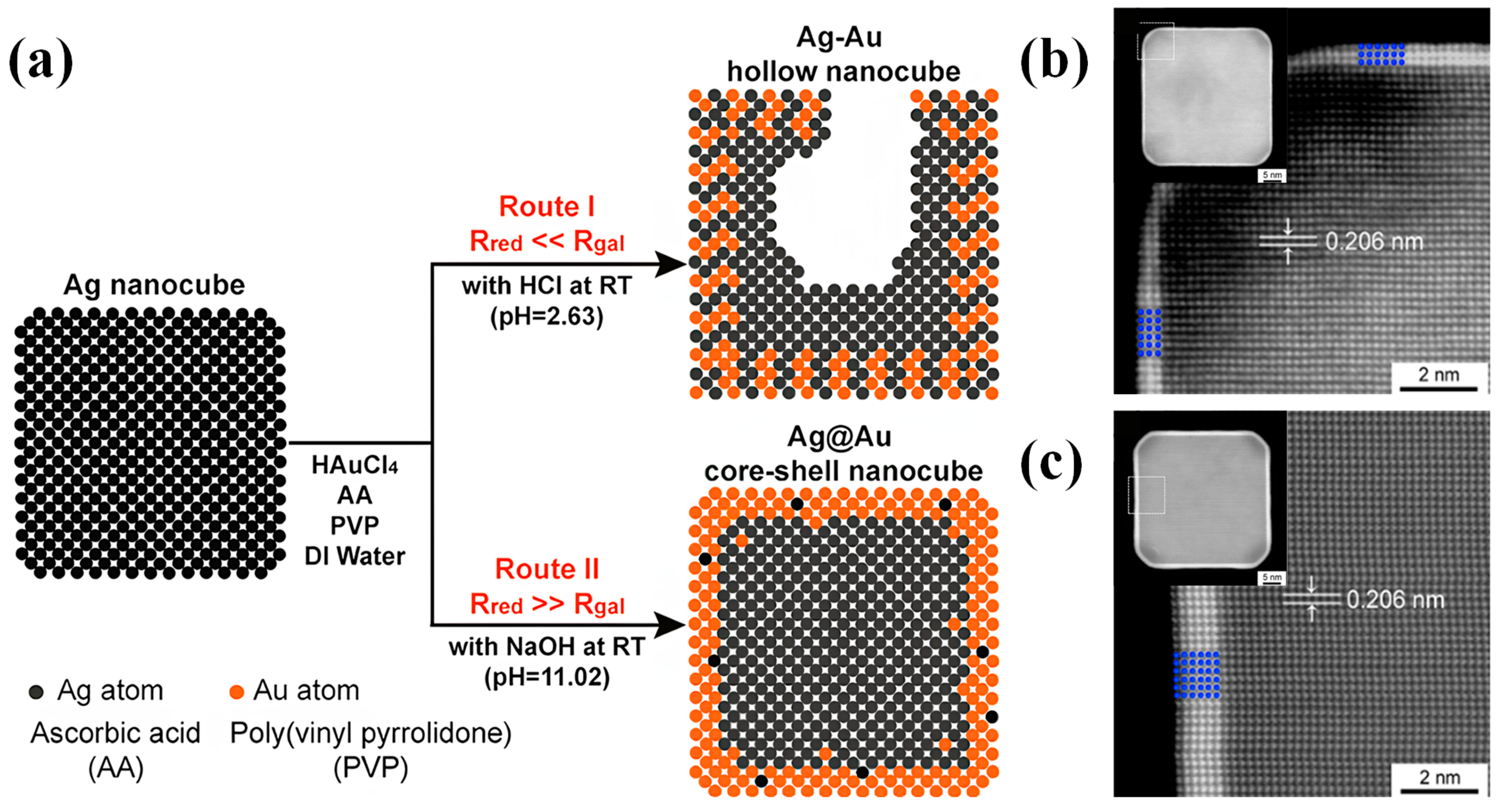


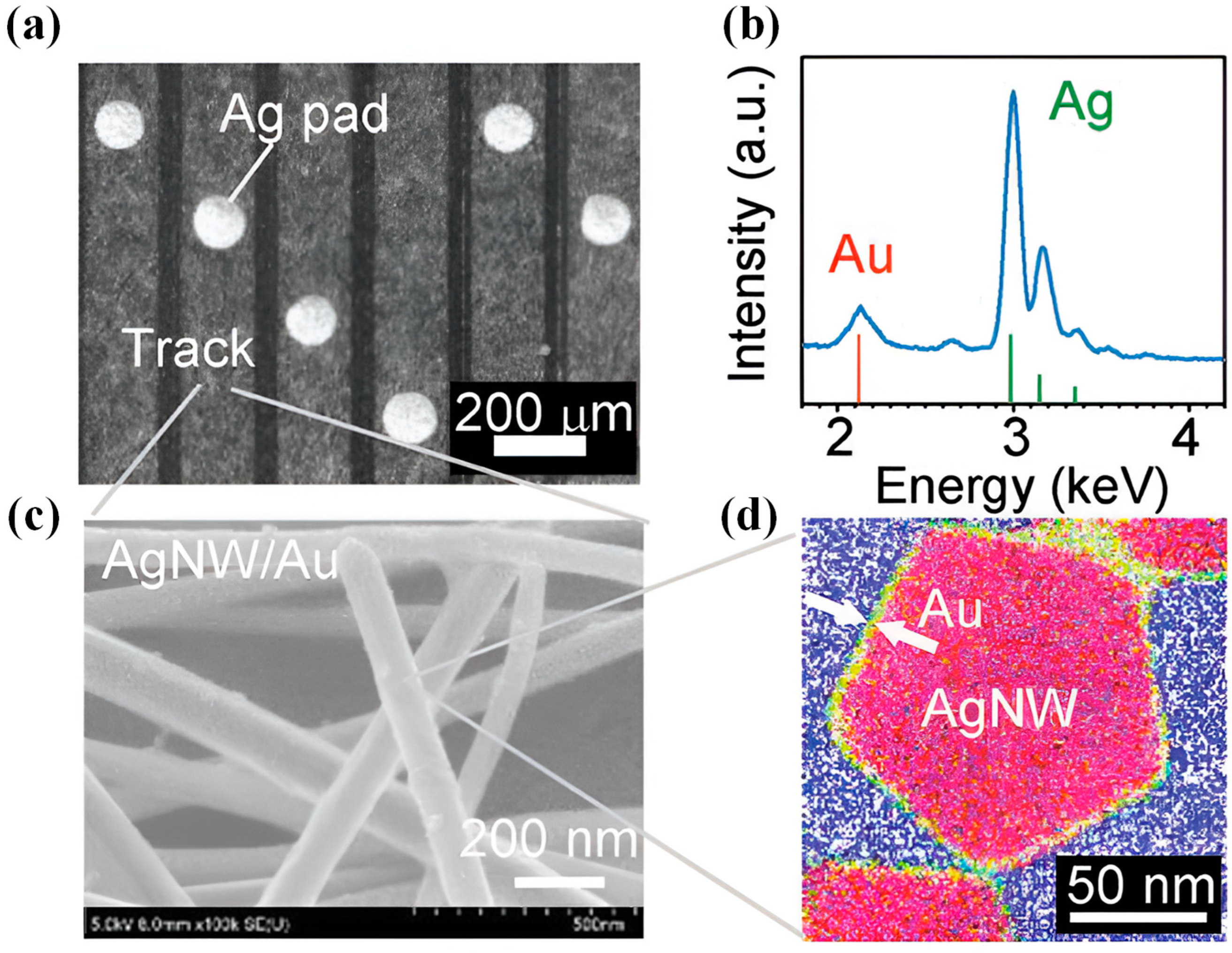
| Nanostructure | Size | Application | Effect | Reference |
|---|---|---|---|---|
| Nanosphere | Ag Core: 15 ± 3 nm Au Shell: 4.5 ± 1.0 nm | Detection of Ochratoxin A (OTA) and aflatoxin B1 (AFB1). | The LOD for OTA: 0.006 ng/mL. The LOD for AFB1: 0.03 ng/mL. | [43] |
| Nanosphere | 2.0 nm | Catalysts of aerobic oxidation of glucose in water. | The catalytic activity is 3 times higher than that of pure Au nanoparticles. | [44] |
| Nanosphere | Ag Core: 43 ± 6 nm Au Shell: 7 nm | Monitoring the 4-NTP to 4-ATP reaction while simultaneously acting as the catalyst to drive this conversion process. | The SERS sensitivity is 37 times higher than that of pure Au nanoparticles. | [32] |
| Nanosphere | 18.0 ± 5.9 nm | Colorimetric sensing for cyanide detection. | The LOD for cyanide: 0.16 ug/mL. | [45] |
| Nanosphere | 85 nm | Removal of methylene blue (MB) as a model dye pollutant under visible light. | The removal efficiency reaches 96.3% for MB, outperforming pure Ag with its 88% efficiency. | [46] |
| Nanocube | Ag Core: 38 nm in average edge length Au Shell: 0.6 nm/1.2 nm | Surface-enhanced Raman scattering (SERS) sensitivity enhancement. | The SERS sensitivity is 5.4 times higher than pure Ag nanocubes. | [37] |
| Nanocube | Ag Core: 100 ± 10 nm Au Shell: branched Au nanorods | Photothermal tumor therapy. | The photothermal conversion efficiency reaches 87.28%. | [47] |
| Nanowire | Porous surface (pore size ranging from 1 to 10 μm) | 3D porous electrodes. | High electrical conductivity (99.33–753.04 S/m) and mechanical stability. | [34] |
| Nanowire | Diameter of 130 nm and length of 2~3 um | Catalysts for CO2 to CO electrochemical reduction reaction. | Nearly 100% Faraday efficiency in 0.1 M KCl electrolyte at an overpotential of ca. −1.0 V. | [48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, S.; Tang, Z.; Huo, T.; Wu, D.; Tang, J.H. Ag/Au Bimetallic Core–Shell Nanostructures: A Review of Synthesis and Applications. J. Manuf. Mater. Process. 2025, 9, 131. https://doi.org/10.3390/jmmp9040131
He S, Tang Z, Huo T, Wu D, Tang JH. Ag/Au Bimetallic Core–Shell Nanostructures: A Review of Synthesis and Applications. Journal of Manufacturing and Materials Processing. 2025; 9(4):131. https://doi.org/10.3390/jmmp9040131
Chicago/Turabian StyleHe, Shuyue, Ziyu Tang, Tianhang Huo, Di Wu, and Jasper H. Tang. 2025. "Ag/Au Bimetallic Core–Shell Nanostructures: A Review of Synthesis and Applications" Journal of Manufacturing and Materials Processing 9, no. 4: 131. https://doi.org/10.3390/jmmp9040131
APA StyleHe, S., Tang, Z., Huo, T., Wu, D., & Tang, J. H. (2025). Ag/Au Bimetallic Core–Shell Nanostructures: A Review of Synthesis and Applications. Journal of Manufacturing and Materials Processing, 9(4), 131. https://doi.org/10.3390/jmmp9040131






